Co-positivity of anti-dengue virus and anti-Japanese encephalitis virus IgM in endemic area: co-infection or cross reactivity?
Department of Microbiology, K.G. Medical University, Lucknow-226003, India
Co-positivity of anti-dengue virus and anti-Japanese encephalitis virus IgM in endemic area: co-infection or cross reactivity?
Kaleshwar Prasad Singh, Gitika Mishra, Parul Jain, Nidhi Pandey, Rachna Nagar, Shikha Gupta, Shantanu Prakash, Om Prakash, Danish Nasar Khan, Sakshi Shrivastav, Desh Deepak Singh, Amita Jain*
Department of Microbiology, K.G. Medical University, Lucknow-226003, India
Objective: To report high co-positivity of anti-dengue virus (DV) and anti-Japanese encephalitis virus (JEV) IgM in an area endemic for both the viruses and to discuss the possibilities of coinfection. Methods: Serum samples from the patients who presented with fever, suspected central nervous system infection and thrombocytopenia, were tested for anti-DV IgM and anti-JEV IgM antibodies. Conventional reverse transcriptase polymerase chain reaction was done for detection of DV RNA and JEV RNA. Results: Of 1 410 patient sera tested for anti-DV and anti-JEV antibodies, 129 (9.14%) were co-positive for both. This co-positivity was observed only in those months when anti-JEV IgM positivity was high. Titers of both anti-DV IgM and anti-JEV IgM were high in most of the co-positive cases. Among these 129 co-positive cases, 76 were tested by conventional reverse transcriptase polymerase chain reaction for both flaviviruses, of which eight cases were co-positive for DV and JEV. Conclusions: Co-infection with more than one flavivirus species can occur in hyperendemic areas.
ARTICLE INFO
Article history:
Received 10 October 2013
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Co-infection
Co-circulation
Dengue virus
IgM
Japanese encephalitis virus
Reverse transcriptase polymerase chain reaction
1. Introduction
Flavivirus infections are a significant public health problem. The two medically important and closely-related members of Flaviviridae family include dengue virus (DV) and Japanese encephalitis virus (JEV). In the most areas of Asia, DV and JEV co-circulate[1]. In the Indian subcontinent and Southeast Asia, they are important causes of human disease and mortality. JEV and DV exhibit significant serological cross-reactivity, which can complicate assessment of the relative burdens of each in co-endemic areas and also make the serological diagnosis of both the diseases difficult[2-4]. DV (types 1, 2, 3 and 4) typically cause dengue fever (DF) and dengue haemorrhagic fever/ dengue shock syndrome (DHF/DSS), while JEV typically causes acute encephalitis syndrome. However, it has been reported that in some cases, DV causes encephalopathy[5-7], while JEV causes an undifferentiated febrile illness[8]. As the diagnosis of viral infection on the basis of clinical syndromes is not reliable, laboratory diagnosis is essential for discrimination between DV and JEV infections, especially in endemic areas where both the viruses are cocirculating.
Co-infection with JEV and DV is possible in endemic areas, hence, co-positivity for both anti-DV IgM and anti-JEV IgM antibodies should be investigated to differentiate co-infection from cross-reactivity. We have been observing the co-positivity of anti-DV and anti-JEV IgM in cases with overlapping clinical symptoms of DV infection and JEV infection. To provide the evidence of true co-infection, we retrospectively tested the samples from these cases and analyzed the laboratory data.
2. Materials and methods
We retrospectively retrieved the clinical samples [serum and/or cerebrospinal fluid (CSF)] from the patients who presented with a suspected central nervous system infection and thrombocytopenia (platelet counts less than 100 000/mL) during July 2008 to October 2011. Central nervous system infections were suspected in patients with fever and at least one of the followings: altered sensorium, focal neurological signs, or convulsions. Samples were stored at -70 ℃ at Grade 1 viral diagnostic laboratory. Relevant clinical and demographic data were retrieved from the laboratory records. All the serum samples were tested for anti-DV IgM antibody by IgM antibody capture ELISA (MAC ELISA) kit from IVD Research Inc. and for anti-JEV IgM antibody by MACELISA kit (JEV-Chex-Xcyton), according to manufacturer’s instructions. For anti-DV IgM antibody, samples with optical absorbance values ≥0.5 were considered positive, while for anti-JEV IgM antibody, samples having a value of ≥100 ELISA units were considered positive. Convalescent samples were not available from patients, since it was a retrospective study.
Selected CSF and serum samples from patients showing co-positivity were tested for both DV RNA and JEV RNA. Criteria for selection are as follows: duration of illness <7 days and availability of >250 μL volume of both serum and CSF. Only 76 cases fulfilled our selection criteria. RNA was extracted using Trizol (Sigma-Aldrich) according to the manufacturer’s recommendations and two-step reverse transcriptase polymerase chain reaction (RT-PCR) for identification of DV was carried out following the protocol of Lanciottiet al[9]. For the detection of JEV RNA, extracted RNA samples were amplified using method of Yanget al[10]. PCR products were visualized after electrophoresis on 2% agarose gel.
3. Results
During a period of 3.25 years, total 1 410 cases fitted our clinical case definition, of which 423 (30.00%) were positive for anti-DV IgM antibody alone, 160 (11.34 %) were positive for anti-JEV IgM antibody alone and 129 (9.14%) were positive for both anti-DV IgM and anti-JEV IgM antibodies. Age wise analysis of anti-DV IgM and anti-JEV IgM positivity is detailed in Table 1. Demographic, geographic and clinical details of the patients were analyzed in three groups: group 1, positive for anti-DV IgM alone; group 2, positive for anti-JEV IgM alone; and group 3, positive for both anti-DV IgM and anti-JEV IgM. Fever was the most common presenting symptom followed by focal neurological deficit, altered sensorium, seizures, headache and vomiting. A few cases also had haemorrhage, petechial rashes, joint pain and breathlessness. For geographical analysis, the state of Uttar Pradesh was divided into five zones, that is, western, eastern, central, northern and southern, as shown in Figure 1. The detail of demographic, geographical and clinical analysis in all the three groups is shown in Table 2. There was no significant difference in clinical/demographic features of DV/JEV infected/co-infected cases (Table 2).

Figure 1. Map of Uttar Pradesh depicting the five zones.
Cases positive for anti-DV IgM were seen throughout the year with a peak in July, September and October, while cases positive for anti-JEV IgM were seen only during September and October. Highest co-positivity was also seen in September (Figure 2).

Table 1 Age wise distribution of patients positive for both anti-DV IgM and anti-JEV IgM.

Table 2 Characterization of DV/JEV suspected cases.
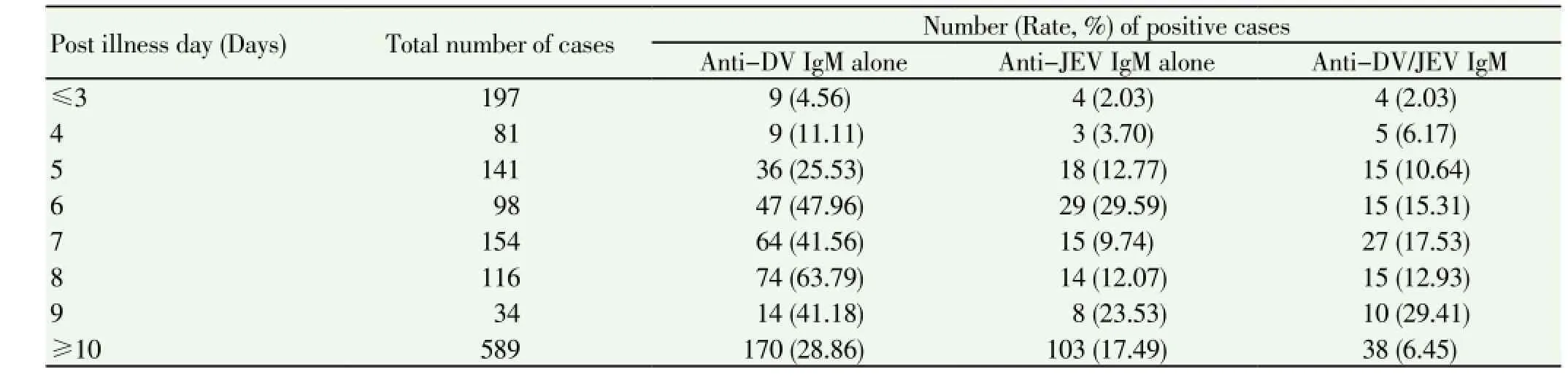
Table 3 Distribution of DV/JEV suspected cases according to post illness day.

Figure 2. Line graph showing anti-DV/JEV IgM positivity during July 2008-October 2011.
Results of IgM detection in relation to duration of illness are shown in Table 3. Total 278 cases presented with illness of <5 days duration, of which 59.89% cases were positive for antibody detection, while of 1 132 cases who presented with illness of 5 or more days, 12.23% cases were positive.
During the study period, 3 168 samples were tested by the Grade 1 Viral diagnostic laboratory for anti-DV IgM alone, of which 1 506 (47.54%) were positive, and 2 015 samples from suspected Japanese encephalitis cases were tested for anti-JEV IgM antibody, of which 463 (22.98%) were positive (data retrieved from laboratory results. Details not shown). The positivity rate for both JEV and DV in these samples was comparable to the positivity rate (39.21% and 20.63%) seen in the 1 410 study samples.
IgM antibody levels of patients co-positive for both anti-DV and anti-JEV IgM antibodies were plotted on a scatter graph (Figure 3). It can be seen that in most of the patients, levels of both anti-DV/JEV IgM antibodies were much higher than the cut off value.
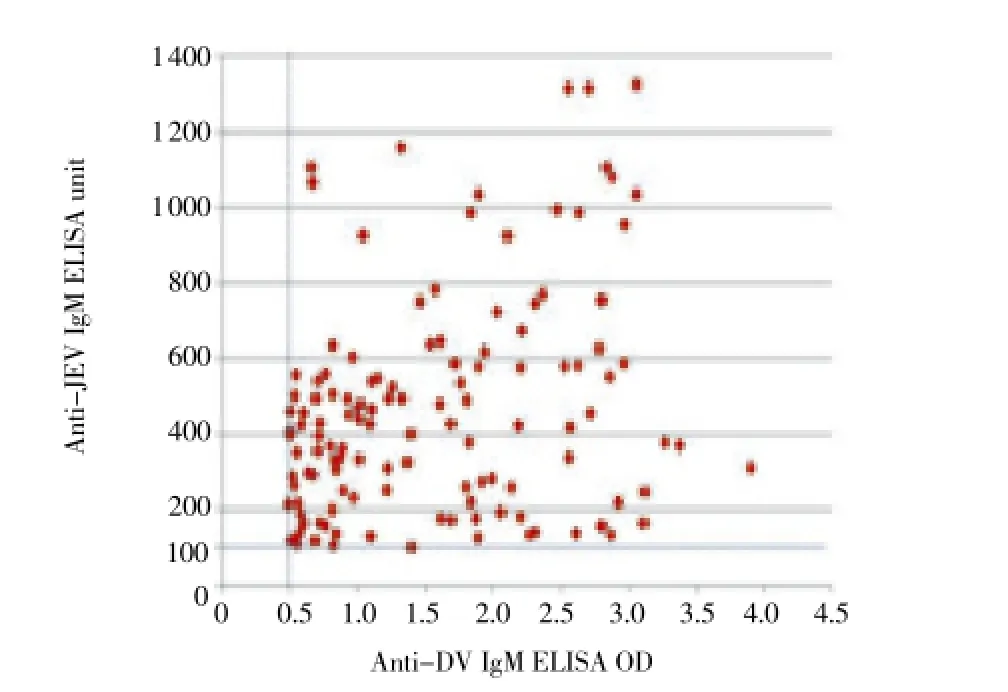
Figure 3. Scattered graph of DV IgM ELISA optical density (OD) and JEV IgM ELISA unit from 129 acute phase patients who were tested positive for both anti-DV and anti-JEV IgM antibodies.The cut-off value for was ≥0.5, while that for JEV was≥100 ELISA unit.
Both CSF and sera from 76 cases were tested for both DV/ JEV RNA by conventional RT-PCR. Thirty patients were tested positive, eight cases were co-positive for both DV/ JEV RNA (Six in serum only and two in both serum and CSF), twelve were positive for DV RNA alone (ten from serum and two from CSF), and ten were positive for JEV RNA alone (six from serum and four from CSF) (Table 4). No nucleic acid was detected from remaining 46 cases. Figure 4 shows the gel picture of bands of both DV and JEV RNA in CSF and serum of a case co-positive for both anti-DV IgM and anti-JEV IgM (case ID: V802). All the DV detected were of serotype 2. Of the eight co-positive cases, seven were positive for anti-DV IgM and seven were positive for anti-JEV IgM, with low to moderate values; high values were not seen.

Figure 4. Agarose gel analysis of amplified products from CSF and serum samples of a patient positive for both DV and JEV RNA (Case ID: V802).Lane M: 100 bp DNA Ladder (100-1 200 bp); Lane 1: Serum sample showing amplified product of 119 bp (positive for DEV serotype 2 RNA); Lane 2: CSF sample showing amplified product of 119 bp (positive for DEV serotype 2 RNA); Lane 3: Serum sample showing amplified product of 146 bp (positive for JEV RNA); Lane 4: CSF sample showing amplified product of 146 bp (positive for JEV RNA); Lane 5: Negative serum sample.
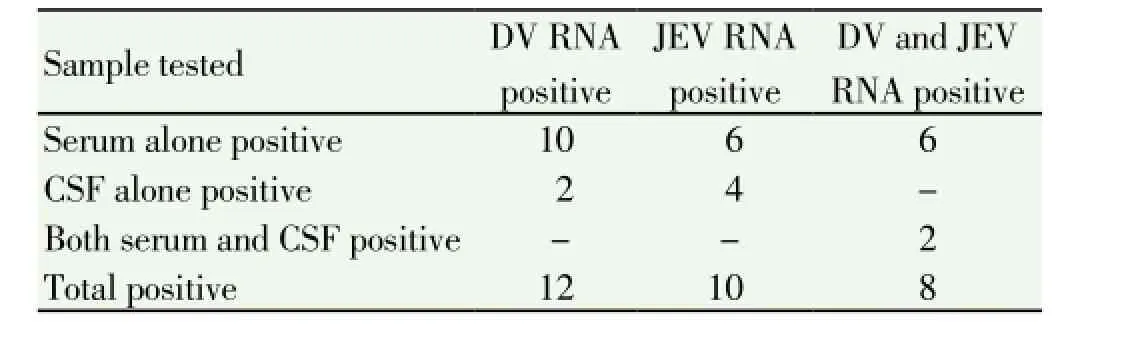
Table 4 RT-PCR results of CSF and serum samples from 76 patients tested positive for both anti-JEV and anti-DV IgM.
4. Discussion
DV and JEV are common and phylogenetically related flaviruses. Clinical features, seasonality and geographical locations for DV and JEV infections overlap. Uttar Pradesh, India is a hyperendemic area for both the infections[11,12]. Both are mosquito borne illnesses showing the same seasonality, and antibodies produced against flavivirus infections are known to cross react with each other[13]. JEV infection usually manifests as acute encephalitis syndrome (AES), while DV infection presents as DF or DHF/DSS. As reported earlier in the recent years, a significant proportion of children infected with DV in North India presented with encephalopathy[14,15]. We observed high co-positivity of anti-DV and anti-JEV IgM in sera from the patients presenting with features of AES. In a hyperendemic region, if a case is positive for both anti-DV IgM and anti-JEV IgM antibodies, there are three possibilities: cross-reaction, sequential infection and co-infection[16].
For demonstrating co-infection, (i) either both the pathogens should grow in sample, or (ii) nucleic acid of both the pathogens should be detected in clinical sample, or (iii) rising antibody titre for both the pathogens should be demonstrated in paired sera, or (iv) comparing IgM specific to DV versus JEV, by measuring neutralizing antibodies[16]. Laboratory diagnosis of viral infections is mostly made either by serology or by molecular methods[17]. Use of culture for diagnosis is limited to very rare situations. Getting paired sera for confirming the serological diagnosis is difficult in routine clinical settings. Detection of nucleic acid depends on post illness day as viral load starts getting lower once antibodies start appearing; moreover, viraemia is seen in early stages of illness. By the time patient comes to hospital, viral load in serum is low[17].
Our discussion of co-infection vs cross reactivity is based on following findings: (a) high antibody titres of both anti-DV and anti-JEV IgM antibodies, much higher than the cut off level, (b) seasonality of co-infection matching to the period when both the viruses are detected in population and (c) confirmation of co-infection by PCR in patients samples. DV and JEV share antigenic epitopes in the major envelope (E) protein, which elicit antibodies commonly referred to as cross-reacting antibodies[16,18]. Studies have demonstrated that the two viruses can be differentiated by comparing the adjusted optical absorbance of anti-DV IgM with that of anti-JEV IgM[16,18]. The virus for which titres are high isconsidered to be the infecting virus[16]. Since high antibody titres of both anti-DV and anti-JEV IgM antibodies, much higher than the cut off level, were seen in majority of copositive patient sera in this study, it is suggested that these antibodies were due to DV/JEV co-infection but not to cross-reaction secondary to endemicity or immunization as reported earlier[13,18]. In a study on 258 confirmed dengue cases, 24 (9%) cases had anti-JEV IgM, the levels of anti-JEV IgM were lower than those of anti-DV IgM and the average level of anti-JEV IgM was 19% of that of anti-DV IgM[13]. In a study done for identification of Dengue type 1, 2, 3, Japanese encephalitis and West Nile viruses by hemagglutination inhibition test, compliment fixation test and neutralization test, serologic responses were found to be non-specific[19]. Most often high heterologous titers were noted with JEV and West Nile antigens. Traditionally, the hemagglutination inhibition assay has been used as goldstandard serological test, but extensive cross-reaction has compromised the general applicability of this assay. Similarly, compliment fixation test is not widely used currently due to requirement of highly trained personnel and cross-reactivity. Plaque reduction and neutralization test (PRNT) is the most specific assay for determination of DV[20] and JEV[21] neutralizing antibodies and remains the ultimate tool for distinguishing between the two viruses. As per CDC guidelines, a positive MAC-ELISA result together with a virus-specific neutralizing antibody titre by PRNT, of more than or equal to 1:4 and >4-fold higher than the titre to the other virus is considered positive for that virus; a positive or equivocal MAC-ELISA result with a virusspecific neutralizing titre more than or equal to 1:4 but not 4-fold higher than the titre to the other virus, is interpreted as presumptive; and an equivocal MAC-ELISA result with no detectable PRNT titer is considered negative[22]. However, this technique has several limitations, such as timeconsuming detection, a long incubation period and labour intensive work, and it is therefore recommended for use only in reference laboratories with experience in this assay and for samples which cannot be easily differentiated by ELISA method[21]. For these reasons, ELISA has been proposed as a simpler and more rapid alternative[20,21]. The IVD research kit for anti-DV IgM (as per the manufacturer) and XCyton kit for anti-JEV IgM[23] have reported sensitivities of 100% and 95% and specificities of 85.9% and 97.5%, respectively.
In ELISA, IgM antibodies are more specific than IgG[24]. When serum samples were tested for IgM and IgG antibodies against fourFlavivirusantigens, West Nile virus, DV, yellow fever virus and tick-borne encephalitis virus, it was detected that IgM responses were monotypic, while IgG antibodies were cross reactive[24]. Recent studies have shown that IgG responses against the pre-membrane protein and can distinguish between previous infection with DV and JEV[1].
Recent primary DV infection can be confirmed by simultaneous presence of IgM and IgA[25] or non-structural protein 1 antigen in serum samples. A recent study done on fatal dengue meningoencephalitis cases showed that nonstructural protein 1 antigen when used alone had sensitivity and specificity of 50% and 100% in CSF, but when used in combination with IgM, the detection rate rose to 92.3%[26].
The pattern of DV infections have changed from seasonal to hyperendemic in the Indian continent due to circulation of all the four serotypes round the year[27,28], while the JEV infections are seasonal occurring mainly in post monsoon season[29]. Co-positivity of anti-DV and anti-JEV IgM, predominantly in those months when both anti-JEV and anti-DV IgM positivity is present in population, is selfexplanatory. Co-positivity in those months when only anti-DV IgM positivity is present is not observed. A similar trend was seen geographically; co-positive cases were seen only in areas where both DV and JEV were circulating.
The definite proof of current infection of DV or JEV is detection of nucleic acid by RT-PCR[20,21], which can be detected in blood in approximately the first 5 days of onset of symptoms. In this study, RNA of both DV and JEV were detected in eight patient samples, two of which were positive in both CSF and serum, which confirms co-infection by both the viruses.
Co-infection of both DV and JEV has also been reported previously from the paddy growing Terai regions of Uttar Pradesh[11,12] where the environmental conditions are very favourable for the mosquito vector. Capability of simultaneous multiplication of JEV and DV is also reported by Kanthonget al[30] who demonstrated simultaneous coinfection of JEV, DV andAedes albopictusdensovirus in C6/36 mosquito cell lines in laboratory conditions.
All the DV detected in this study were of serotype 2; it is known that patients with DV-2 infections experience more severe disease than those infected with other serotypes[31]. The clinical implications of co-infection with DV and JEV are not well understood. However, a recent study indicates that the probability of symptomatic DV-2 illness increases due to the prior existence of JEV neutralizing antibodies[32]. The antibodies may not be detectable in clinical samples within the first few days of illness. The sensitivity of MACELISA is approximately 90% when used in samples collected after 5 or more days of onset of illness[20,21]. A similar pattern was seen in this study.
This retrospective study has limitations like analysis of data is done on the basis of routine records, and all possible tests could not be done on all the samples due to limitations on volume and availability of desired samples. It is not always possible to obtain paired serum sample from patients because of several practical reasons: a) late presentation of patients to the hospital meaning acute phase sera is not available, (b) patient not consenting to give a second blood sample, and (c) decline in interest of treating physician, once the patient improves.
We reported an observation of co-positivity with anti-JEV and anti-DV IgM in a hyperendemic area of Northern India. This article throws a light on how frequently co-positivity is detected in clinical samples from AES cases.
Conflict of interest statement
The authors declare they have no conflict of interest.
Acknowledgments
Financial support from Indian Council of Medical Research, New Delhi and Council of Scientific and Industrial Research, New Delhi is acknowledged.
[1] Cardosa MJ, Wang SM, Sum MSH, Tio PH. Antibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis viruses. BMC Microbiol 2002; 2: 9.
[2] Martin DA, Biggerstaff BJ, Allen B, Johnson AJ, Lanciotti RS, Roehrig JT. Use of immunoglobulin M cross-reactions in differential diagnosis of human flaviviral encephalitis infections in the United States. Clin Diagn Lab Immunol 2002; 9(3): 544-549.
[3] Makino Y, Tadano M, Saito M, Maneekarn N, Sittisombut N, Sirisanthana V, et al. Studies on serological cross-reaction in sequential flavivirus infections. Microbiol Immunol 1994; 38(12): 951-955.
[4] Robinson JS, Featherstone D, Vasanthapuram R, Biggerstaff BJ, Desai A, Ramamurty N, et al. Evaluation of three commercially available Japanese encephalitis virus IgM enzyme-linked immunosorbent assays. Am J Trop Med Hyg 2010; 83(5): 1146-1155.
[5] Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg 2001; 65(6): 848-851.
[6] Kalita J, Misra UK. EEG in dengue virus infection with neurological manifestations: a clinical and CT/MRI correlation. Clin Neurophysiol 2006; 117(10): 2252-2256.
[7] Kanade T, Shah I. Dengue encephalopathy. J Vector Borne Dis 2011; 48(3): 180-181.
[8] Watt G, Jongsakul K. Acute undifferentiated fever caused by infection with Japanese encephalitis virus. Am J Trop Med Hyg 2003; 68(6): 704-706.
[9] Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase polymerase chain reaction. J Clin Microbiol 1992; 30(3): 545-551.
[10] Yang DK, Kweon CH, Kim BH, Lim SI, Kim SH, Kwon JH, et al. Taq Man reverse transcription polymerase chain reaction for the detection of Japanese encephalitis virus. J Vet Sci 2004; 5(4): 345-351.
[11] Mathur A, Chaturvedi UC, Tandon HO, Agarwal AK, Mathur GP, Nag D, et al. Japanese encephalitis epidemic in Uttar Pradesh, India during 1978. Indian J Med Res 1982; 75: 161-169.
[12] Saxena SK, Mishra N, Saxena R, Singh M, Mathur A. Trend of Japanese encephalitis in North India: evidence from thirty-eight acute encephalitis cases and appraisal of niceties. J Infect Dev Ctries 2009; 3(7): 517-530.
[13] Nuegoonpipat AA, Panthuysori N, Anantapreecha S, Chanama S, Sa-Ngasang A, Sawanpanyalert P, et al. Cross-reactive IgM responses in patients with dengue or Japanese encephalitis. J Clin Virol 2008; 42(1): 75-77.
[14] Kumar R, Tripathi S, Tambe JJ, Arora V, Srivastava A, Nag VL. Dengue encephalopathy in children in northern India: Clinical features and comparison with non dengue. J Neurol Sci 2008; 269(1-2): 41-48.
[15] Chandrakanta, Kumar R, Garima, Agarwal J, Jain A, Nagar R. Changing clinical manifestations of dengue infection in North India. Dengue Bulletin 2008; 32: 118-125.
[16] Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 1989; 40(4): 418-427.
[17] Fischer M, Lindsey N, Staples JE, Hills S. Japanese Encephalitis Vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Morbidity and Mortality Weekly Report (MMWR). 2010; 59(RR01): 1-27. [Online]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5901a1.htm.
[18] Garg RK, Malhotra HS, Gupta A, Kumar N, Jain A. Concurrent dengue virus and Japanese encephalitis virus infection of the brain: is it co-infection or co-detection? Infection 2012; 40: 589-593.
[19] Carey DE, Myers RM, Reuben R, Rodrigues FM. Studies on dengue in Vellore, South India. Am J Trop Med and Hyg 1966; 15(4): 580-587.
[20] Guzman MG, Kouri G. Dengue diagnosis, advances and challenges. Int J Infect Dis 2004; 8(2): 69-80.
[21] World Health Organization. Manual for the laboratory diagnosis of Japanese encephalitis virus infection. Geneva: World Health Organization; 2007.
[22] Gubler DJ, Campbell GL, Nasci R, Komar N, Petersen L, Roehrig JT. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunol 2000; 13(4): 469-475.
[23] Vasanthapuram R, Robinson JS, Russell BJ, Desai A, Ramamurty N, Featherstone D, et al. Evaluation of IgM antibody capture enzyme-linked immunosorbent assay kits for detection of IgM against Japanese encephalitis virus in cerebrospinal fluid samples. Am J Trop Med Hyg 2009; 81(6): 1144-1150.
[24] Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. Evalutation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infections. J Clin Microbiol 2000; 38(6): 2232-2239.
[25] Nawa M, Takasaki T, Ito M, Inoue S, Morita K, Kurane I. Immunoglobulin A antibody responses in dengue patients: a useful marker for serodiagnosis of dengue virus infection. Clin Diagn Lab Immunol 2005; 12(10): 1235-1237.
[26] Araujo FM, Brilhante RS, Cavalcanti LP, Rocha MF, Cordeiro RA, Perdigao AC, et al. Detection of the dengue non-structural 1 antigen in cerebral spinal fluid samples using a commercially available enzyme-linked immunosorbent assay. J Virol Methods 2011; 177(1): 128-131.
[27] Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, et al. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J 2008; 5: 1.
[28] Dar L, Gupta E, Narang P, Broor S. Co-circulation of dengue serotype, Delhi, India, 2003. Emerg Infect Dis 2006; 12: 352-353.
[29] Centre for Disease Control and Prevention (CDC). Risk of Japanese Encephalitis by country, region and season-CDC 2010. [Online]. Available from: http://wwwn.cdc.gov/travel/ yellowBookCh4-apaneseEncephalitis.aspx.
[30] Kanthong N, Khemnu N, Pattanakitsakul SN, Malasif P, Flegel TW. Persistent, triple-virus co-infections in mosquito cells. BMC Microbiol 2010; 10: 14.
[31] Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181(1): 2-9.
[32] Anderson KB, Gibbons RV, Thomas SJ, Rothman AL, Nisalak A, Berkelman RL, et al. Preexisting Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in Thailand. PLoS Negl Trop Dis 2011; 5(10): e1311.
*Corresponding author: Prof. Amita Jain, M.B.B.S., M.D. (Path and Bact), Department of Microbiology, K.G. Medical University, Lucknow-226003, India.
Tel: 0522-2258633
Fax: 0522-2258633
E-mail: amita602002@yahoo.com
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Hepatic effect of NAC on sevear acute pancteatise of rats
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
