A review of age-old antimalarial drug to combat malaria: efficacy upgradation by nanotechnology based drug delivery
Immunology and Microbiology Laboratory, Department of Human Physiology with Community Health, Vidyasagar University, Midnapore-721 102, West Bengal, India
A review of age-old antimalarial drug to combat malaria: efficacy upgradation by nanotechnology based drug delivery
Satyajit Tripathy, Somenath Roy*
Immunology and Microbiology Laboratory, Department of Human Physiology with Community Health, Vidyasagar University, Midnapore-721 102, West Bengal, India
Malaria is uncontrolled burden in the world till now. Despite of different efforts to develop antimalarial drug for decades, any anti-malarial drug can able to eradicate completely till now. Many anti-malarial substances are practically ineffectual because of their physicochemical limitations, cytotoxicity, chemical instability and degradation, and limited activities against intracellular parasites. Taking into consideration, the amount of research is going to conduct in the field of nanoparticle based drug delivery systems, lead to new ways of improving the treatment of infectious diseases. The study has focused on the progress and advancement of research on nanotechnology based drug delivery to eradicate the malaria. We like to focus the efficacy of nanotechnology based drug application for the opening out of novel chemotherapeutics in laboratory research, which may show the way to better use with age-old antimalarial drug and may draw the attention of pharmaceutical industries for the improvement and designing of effective anti-malarial drugs in future.
ARTICLE INFO
Article history:
Received 10 May 2014
Received in revised form 15 June 2014
Accepted 15 July 2014
Available online 20 September 2014
Nanotechnology
Age-old drug
Drug efficacy
Antimalaria
Nanomedicine
1. Introduction
Malaria is till now one of the burdens in the world. It is leading cause of sickness and death in the developing world. The clinical manifestations of malaria are fever, chills, prostration, and anemia, whereas in severe malarial infection, metabolic acidosis, cerebral malaria, multi-organ failure, coma and death may also chase. In the world >40% population lives with some danger of constricting malaria[1,2]. About 3.3 billion people-half of the world’s population are at risk of malaria and primarily among children and pregnant women. The World Health Organization (WHO) reports that a child dies from malaria every 30 s, hence about 3 000 children who are under the age of five die each day, summing up to over 1 million deaths per year.
In the life cycle of plasmodium parasites, sporozoites infect hepatocytes and proliferate into thousands of merozoites in the liver[3]. Merozoites rip apart from the hepatocytes, come in to the blood circulation and invade red blood cells (RBCs), where they expand first into rings and then into the late forms, trophozoites and schizonts. Schizont-infected RBCs burst and liberate more merozoites, which begin the blood cycle again. Because the blood stage infection is to blame for all symptoms and pathologies of malaria.
Numbers of drug have launched by FDA against the disease. Plasmodium infected RBCs (pRBCs) are main chemotherapeutic target[4]. Several drugs are evidence for different degrees of toxicity, which limits their use as recent administration forms release the free compound in the blood and offer slight specificity regarding the targeted cells[5]. Consequently, to achieve therapeutic levels that enlarge eventually, the first concentration of the drug in the body should be elevated. Alternatively, if the administered chemical has unspecific toxicity, the low doses essential contribute to the maturity of resistant parasite strains[6].
To trim down the unfavorable effects arising during the treatment, the main entity of the modern parasitic chemotherapy is to object the drug particularly to the parasite to maximum possible coverage. Before possible thinking about the novel drug designing or drug delivery system, absolute localization of the parasite within the hostorgan, tissue or cells should known during the acute and chronic phase of the disease. Moreover, following factors must be given due thoughtfulness[7].
•The relationship between the parasite and host cell
•To reach the target organ, tissue or cells, biological barrier system to be overcome
•Total in sequence about the receptors, present on the infected cells
•About the presence of antigens or receptors on the surface of the parasite
•Pathophysiological studies of the disease
Recent investigations into the balanced delivery and targeting of pharmaceutical, curative, and analytical mediator are nanotechnology based drug delivery. These involve the recognition of accurate targets (cells and receptors) associated to definite clinical circumstances, and picking of the fitting nanocarriers to pull off the obligatory responses while reducing the side effects[8]. Till now various types of devices and strategies based on nanotechnologies suitable for drug delivery have been projected. In general, these devices may show the property of (Ⅰ) enhancement of drug absorption by facilitating diffusion through epithelium, (Ⅱ) safeguard of drug from degradation, (Ⅲ) adjustment of pharmacokinetics of drug and tissue distribution profiles, or/ and (Ⅳ) improvement of penetration and distribution into the cell[9].
Due to the intracellular nature and scattered locations of malaria, leishmaniasis, and trypanosomiasis, parasites create a great challenge to scientists minded with drug discovery and represent a significant global burden. Most of the recent researches in the field of drug development for parasitic diseases are paying attention on the biological and biopharmaceutical issues to consider in the design of delivery strategy for illuminating these parasitic infections. In addition, the role of the colloidal carriers’ liposomes, polymeric nanoparticles, and lipid nanoparticles including lipid drug conjugate (LDC) nanoparticles in optimizing the delivery of anti-malaria, anti-leishmania and antitrypanosomia agents were studied[10]. In this study we found that age-old antimalarial drugs play more effective activity when it is delivered with applying the nanotechnology.
2. Current therapeutic regimen against malaria
Malaria diseases not only cause an escalating risk to human health, but also remains a constant threat to the economic affluence of mankind. The limitation of available tools for the therapeutic treatment of most parasitic diseases has created an insecure scenario. One major limitation is drug resistance. Now, Artimisinine Combination Therapy (ACT) is frontline drug against malaria disease.
In particular, the artemisinins have the ability to kill a broad range of asexual parasite stages at safe concentrations that are repeatedly again achievable via standard dosing regimens[11]. Artemisinins have been used in traditional Chinese herbal medicine for more than 2 000 years but were not subjected to scientific inspection until the 1970s[12]. In the effort to minimize the risk of developing resistance, WHO has recommended artemisinin to use to treat uncomplicated malaria in combination with other antimalarials as artemisinin combination therapies (ACTs). With the deployment of ACTs in 2005/2006 have been found as firstline treatment in several endemic countries in Africa, the malaria cases and deaths have been reported to be on the decline[13]. Artemesinin has a very different mode of action than conventional anti-malarials, this makes is particularly useful in the treatment of resistant infections, however in order to put a stop to the development of resistance to this drug it is only recommended in combination with another non-artemesinin based therapy (Table 1). It produces a very rapid reduction in the parasite biomass with an associated reduction in clinical symptoms and causes a lessening in the transmission of gametocytes thus decreasing the potential for the multiply of resistant alleles[14].
But, it is reported, parasites that have low sensitivity to ACTs in Combodia as well as South East Asia threaten these advances[15,16]. In the past, parasites that are resistant to CQ and anti-folate emerged from this region and spread to East Coast of African and from there to the rest of Africa. If resistance emerges against ACTs, then, the result will be catastrophic considering the limited number of anti-malarial drug currently available. Furthermore, the artemisinins are known for their very short half-life[16,17]. Under considering the conclusion above, there is an urgent need to develop formulations able to reduce the side effects and also to limit the progression of drug resistance.
3. Nanotechnology based strategy against malaria
To overcome the challenge posed by malarial parasites as well as multi-drug resistant parasites, different strategies have taken to test for intracellular diagnosis and antimalarial delivery, based on nanoparticles; like functionalized nanoparticles, dendrimers, polymeric nanoparticles, liposomal nanoparticles, quantum dots, carbon nanotubes. Many scientist as well as researcher of different part of the world proposes the use of nano-medicine to address the current shortfalls of malaria therapies.
3.1. Diagnosis of malaria with nanoscience
For pure treatment of every disease, proper and precise diagnosis is highly essential. Although, the wide spread malaria treatment is started by clinical diagnostic study, but most of clinical signs like, headache, fever, anorexia, malaise, etc. are not only symptom of malaria but these overlap with other diseases[18]. In this connection, a case study shows that 1 945 children and 2 885 adult were suffering from fever but when their blood was kept undermicroscope, only 7% children and 12% adult were malaria infected[19]. In such situations, people may be treating for malaria when they do not have malaria and it may contribute high death toll in malaria endemic zone[20,21]. So, it is difficult to set up the level of parasitemia by clinical diagnosis as a consequence of various level of tolerance by different patients[22]. Clinical diagnosis should therefore be backed by other diagnostic approaches. To avoid such problem, recently researchers have evaluated few diagnostic devices, like, dendrimer-based devices, quantum dots, magnetic nanoprobes, nanoshells, and nanotubes[23].
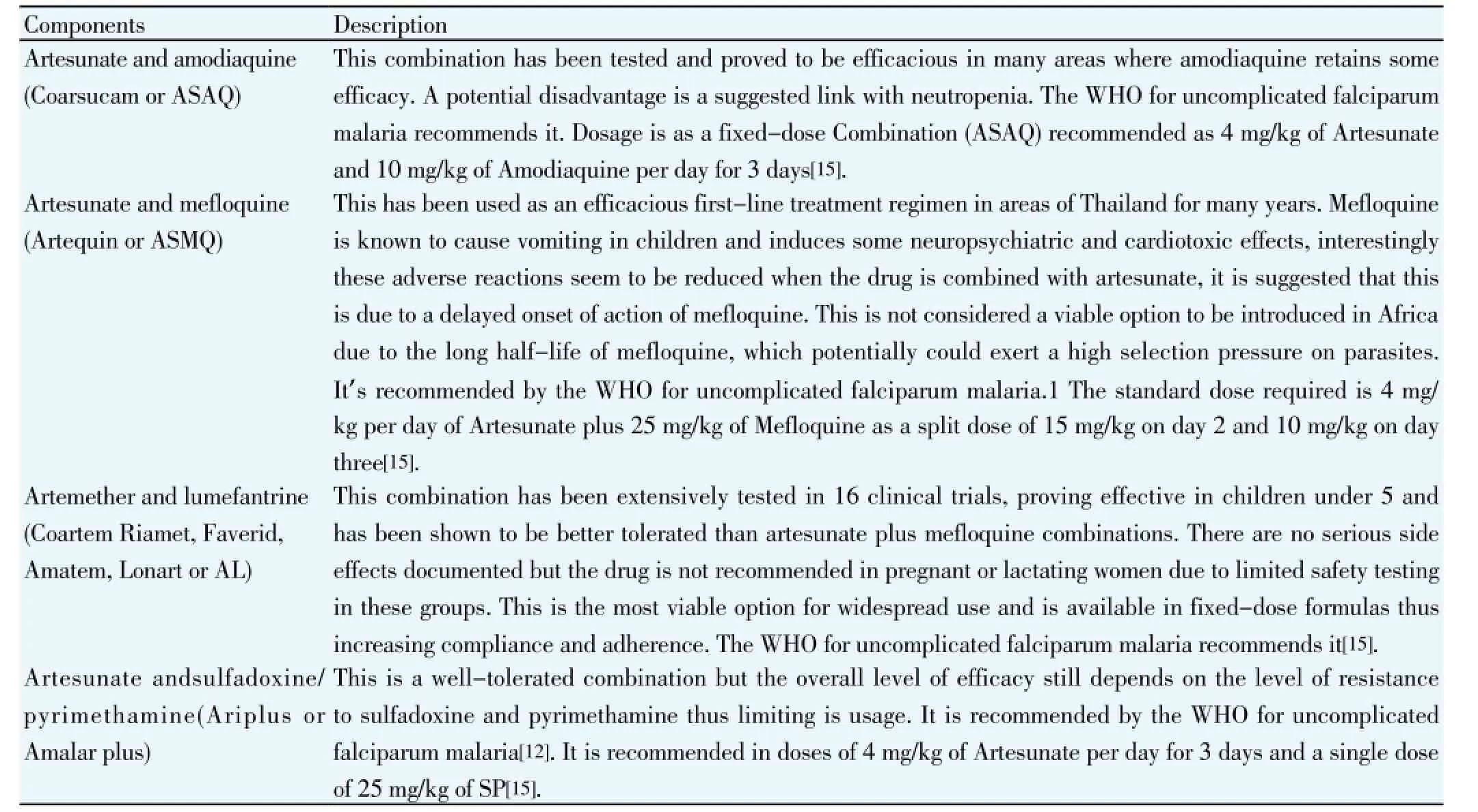
Table 1 Briefly description of recently use artimisinin combination therapy (ACT).
Malaria, a disease that targets liver and red blood cells, has a dormant phase in the liver, which may lead to recurrence after several years[24]. Though the availability of antimalarial drugs is not an issue in endemic zone, proper diagnosis is the main issue. Apart from microscopic detection, lateral flow immune-chromatographic tests (ICT) are available to overcome the confines of microscopic diagnosis. The analysis also requires highly skilled and practiced staff, and detection at low-level parasitemia is often tricky. This technique is comparable to the microscopic diagnosis and is inexpensive, even though there are concerns of false negatives. PCR-based techniques are used for the detection of low-level parasitemia[25]. Various groups are working towards the development of an inexpensive diagnostic technique that will incorporate micro fluidics and genomics integrated with nanotechnology, in order to diagnose not only the type of malaria parasite but also determine drug resistance.
3.2. Therapeutic application of age-old antimalarial drugs with nanoscience
The main drawbacks of straight malaria chemotherapy is the development of multiple drug resistance and the wandering off the point localization to intracellular parasites, resulting in high dose requirements and subsequent intolerable side effect which eventually leads to patient non compliance[26]. Different strategies to deliver anti-malarial drugs via nano particles/carriers have been evaluated. Recently, the focus is placed mainly on lipid-based (e.g., liposomes, solid lipid nanoparticles and microemulsions) and polymer-based nanodrug delivery (nanocapsules and nanospheres)[11]. These nanocarriers are known to improve the efficacy of currently available anti-malarial drugs and contribute to the formulation and delivery of new chemical entities[11]. Targeting drugs specifically to their site of action have great advantage in malaria since malaria parasites frequently develop drug resistance due to the administration of low drug concentrations in the presence of a high parasitic count. Furthermore, nanomedicine has the potential to restore the use of old and toxic drugs by modifying their bio-distribution, improve bioavailability and reducing toxicity[11]. This advantage is particularly important in malaria therapy, since the development of new dosage forms for delivering drugs to parasite-infected cells is urgently needful, especially for the anti-malarial in clinical use[27]. Nano-carriers may also allow the use of potentially toxic anti-malarial, which are still under development.
Both passive and active nanotechnology-based drug delivery systems have evaluated for malaria using thedifferent route since they are able to deliver the drug to the specific target in the human body where the malaria parasite is located. In passive targeting, conventional nanocarriers (e.g. liposomes, hydrophobic polymeric nanoparticles), or surface-modified long-circulating nanocarriers (e.g. polyethylene glycol coated particles) can be used. The drugloaded carrier accumulates at a specific site in the body, especially mononuclear phagocyte system (MPS) cells, due to physicochemical or characteristic of the nanocarries. In passive targeting red blood cells (RBCs), which are not, phagocytotic or endocytotic cells do not act as target[11,28]. In the following it has been described about different nanoparticles based different age old anti-malarial drugs delivery and improvement of their efficacy in laboratory research.
3.2.1. Therapeutic approach with quinine
Quinine discovered from the cinchona tree, and the potential uses of its bark and collection of its derivatives is used frequently in the prevention and treatment of malaria. Quinine is an alkaloid (Figure 1a) and the treatment regimen of quinine is complex. The World Health Organization recommendation for quinine is 20 mg/kg first times and 10 mg/kg 8 h for 5 d where parasites are sensitive to quinine, combined with doxycycline, tetracycline or clindamycin. The recommended method depends on the urgency of treatment and the available resources (i.e. sterilized needles for IV or IM injections). Use of quinine is characterized by a frequently experienced syndrome called cinchonism. Tinnitus (a hearing impairment), rashes, vertigo, nausea, vomiting and abdominal pain are the most common symptoms. Besides it, neurological effects have been found in some cases due to the drug’s neurotoxin properties because the drug’s interactions cause a decrease in the excitability of the motor neuron end plates and often results in functional impairment of the eighth cranial nerve, resulting in confusion, delirium and coma. Hypoglycaemia is also result of quinine treatment and therefore it is advised that glucose levels are monitored in all patients every 4-6 hours. During pregnancy, additional care in administering and monitoring the dosage is essential. Repeated or over dosage can result in renal failure and death through depression of the respiratory system.
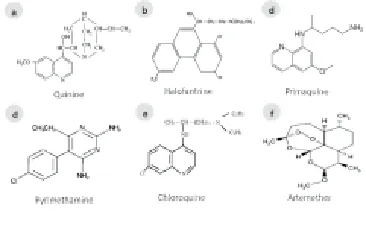
Figure 1. Chemical structure of anti-malarial drugs.
A report suggests that, intravenous administration of QN-loaded nanocapsules prepared by interfacial deposition of poly-ε-caprolactone showed more efficacy than only quinine. At 75 mg/kg/day to infected rats resulted in 100% survival, representing an almost 30% reduction compared with the free QN effective dose (105 mg/kg/day) and the pharmacokinetic parameters of nano-encapsulated QN were not significantly different from those estimated for free drug (alpha=0.05). The QN partition coefficient into infected erythrocytes doubled (6.25±0.25) when the drug was nano-encapsulated compared with the free drug (3.03± 0.07). Therefore, nano-encapsulation amplified the dealings between QN and the erythrocyte and this mechanism is responsible for the drug’s greater than before efficiency when nano-encapsulated[29]. Another study carry that, transferrin-conjugated solid lipid nanoparticles were prospective for targeted delivery of quinine di-hydrochloride to the brain for cerebral malaria medication[30].
3.2.2. Therapeutic approach with halofantrine
In 1960, the Walter Reed Army Institute of Research developed halofantrine. It is a phenanthrene methanol (Figure 1b). Its mechanism of action is similar to other anti-malarials. The cytotoxic complexes formed with ferritoporphyrin XI that cause plasmodial membrane damage. It was prescribed by physicians against drug resistant parasites infection but it is not commonly used in the treatment (prophylactic or therapeutic) of malaria due to its high cost and it has variable bioavailability and shown to have potentially high levels of cardio toxicity.
Nanotechnology has become able to reduce the bad effects of the free drug. The reduction of the cardiovascular alterations has found after injection of halofantrine nanocapsule (Hf-NC) in Plasmodium berghei infected mice. This was probably due to the ability of nanocarriers by modifying the distribution of the entrapped drug into the body. The reduction of toxic effects resulting from nanoencasulated halofentrine treatment that could be accredited to a lower free fraction of the drug available for association with the cardiac tissue as compared with the administration of the drug as a solution. Few studies reveal that the free Hf binds extensively to lipoproteins, mainly low- and highdensity lipoproteins and results the association of the Hf with the oily core of nanocapsules reduces the amount of drug bound to lipoproteins in the blood and consequently lowers the fraction that would be transported to the heart tissue through LDL receptors. However, when halofantrine is nanoencapsulated with poly-ε-caprolactone nanocapsules, leading to lowering the dose and increased solubility, significant reduction in cardiac toxicity and improved bioavailability was observed in comparison to its conventional form. It concluded that distribution and circulation time of drugs could be improved by these nanocarriers[26,31].
3.2.3. Therapeutic approach with primaquine
Primaquine, a highly active 8-aminoquinolone was use to treat malaria infection (Figure 1c). However, the drug was use in conjunction with another effective blood schizonticidal drug but there are few significant side effects. It has seen that primaquine may cause anorexia, nausea, vomiting, cramps, chest weakness, anaemia, some suppression of myeloid activity and abdominal pains. In cases of overdosage granulocytopenia may occur. Primaquine is one of the most widely used second line antimalarial acting specifically on the pre-erythrocytic schizonts. Ultimately, PQ is characterized by poor oral bioavailability and drugrelated side effects that can lead to hemolytic anaemia, gastrointestinal disturbances, heart failure and abdominal cramps[32-34].
To overcome the drawbacks of PQ, a group of researcher incorporated the drug into an oral lipid nanoemulsion with particle size in the range of 10-200 nm. The efficacy of nanodrug have been evaluated against Plasmodium berghei in infected mice and was observed at 25% lower dosage levels than the unencapsulated form.63 Lipid nanoemulsion of PQ exhibited improved oral bioavailability and was taken up preferentially by the liver with drug concentration higher at least by 45% as compared with the unencapsulated drug. It was concluded that, lipid nanoemulsion embrace an enormous promise for delivery of PQ to the liver with potential to treat latent stage malaria and reduced toxicity[7,35].
3.2.4. Therapeutic approach with pyrimethamine
Pyrimethamine is a standard antiprotozoal drug recommended for prophylaxis and treatment of malarial infections. Limited bioavailability, slow onset of action, and life-threatening side effects restrict its use. Pyrimethamine (Figure 1d) was used for the treatment of uncomplicated malaria. The mode of action of the drug is to inhibit dihydrofolate reductase in the parasite. It prevents the biosynthesis of purines and pyrimidines.
Ionically cross-linked chitosan/tripolyphosphate microparticles containing pyrimethamine has formulated[36]. The spectral analyses confirm the occurrence of microencapsulation with the outcome of stable microparticles. These microparticles should investigate further for clinical application as a controlled release matrix for the drug pyrimethamine.
An additional study showed pyrimethamine nanosuspension was prepared with the objective to improve its dissolution rate and pharmacokinetic profile. Stable pyrimethamine nanosuspension with submicron particle size was prepared by nanoprecipitation and high-pressure homogenization techniques. Nanosizing and stabilizers modified the surface characteristics of drug particles resulting in considerable increase in the dissolution rate. The in vivo pharmacokinetic profiling of pyrimethamine nanosuspension in rats showed higher AUC 0-24 hand C max compared to the plain and marketed pyrimethamine suspensions. In contrast to its plain and marketed formulation, pyrimethamine nanosuspension showed rapid onset of action (T max 0.5 h vs. 2 h). Thus, improved in vitro - in vivo kinetics indicated that nanosuspension proved to be a suitable strategy for elevating the therapeutic profile of pyrimethamine.
3.2.5. Therapeutic approach with chloroquine
Chloroquine was the more effective against malaria for long time. Chloroquine (CQ) was one of the most useful drugs ever discovered[8] CQ (Figure 1e) has numerous pharmacokinetic and pharmacological advantages over all other anti-malarial drugs over eight decades of malaria therapy. Above all CQ is cheap, relatively safe, easy to administer and was extremely effective. The immense majority of widely used antimalarial therapies have lost their efficacy over time. It has become resistant to Plasmodium falciparum (P. falciparum). CQ also now use as first line drug against other species besides a report suggest that CQ is returning to combat malaria. Malawi, a country in Africa became to replace chloroquine with the combination of sulfadoxine and pyrimethamine to treat the malaria in the year of 1993. At that time, the clinical efficacy of chloroquine was less than 50%. The molecular marker of chloroquine-resistant P. falciparum malaria subsequently declined in prevalence and was undetectable by 2001. This is suggesting that chloroquine might once again be effective in Malawi[37].
For treatment of infections by the Apicomplexan parasites, a major goal is to reach the parasitophorous vacuoles, the intracellular niche for parasite growth. In this regard, lipidbased nanocarriers and polymer- based nanovehicles have drawn considerable attention. Conventional, negatively charged chloroquine phosphate (a traditional anti-malarial) was prepared using dipalmitoylphosphatidylcholine and di palmitoylphosphatidylglycerol, leading to either gel or fluid state liposomes, which were then tested in mice infected with P. berghei. An enhanced therapeutic effect observed in case of drug encapsulated in gel state liposomes, when compared to the fluid state[38].
In this association, it is reported that chitosan tripolyphosphate conjugate nanochloroquine shows their improved efficacy than only drug against P. berghei NK65 infection in Swiss mice[39]. Interesting study has been shown by same researcher that chitosan conjugated CQ delivery is more potent then only CQ to attenuate the parasitemia and also host pathology. The study have showed that CQ in 68.5 mg/kg body weight was less effective to attenuate the parasitemia and to scavenge the oxidative stress than same amount CQ conjugated chitosan NPs[40]. So in future it may play a positive role brightly against malaria where CQ is treated and where CQ is resistant.
Researchers have also explored the helpfulness of the polymeric nanosystems as adjuvants for old antimalarial drugs that selectively target the pathogen. The antimalarial activity of the polymeric salt, chloroquine salts in polyamidoamine polymers inhibits the growth of P. falciparum and bind preferentially to Plasmodium-infected red blood cells compared to uninfected cells[41].
3.2.6. Therapeutic approach with artemether
Artemisinin, a chinese herb (qinghaosu) is used for the majority of its anti-malarial action. At present, it has been controlled by WHO guidelines. It have been used as early warning signals of impending resistance, such as delayed parasite clearance, low parasite reduction ratio, and increased gametocytemia[42]. It is also only given in combination with other anti-malarials. Furthermore artemether (AM) (Figure 1f), an inject able drug which is characterized by low bioavailability due to poor solubility and degradation in the acidic environment of the stomach and on the other hand injected AM is not very effective for rapid clearance of the parasite in severe malaria.
Other than, when (AM) was nano-encapsulated and injected, significant antimalaria activity was observed compared to free injected AM. This observation attributed to slow and sustained release of AM over a day. In active targeting, the surface of the nanocarriers can modify with cell-specific ligands (e.g., carbohydrates, proteins, peptides or antibodies)[43]. This will allow partisan accumulation of the drug in the target tissue or cell. The main goal of malaria therapy is to promote a high drug concentration in the intracellular parasitophorous vacuoles. Antimalarial drugs have to traverse through membranes (barriers) in order to access parasite targets within the infected RBC. The ability of a nanocarrier to stay in the blood stream for long time in order to perk up the interaction with infected RBCs and parasite membranes is important. This was confirmed when AM was entrapped in a long-circulating and site-specific system prepared with PEG-lysine type dendrimer and chondroitinsulfate A (CSA) for sustained and controlled delivery of the drug via the intravenous route of administration and the mean residence time increased by four folds compared to free AM.
Another report suggests that both in vitro and in vivo studies showed enhanced uptake of nano-capsuled drugs compared to free drug. Artemether, a potent and very hydrophobic antimalarial drug, was solubilized in dendrimers. Successful synthesis of dihydroartemisinin nanosuspension was done by dispersing ternary ground mixtures of polyvynilpyrolidone, sodium deoxycholate and dihydroartemisinin. Although the system did not yield a reduced particle size suspension, a more physically stable nanosuspension obtained[44].
4. Conclusions
The application of nanotechnology to drug delivery has previously had a positive relevant on many areas of medication. With continual research and expansion efforts, nanotechnology is projected to have a remarkable impact on medicine for decades to draw closer. Pharmaceutical research stands for a major tactic for discovering and developing new drugs. Current malaria therapeutics commands strategies capable to selectively deliver drugs to Plasmodium - infected red blood cells (pRBCs) in order to limit the appearance of parasite resistance. Nanoparticle coordination to anti-malarial drugs/biologically active molecules can be employed as a strategy to enhance their activity and overcome resistance to the scientific assessment of nanotechnology based remedies that might be safer, cheaper, and less toxic than current prescription medicines. The expansion of efficacy of age-old drug by using nanovehicles is still an open area for research.
Conflict of interest statement
There are no conflicts of interest to declare.
[1] WHO. World Malaria Report. [Online]. Available from: http:// www.who.int/malaria/world_malaria report2009/en/]. [Accessed on 2009].
[2] Kappe SH, Vaughan AM, Boddey JA, Cowman AF. That was then but this is now: malaria research in the time of an eradication agenda. Science 2010; 328: 862-866.
[3] Tuteja R. Malaria - an overview. FEBS J 2007; 274: 4670-4679.
[4] Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA 2007; 297: 2264-2277.
[5] Na-Bangchang K, Karbwang J. Current status of malaria chemotherapy and the role of pharmacology in antimalarial drug research and development. Fundam Clin Pharmacol 2009; 23: 387-409.
[6] White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother 1997; 41: 1413-1422.
[7] Date AA, Joshi MD, Patravale VB. Parasitic diseases: Liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv Drug Deliv Rev 2007; 59(6): 505-521.
[8] Langer R. Drug delivery and targeting (Review). Nature 1998; 392(6679 Suppl): 5-10.
[9] Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res 2006; 23:1417-1450.
[10] Chatelain E, Ioset JR. Drug discovery and development for neglected diseases: Preclinical drug development. Genesis 2009; 52-61.
[11] Santos-Magalhães NS, Mosqueira VCF. Nanotechnology applied to the treatment of malaria. Adv Drug Del Rev 2010; 62: 560-575.
[12] Maude RJ, Beare NAV, Abu Sayeed A, Chang CC, Charunwatthana P, Faiz MA, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans Royal Soc Trop Med Hyg 2009; 103: 665-671.
[13] O’Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 2205; 372: 1555-1562.
[14] WHO. Guidelines for the treatment of malaria. Second edition. [Online]. Available from: http://www.who.int/malaria/publications/ atoz/9789241547925/en/index.html [Accessed on 2010].
[15] Lim P, Alker AP, Khim N, Shah NK, Incardona S, Doung S, et al. Pfmdr1 copy number and arteminisin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar J 2009; 8: 11.
[16] Lindegardh N, Hanpithakpong W, Kamanikom B, Singhasivanon P, Socheat D, Yi P, et al. Major pitfalls in the measurement of artemisinin derivatives in plasma in clinical studies. J Chromato 2008; B 876: 54-60.
[17] Thanh NV, Cowman AF, Hipgrave D, Kim TB, Phuc BQ, Cong LD, et al. Assessment of susceptibility of Plasmodium falciparum to chloroquine, quinine, mefloquine, sulfadoxine-pyrimethamine and artemisinin in southern VietNam. Trans Royal Trop Med Hyg 2009; 95: 513-517.
[18] Bojang KA, Obaro S, Morison LA, Greenwood BM. A prospective evaluation of a clinical algorithm for the diagnosis of malaria in Gambian children. Trop Med Int Health 2000; 5: 231-236.
[19] Chandramohan D, Carneiro I, Kavishwar A, Brugha R, Desai V, Greenwood B. A clinical algorithm for the diagnosis of malaria: results of an evaluation in an area of low endemicity. Trop Med Int Health 2001; 6: 505-510.
[20] Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, et al. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ 2007; 334: 403.
[21] Luxemburger C, Nosten F, Kyle DE, Kiricharoen L, Chongsuphajaisiddhi T, White NJ. Clinical features cannot predict a diagnosis of malaria or differentiate the infecting species in children living in an area of low transmission. Trans R Soc Trop Med Hyg 1998; 92: 45-49.
[22] Rougemont A, Breslow N, Brenner E, Moret AL, Dumbo O, Dolo A, et al. Epidemiological basis for clinical diagnosis of childhood malaria in endemic zone in West Africa. Lancet 1991; 338: 1292-1295.
[23] Freitas Jr RA. Current status of nanomedicine and medical nanorobotics. J Comput Theor Nanosci 2005; 2: 1-25.
[24] Todd CW, Venkatachalam U, Escalante AA, Lal AA. Encyclopedia of infectious diseases: modern methodologies. In: M. Tibayrenc (Ed.), Malaria vaccines. New York: John Wiley & Sons; 2007, p. 137-150.
[25] Drakeley C, Reyburn H. Out with the old, in with the new: the utility of rapid diagnostic tests for malaria diagnosis in Africa. Trans R Soc Trop Med Hyg 2009; 103: 333-337.
[26] Obonyo CO, Juma EA, Ogutu BR, Vulule JM, Lau J. Amodiaquine combined with sulfadoxine/pyrimethamine versus artemisininbased combinations for the treatment of uncomplicated falciparum malaria in Africa: a meta-analysis. Trans Royal Soc Trop Med Hyg 2007; 101: 117-126.
[27] Sosnik A, Amiji M. Nanotechnology solutions for infectious diseases in developing nations. Adv Drug Deliv Rev 2010; 62: 375-377.
[28] Soma CE, Dubernet C, Barratt G, Benita S, Couvreur P. Investigation of the role of macrophages on the cytotoxicity of doxorubicin and doxorubicin-loaded nanoparticles on M5076 cells in vitro. J Cont Rel 2005; 68: 283-289.
[29] Haas SE, Bettoni CC, de Oliveira LK, Guterres SS, la Costa T. Nanoencapsulation increases quinine antimalarial efficacy against Plasmodium berghei in vivo. Int J Antimicrob Agent 2009; 34: 156-161.
[30] Gupta Y, Jain A, Jain SK. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J Pharm Pharmacol 2007; 59: 935-940.
[31] Leite EA, Grabe-Guimarães A, Guimarães HN, Machado-Coelho GLL, BarrattG, Mosqueira VCF. Cardiotoxicity reduction induced by halofantrine entrapped in nanocapsule devices. Life Sci 2007; 80: 1327-1334.
[32] Gaspar R, Préat V, Roland M. Nanoparticles of polyisohexylcyanoaerylate (PIHCA) as carriers of primaquine: formulation, physico-chemical characterization and acute toxicity. Int J Pharmace 1991; 68: 111-119.
[33] Pirson P, Steiger R, Trouet A. The disposition of free and liposomally encapsulated antimalarial primaquine in mice. Biochem Pharmacol 1982; 31: 3501-3507.
[34] Stjärnkvist P. Biodegradable microspheres: XIV. Effect of microparticle-bound primaquine on L. donovani in mice. Int J Pharmace 1993; 96: 23-32.
[35] Singh KK, Vingkar SK. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int J Pharmace 2008; 347: 136-143.
[36] Ibezim EC, Andrade CT, Barretto CMB, Odimegwul DC, Lima FFD. Ionically Cross-linked Chitosan/Tripolyphosphate Microparticles for the Controlled Delivery of Pyrimethamine. Ibnosina J Med Biomed 2011; 3: 77-87.
[37] Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, et al. Return of Chloroquine Antimalarial Efficacy in Malawi. New England J Med 2006; 355: 1959-1966.
[38] Semete B, Kalombo L, Katata L, Swai H. Nano-drug delivery systems: Advances in TB, HIV and Malaria treatment. In: AK Mishra, A. Tiwari, S.B. Mishra eds. Smart biomolecular medicine. Allahabad: VBRI Press; 2010, p. 15-52.
[39] Tripathy S, Das S, Chakraborty SP, Sahu SK, Pramanik P, Roy S. Synthesis, characterization of chitosan-tripolyphosphate conjugated chloroquine nanoparticle and its in vivo anti-malarial efficacy against rodent parasite: A dose and duration dependent approach. Int J Pharmaceu 2012; 434: 292-305.
[40] Tripathy S, KarMahapatra S, Chattopadhyay S, Das S, Dash SK, Majumder S, et al. A novel chitosan based antimalarial drug delivery against Plasmodium berghei infection. Acta Trop 2013; 128: 494-503.
[41] Urbán P, Valle Delgado JJ, Mauro N, Marques J, Manfredi A, Rottmann M, et al. Use of poly (amidoamine) drug conjugates for the delivery of antimalarials to Plasmodium. J Control Release 2014; 177: 84-95.
[42] Laufer MK. Monitoring Antimalarial Drug Efficacy: Current Challenges. Curr Infect Dis Rep 2009; 11(1): 59-65.
[43] Soma CE, Dubernet C, Barratt G, Benita S, Couvreur P. Investigation of the role of macrophages on the cytotoxicity of doxorubicin and doxorubicin-loaded nanoparticles on M5076 cells in vitro. J Contr Rel 2000; 68: 283-289.
[44] Chingunpitak J, Puttipipatkhachorn S, Chavalitshewinkoon-Petmitr P, Tozuka Y, Moribe K, Yamamoto K. Formation, physical stability and in vitro antimalarial activity of dihydroartemisinin nanosuspensions obtained by co grinding method. Drug Dev Ind Pharm 2008; 34: 314-322.
ment heading
10.1016/S1995-7645(14)60115-2
*Corresponding author: Dr. Somenath Roy, Professor, Immunology and Microbiology Laboratory, Department of Human Physiology with Community Health, Vidyasagar University, Midnapore-721 102, West Bengal, India.
Tel/Fax: (91) 3222-275329
E-mail: sroy.vu@hotmail.com
 Asian Pacific Journal of Tropical Medicine2014年9期
Asian Pacific Journal of Tropical Medicine2014年9期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Protective effect and mechanism of lithium chloride pretreatment on myocardial ischemia-reperfusion injury in rats
- Nerve protective effect of rhTPO and G-CSF on hypoxic ischemic brain damage in rats
- Protective effects of Ginseng mixture on myocardial fibrosis in rats
- Effect of siRNA interference on nerve growth factor in intervertebral disc inflammation rats
- Runx3 might participate in regulating dendriti cell function in patients with irritable bowel syndrome
- Sonic Hedgehog signaling pathway in primary liver cancer cells
