Correlation study between the polymorphism of repetitive sequence in gene CAG of androgen receptor and the occurrence and progression of prostate cancer
Xiao-Lei Zhai, Xiao-Wei Qu, Liang Guo, Qian-He Ha
Department of Urology, People’s Hospital of Zhengzhou, Henan Province, China
Correlation study between the polymorphism of repetitive sequence in gene CAG of androgen receptor and the occurrence and progression of prostate cancer
Xiao-Lei Zhai, Xiao-Wei Qu, Liang Guo, Qian-He Ha*
Department of Urology, People’s Hospital of Zhengzhou, Henan Province, China
Objective: To explore the relation between the polymorphism of repetitive sequence in gene CAG of androgen receptor (AR) and the susceptibility and clinical stages as well as pathological grading of prostate cancer among Han population. Method: Sixty-eight cases with prostate cancer hospitalized in Urinary Surgery Department from Feb. 2010 to Feb. 2012 and 60 healthy cases were chosen as research subjects. Methods of PCR and direct sequencing were adopted to detect DNA sequence of AR gene and the length of repetitive sequence in CAG. Results: The lengths of repetitive sequence in CAG of patients with prostate cancer and healthy people were (22.3±4.6) and (23.0±4.9), respectively showing no statistical significance. Comparing length (repetitive sequence of CAG)>22, those with that < 22 suffer a remarkably higher risk of prostate cancer (P<0.05). The number of repetitive sequence in CAG of patients at clinical stage C-D was less than that of patients at stage B, and the number of repetitive sequence in CAG of patients with poorly differentiated prostate cancer was also less than that of patients with moderately and highly differentiated prostate cancer. But there was no statistical significance int the difference (P>0.05); the proportion of patients with length <22 at clinical stage C-D was much larger than that of patients at clinical stage B (P<0.05), and as the aggravation of pathological grading, the proportion of patients with the length <22 was also remarkably increased and there was significant difference between patients with highly differentiated prostate cancer and those with poorly differentiated prostate cancer (P<0.05). Conclusions: There is correlation between the occurrence and development of prostate cancer in Han population and the polymorphism of repetitive sequence in gene CAG of androgen receptor. The less the number of repetitive sequence in CAG is, the higher the risk of prostate cancer will be and the more severe the clinical stage and pathological grading will be.
ARTICLE INFO
Article history:
Received 10 September 2013
Received in revised form 15 October 2013
Accepted 15 December 2013
Available online 20 April 2014
Prostate cancer
1. Introduction
Prostate cancer (Pca) is a malignant tumor of urinary system with remarkably biological genetic characteristics and the rate of its occurrence is high in Europe and the United State[1] but it is low in Asia. So far, its exact pathogenesis and the reason of different prevalence among different races are not clear. Studies in recent years showed that the gene polymorphism of androgen receptor (AR), especially the polymorphism of repetitive sequence in CAG (the first exon), is closely related to the occurrence and development of prostate cancer[2-5]. But most of the studies focused on the population in Europe and the United State where the risk of prostate cancer is high. Study reports about the polymorphism of related susceptibility gene of prostate cancer in Han population in our country are few. To make a further study about the relation between the polymorphism of repetitive sequence in CAG of androgen receptor in Han population and the biological characteristics of prostate cancer, we made a comparison about the polymorphism of repetitive sequence in CAG among the patients at the same clinical stage and with different pathological grading and healthy people.
2. Materials and methods
2.1. General materials
Sixty-eight patients who came to urinary surgery department of our hospital during the period from Feb. 2010 to Feb. 2012 were enrolled. They were all diagnosed as prostate cancer by biopsy or operation and pathological examination. The range of their ages was 54-86 years and the average age was (67.4±7.8) years according to Whitmore-Jewett, there are 42 cases at stage B, 18 cases at stage C and 8 cases at stage D; according to Gleason evaluation standards, there are 48 cases with highly differentiated Pca (2-4 scores), 11 cases with moderately differentiated Pca (5-7 scores) and 9 cases with poorly differentiated Pca (8-10 scores). Sixty healthy cases (without the history of hyperplasia of prostate gland and prostate cancer) who came to the examination center in our hospital during the same period were chosen as control group. The range of their ages was 53-87 and the average age was (67.9±8.9) years. All the subjects were Han population and agreed to participate in the study.
2.2. Methods
2.2.1. Extraction of DNA in peripheral blood
Peripheral venous blood 4 mL was collected from each subject with a empty stomach. 0.2% EDTA-Na2 was used for anti-freezing. Improved salting out method[6] was used to extract DNA (Rapid extraction of genomic DNA Kit, Beijing SaiBaiSheng Gene Technology Co., Ltd.) and DNA was stored in fridge at -70 ℃.
2.2.2. PCR amplification of repetitive sequence in CAG
Two pairs of primers were synthesized according to Irvine RA methods[7,8] and two rounds of PCR reaction were performed on them. The upstream primer F1 was 5’-GTGCGCGAAGTGATCCAGAA-3’ and R1 was 5’-TCTGGGACGCAACCTCTCTC-3’; the downstream primer F2 was 5’-AGAGGCCGCGAGCGCAGCAC-CTC-3’ and R2 was 5’-GCTGTGAAGGTTGCTGTTCCTCAT-3’. PCR reaction system 50 μL contains genomic DNA 100ng. Amplification parameters: the reaction conditions of the first round were 60s denaturation at 94 ℃, 60s annealing at 55 ℃ and stretch 90s at 72 ℃ and there were 17 circulations in total; the reaction conditions of the first round were 60s denaturation at 94 ℃, 60s annealing at 66 ℃ and stretch 90s at 72 ℃ and there were 28 circulations in total.
2.2.3. Analysis of amplified products
PCR products were placed on agarose gel for electrophoresis. The existence of objective gene fragment (200-250 bp) was confirmed. The products were purified (OMEGA Bio-tek Company Kit). The DNA sequence of products and the length of repetitive sequence in CAG were measured by ABI377DNA sequencer (Shanghai Boya Ltd.).
2.3. Statistic analysis
Software SPSS15.0 was used for statistical processing, measurement data were symbolized byas well as single factor analysis of variance were used. Difference were considered as significant at P<0.05.
3. Results
3.1. Relation between polymorphism of repetitive sequence in CAG of AR and the susceptibility of Pca
The length of repetitive sequence in CAG of 68 patients with Pca was 16-31 and the average length was (22.3±4.6); length of that of healthy cases was 18-31 and the average length was (23.0±4.9). There was no significant difference in the average length of repetitive sequence between CAG of patients and that of healthy people (t=0.83, P>0.05). Using 22 as the critical value[9], we divided them into short CAG group (the repetitive sequence of CAG <22) and long CAG group (the repetitive sequence≥22 ). There was significant difference between the two groups (x2=3.97, P<0.05) (Table 1).
3.2. Relation between the polymorphism of repetitive sequence in CAG of AR and the clinical stage of Pca
The average length of repetitive sequence in CAG of patients with prostate cancer at stage B was (22.8±3.1) and the average length of repetitive sequence in CAG of patients with prostate cancer at stage C-D was (21.5±3.5). There was no statistical significance in the difference (t=1.58, P>0.05). There were 15 cases (35.7%) in short CAG group at stage B and 27 cases (64.3%) in long CAG group; there are 17 cases (65.4%) in short CAG group at stage C-D and 9 cases (34.6%) in long CAG group. There was remarkable difference in the comparison between patients at stage B and those at stage C-D (x2=4.55, P<0.05) (Table 2).
3.3. Relation between the polymorphism of repetitive sequence in CAG of AR and the pathological grading of Pca
The lengths of repetitive sequence in CAG of patients with pooly, moderately and highly differentiated prostate cancer were (20.3±3.6), (21.3±3.8) and (22.9±4.3), respectively. The length of repetitive sequence in CAG of patients with poorly differentiated prostate cancer was shorter than that of those with moderately and highly differentiated prostate cancerwhile there was no statistical significance in the difference (t=1.14, 1.70, P>0.05). The distribution of repetitive sequence in CAG of AR in short CAG group expanded as the pathological grading of prostate cancer aggravated. There was significant difference in the comparison between patients with highly differentiated Pca and those with poorly differentiated Pca (x2=6.13, P<0.05) (Table 3).

Table 1 Comparison between the polymorphism of repetitive sequence in CAG of AR of patients with Pca and healthy people.
4. Discussion
Prostate cancer is one of the malignant tumors in males. Its pathogenesis is closely related to androgen and the function of androgen is adjusted by androgen receptor (AR). AR is the member of the superfamily of steroid receptor and it mainly plays its role in N gene. The most remarkable characteristic of AR is that it contains one CAG with triad nucleotide repetitive sequences. The number of triad nucleotide repetitive sequences in CAG is 9-36 in healthy people[10-11]. The American Blacks have smallest number of that, the Whites rank the second and Asian Americans have the largest number of that, which just conforms to epidemiological investigation which showed the risk of prostate cancer in different races[12-14]. This may show that the polymorphism of repetitive sequence in CAG of AR is relevant with the occurrence and development of prostate cancer.
Currently, disputes about the relation between the polymorphism of repetitive sequence in CAG of AR and prostate cancer exist in both domestic and foreign studies. Some scholars argued that the less the number of repetitive sequence in CAG was, the higher the risk of prostate cancer would be[15-17]. Studies conducted by Akinloye and his colleagues[18] showed that every time the CAG fragment was increased by one, the risk of prostate cancer would be decreased by 3%. But other scholars believed that the length of repetitive sequence in CAG was not the risk factor of prostate cancer[19,20] and was not significantly related to the clinical stages and pathological grading of prostate cancer[21-23]. The studies on the population of Brazil conducted by Silva Neto B and his colleagues[24,25] showed that compared with that of normal people, the length of repetitive sequence in CAG of patients with prostate cancer was not remarkably shortened.
In our study, we firstly made a comparison about the polymorphism of repetitive sequence in CAG of AR between the 68 cases with prostate cancer and 60 healthy cases and found there was no significant difference in the average length of repetitive sequence in CAG in the two groups. To further understand the relation between the polymorphism of repetitive sequence in CAG and the susceptibility of prostate cancer, we, according to the reference and regarding 22 as standard[9], divided them into short CAG group (the repetitive sequence of CAG <22) and long CAG group (the repetitive sequence≥22 ). The results showed that the risk of prostate cancer in short CAG group increased remarkably (P<0.05), which conforms to most of foreign reports[26-30]. Then we studied the relation between the polymorphism of repetitive sequence in CAG of AR and the clinical stages and pathological grading of prostate cancer. The results showed that the number of repetitive sequence in CAG of patients at clinical stage C-D is less than that of patients at stage B, and the number of repetitive sequence in CAG of patients with poorly differentiated prostate cancer is also less than that of patients with moderately and highly differentiated prostate cancer. But there is no statistical significance in the difference, which may be caused by the small amount of samples. Still regarding 22 as the critical value, through comparison, it was found that the proportion of patients at clinical stage C-D in short CAG group is remarkably larger than those at stage B. As the aggravation of pathological grading, the proportion of patients with the length <22 is remarkably increased and there is significant difference between patients with highly differentiated prostate cancer and those with poorly differentiated prostate cancer.
To sum up, our study holds the opinion that there is relation between the occurrence and development of prostate cancer in Han population and the polymorphism of repetitive sequence in CAG of AR. The less the number of repetitive sequence in CAG is, the higher the risk of prostate cancer will be and the more advance the clinical stage and pathological grading will be. However, since the samples are relatively small, there is no statistical difference in the average length of repetitive sequence in CAG among groups, which needs to be further studied with more samples.

Table 2 Relation between the polymorphism of repetitive sequence in CAG of AR and the clinical stage of Pca.
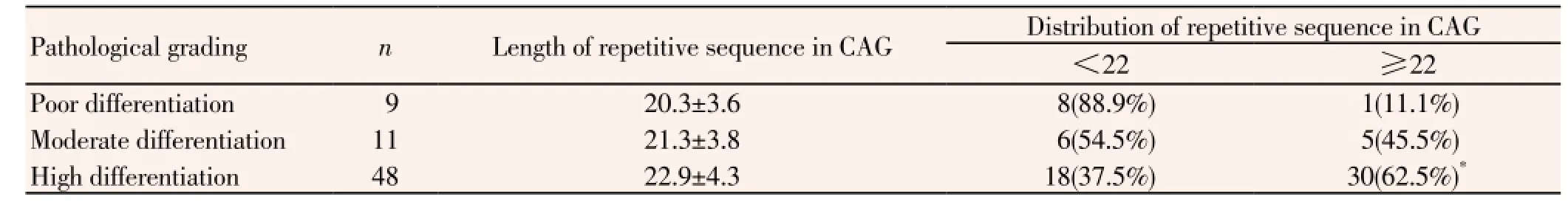
Table 3 Relation between the polymorphism of repetitive sequence in CAG of AR and the pathological grading of Pca.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Chavarro JE, Kenfield SA, Stampfer MJ, Loda M, Campos H, Sesso HD, et al. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am J Epidemiol 2013; 178(8): 1246-1255.
[2] Buchanan G, Need EF, Barrett JM, Bianco-Miotto T, Thompson VC, Butler LM, et al. Corepressor effect on androgen receptor activity varies with the length of the CAG encoded polyglutamine repeat and is dependent on receptor/corepressor ratio in prostate cancer cells. Mol Cell Endocrinol 2011; 342(1-2): 20-31.
[3] Kumar R, Atamna H, Zakharov MN, Bhasin S, Khan SH, Jasuja R. Role of the androgen receptor CAG repeat polymorphism in prostate cancer, and spinal and bulbar muscular atrophy. Life Sci 2011; 88(13):565-571.
[4] Wen S, Niu Y, Lee SO, Chang C. Androgen receptor (AR) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat Rev 2013; 13 (7): 139-144.
[5] Xu H, Yu X, Qu S, Sui D. Juglone, isolated from Juglans mandshurica Maxim, induces apoptosis via down-regulation of AR expression in human prostate cancer LNCaP cells. Bioorg Med Chem Lett 2013; 23(12): 3631-3634.
[6] Molinari J, Eskes C, Andres E. Improved procedures for in vitro skin irritation testing of sticky and greasy natural botanicals. Toxicol In Vitro 2013, 27(1): 441-450.
[7] Semaka A, Kay C, Doty C. CAG size-specific risk estimates for intermediate allele repeat instability in Huntington disease. J Med Genet 2013; 50(10): 696-703.
[8] Lindström S, Ma J, Altshuler D, Giovannucci E, Riboli E, Albanes D, et al. A large study of androgen receptor germline variants and their relation to sex hormone levels and prostate cancer risk. J Clin Endocrinol Metab 2010; 95(9): 121-127.
[9] Sun JH, Lee SA. Association between CAG repeat polymorphisms and the risk of prostate cancer: A meta-analysis by race, study design and the number of (CAG)n repeat polymorphisms. Int J Mol Med 2013; 32(5): 1195-1203.
[10] Zhang T, Liang W, Fang M, Yu J, Ni Y, Li Z. Association of the CAG repeat polymorphisms in androgen receptor gene with polycystic ovary syndrome: a systemic review and meta-analysis. Gene 2013; 524(2): 161-167.
[11] Baculescu N. The role of androgen receptor activity mediated by the CAG repeat polymorphism in the pathogenesis of PCOS. J Med Life 2013; 6(1): 18-25.
[12] Gottlieb B, Alvarado C, Wang C. Making sense of intratumor genetic heterogeneity: altered frequency of androgen receptor CAG repeat length variants in breast cancer tissues. Hum Mutat 2013; 34(4): 610-618.
[13] Mariani S, Musumeci B, Basciani S. Lack of influence of the androgen receptor gene CAG-repeat polymorphism on clinical and electrocardiographic manifestations of the Brugada syndrome in man. Clin Med Insights Cardiol 2012; 6: 145-152.
[14] Folland JP, Mc Cauley TM, Phypers C, Hanson B, Mastana SS. The relationship of testosterone and AR CAG repeat genotype with knee extensor muscle function of young and older men. Exp Gerontol 2012; 47(6): 437-443.
[15] Misra D, Xie W, Regan MM. Germline CAG repeat length of the androgen receptor and time to progression in patients with prostate cancer treated with androgen deprivation therapy. BJU Int 2011; 108(7): 1086-1091.
[16] Kristal AR, Price DK, Till C, Schenk JM, Neuhouser ML, Ockers S, et a1. Androgen receptor CAG repeat length is not associated with the risk of incident symptomatic benign prostatic hyperplasia: results from the Prostate Cancer Prevention Trial. Prostate 2010; 70(6): 584-590.
[17] Yepuru M, Wu Z, Kulkarni A. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin Cancer Res 2013; 19(20): 5613-5625.
[18] Akinloye O, Gromoll J, Simoni M. Variation in CAG and GGN repeat lengths and CAG/GGN haplotype in androgen receptor gene polymorphism and prostate carcinoma in Nigerians. Br J Biomed Sci 2011; 68(3): 138-142.
[19] Buchanan G, Need EF, Barrett JM, Bianco-Miotto T, Thompson VC, Butler LM, et al. Corepressor effect on androgen receptor activity varies with the length of the CAG encoded polyglutamine repeat and is dependent on receptor/corepressor ratio in prostate cancer cells. Mol Cell Endocrinol 2011; 342(1-2): 20-31.
[20] Zeigler-Johnson C, Weber A, Spangler E, Panossian S, Rebbeck TR, Malkowicz SB. A Relationship of obesity, androgen receptor genotypes and biochemical failure after radical prostatectomy. Prostate 2012; 72(9): 984-990.
[21] Simanainen U, Brogley M, Gao YR, Jimenez M, Harwood DT, Handelsman DJ, et al. Length of the human androgen receptor glutamine tract determines androgen sensitivity in vivo. Mol Cell Endocrinol 2011; 342(1-2): 81-86.
[22] Misra D, Xie W, Regan MM, Ross RW, Lee GS, Germain D, et al. Germline CAG repeat length of the androgen receptor and time to progression in patients with prostate cancer treated with androgen deprivation therapy. BJU Int 2011; 108(7): 1086-1091.
[23] Soni A, Bansal A, Mishra AK, Batra J, Singh LC, Chakraborty A, et al. Association of androgen receptor, prostate-specific antigen, and CYP19 gene polymorphisms with prostate carcinoma and benign prostatic hyperplasia in a north Indian population. Genet Test Mol Biomarkers 2012; 16(8): 835-840.
[24] Silva Neto B, Koff WJ, Biolchi V, Brenner C, Biolo KD, Spritzer PM, et al. Polymorphic CAG and GGC repeat lengths in the androgen receptor gene and prostate cancer risk: analysis of a Brazilian population. Cancer Invest 2008; 26(1): 74-80.
[25] Kumar R, Atamna H, Zakharov MN, Bhasin S, Khan SH, Jasuja R. Role of the androgen receptor CAG repeat polymorphism in prostate cancer, and spinal and bulbar muscular atrophy. Life Sci 2011; 88(13): 565-571.
[26] Björk C, Giwercman YL. Androgen receptor CAG repeat length modifies the effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on receptor activity in human prostate cells. Reprod Toxicol 2013; 35: 144-149.
[27] Madjunkova S, Eftimov A, Georgiev V, Petrovski D, Dimovski AJ, Plaseska-Karanfilska D. CAG repeat number in the androgen receptor gene and prostate cancer. Balkan J Med Genet 2012; 15(1): 31-36.
[28] Gu M, Dong X, Zhang X, Goulian BS, Modarressi MH. The CAG repeat polymorphism of androgen receptor gene and prostate cancer: a meta-analysis. Mol Biol Rep 2012; 39(3): 2615-2624.
[29] Ashtiani ZO, Hasheminasab SM, Ayati M. Are GSTM1, GSTT1 and CAG repeat length of androgen receptor gene polymorphisms associated with risk of prostate cancer in Iranian patients. Pathol Oncol Res 2011; 17(2): 269-275.
[30] Alptekin D, Izmirli M, Bayazit Y, Luleyap HU, Yilmaz MB, Soyupak B, et al. Evaluation of the effects of androgen receptor gene trinucleotide repeats and prostate-specific antigen gene polymorphisms on prostate cancer. Genet Mol Res 2012; 11(2): 1424-1432.
ment heading
10.1016/S1995-7645(14)60043-2
*Corresponding author: Qian-He Han, Department of Urology, People's Hospital of Zhengzhou, No. 33, Huanghe Road, Zhengzhou 450003. Henan Province, China.
E-mail: hangqianhe@126.com
Foundation Project: This research was supported by special fund for provincial science and technology cooperation project by Science and Technology Department of Henan province (122106000042).
Androgen receptor
Polymorphism of CAG
Gene
 Asian Pacific Journal of Tropical Medicine2014年4期
Asian Pacific Journal of Tropical Medicine2014年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats
- Correlation of expression of STAT3, VEGF and differentiation of Th17 cells in psoriasis vulgaris of guinea pig
- Effect of anesthesia on cognitive status and MMP-2 expression in rats
- Ultrasonic diagnosis and vasoactive substances examination in patients with cirrhosis
- Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines
- Effect of RSCs combined with COP-1 on optic nerve damage in glaucoma rat model
