Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
Kang Huang, Shi-Juan Lu, Jiang-Hua Zhong, Qun Xiang, Liu Wang, Miao Wu
Department of Cardiology, Haikou People’s Hospital, Haikou 570208, China
Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
Kang Huang, Shi-Juan Lu*, Jiang-Hua Zhong, Qun Xiang, Liu Wang, Miao Wu
Department of Cardiology, Haikou People’s Hospital, Haikou 570208, China
Objective: To investigate the protective effect of different cyclosporin A (CsA) doses on myocardial ischemia/reperfusion injury in rat models. Methods: A rat model of myocardial ischemia/reperfusion injury was established in vivo and the rats were randomly divided into four groups: placebo (PBS; T1), low-dose (CsA dose: 1.0 mg/kg; T2), medium-dose (CsA dose: 2.5 mg/kg; T3), and high-dose (CsA dose: 5.0 mg/kg; T4) groups. Heart function indexes were monitored at different time points, the extent of myocardial infarction was assessed by Evans Blue-TTC staining, and creatine kinase MB mass and cardiac troponin I values were measured by biochemical assays. Results: Compared with the T1 and T2 groups, both the creatine kinase MB mass and cardiac troponin I were significantly lower in the T3 and T4 groups (P<0.05). The mean arterial pressure (MAP) and left ventricular systolic pressure (LVSP) decreased sequentially in each group, with the extending reperfusion time. Significant decreases in LVSP and MAP were observed in the T3 and T4 groups as compared to the T1 and T2 group (P<0.05), and the T2 group showed a significantly lower LVSP and MAP decline than the T1 group (P<0.05). Compared with the T1 group, the rats from the T2, T3, and T4 groups suffered from a significantly lower extent of myocardial infarction (P<0.05). Also, the animals in the T3 and T4 groups had a significantly smaller extent of myocardial infarction than those in the T2 group (P<0.05). Conclusions: Various CsA doses exert different degrees of protection against ischemia/reperfusion injury, and this protective effect peaks at approximately 2.5 mg/kg in rat models.
ARTICLE INFO
Article history:
Received 10 October 2013
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Cyclosporin A
1. Introduction
Myocardial ischemia/reperfusion injury is a common pathological and physiological course of myocardial reperfusion seen in the clinical practice and affects the post-ischemic recovery of the heart function, even endangering patients’ lives[1]. The mitochondrial permeability transition pore (mPTP) acts as a key target organ of the mid- and down-stream ischemia/reperfusion injury pathways. Intracellular calcium overload and ischemia/oxygenation-induced free radicals serve as the underlying mechanism of myocardial ischemia/reperfusion injury, eventually resulting in increased intracellular mPTP permeability and thereby leading to cell death by activating the mitochondrial apoptotic pathway[2-6]. The ischemia/reperfusion injury can be significantly alleviated by inhibiting the opening of mPTPs. Until now, an adequate evidence has demonstrated that cyclosporin A (CsA), an mPTP inhibitor, effectively alleviates ischemia/reperfusion injury. However, the CsA doses used in previous studies varied considerably, and its inhibitory effects on myocardial injury were also inconsistent. In this study, we observed the dose-effect relationship of different CsA dosesin vivousing a rat model of ischemia/reperfusion injury and determined the optimal dose.
2. Materials and methods
2.1. Rat grouping
In total, 32 healthy male Sprague Dawley (SD) rats, weighing 300-320 g, were provided by Dongchuang Laboratory Animals with the certificate number SCXK (Xiang) 2009-0012 (Changsha, Hunan, China). The rats were randomly assigned into four groups: placebo group (PBS; T1 group), low-dose group (CsA dose: 1.0 mg/kg; T2 group), medium-dose group (CsA dose: 2.5 mg/kg; T3 group), and high-dose group (CsA dose: 5.0 mg/kg; T4 group), eight in each group.
2.2. Main instruments and reagents
The instruments and reagents used in this study included an artificial respiration machine for animals (Huaibei Zhenghua Bioinstrumentation Co., Ltd., Anhui, China); a HITACHI 7080 automatic biochemical analyzer (HITACHI, Japan); a Beneview T5 monitor (Mindray); microcatheter (EchelonTM10 microcatheter); CsA, Evans-Blue, 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, U.S.), cardiac troponin Ⅰ (cTnⅠ), and creatine kinase MB (CK-MB, Nanjing Jiancheng Bioengineering Institute, China).
2.3. Establishment of ischemia/reperfusion injury model
Ischemia/reperfusion injury model was established through the ligation of anterior descending branch of left coronary artery[7]. The animals were anesthetized using 1% pentobarbital sodium (40 mg/kg). A catheter was subsequently placed inside the trachea and connected to a breathing machine. First, the rats were sterilized by iodophor after removing the hair from the chest skin, and the skin above the heart was then incised along the left side of the sternum, with a vertical 2.0-2.5 cm cut. Subcutaneous tissues and muscles were detached to expose the left 3rd to 4th ribs until the shadow of the beating heart was seen. Subsequently, a bent-head hemostasis clamp was rapidly inserted into the thorax, in the gap between the 3rd and 4th ribs, and the thorax area was fully exposed. After the chest was opened, the bottom part of the heart was thoroughly exposed and the pericardium was removed with a forceps used in ocular surgery. The heart was gently pushed rightwards using a wet cotton swab, the thymus and left atria were slightly lifted with a cotton stick, and the vena cava was exposed between the left atria and pulmonary artery. The vein was used as the ligation sign. The left coronary artery was ligated by No. 3/8 sutures at 1-2 mm around the root of the left atria with a depth of 1.0-1.5 mm, and the anterior descending branch of the left coronary artery was ligated along with the vena cava. Prior to ligation, two No. 2 suture strings were placed below the ligatures for unknotting[8]. After ligation of the coronary artery, the heart was immediately placed back into the thorax, the thorax was gently pressed to squeeze out the gas, and it was subsequently closed by a vessel clamp. The ischemia time was 30 min and the reperfusion time was 2 h. Criteria for a successful left anterior descending (LAD) blockage were a high-frequency QRS complex, a significantly elevated ST segment, and the darkening of the myocardial color under the ligatures. Criteria for successful reperfusion were a dropping QRS complex or a significant decline of the ST segment, and the red appearance of the myocardial muscle below the ligatures.
2.4. Measurements of blood flow dynamics
Prior to the ischemia, at the beginning of the reperfusion (0 min) and 30, 60, 90, and 120 min afterwards, the heart rate (HR), left ventricular systolic pressure (LVSP), and left ventricular developed pressure (LVDP) were recorded by a monitor and the mean arterial pressure (MAP) were calculated.
2.5. Measurement of the biochemical markers for myocardial injury
After reperfusion, 3 mL of jugular venous blood was collected in a 5 mL syringe and injected into a biochemical tube and a tube for the myocardial calcium protein assay, respectively. After centrifugation at 2 000 r/min for 10 min, the serum was prepared for subsequent analysis.
2.6. Evaluation on the extent of myocardial infarction
Following reperfusion and blood sampling, the anterior descending branch of the left coronary artery was repeatedly ligated and stained by the Evans Blue-TTC method. The weight of the left ventricle myocytes and that of the necrotic myocytes were determined using an electronic balance. The degree of ischemia, expressed as the weight of the necrotic myocytes as a proportion by weight of the left ventricle myocytes, was then calculated.
2.7. Statistical analysis
The SPSS 17.0 statistical software was used for statistical analysis. Measurement data were expressed as mean± standard deviation. The dynamics of the blood flow, as data from repeated measurements, were statistically analyzed by ANOVA. Group comparisons were performed by the LSD test.A value ofP<0.05 was considered as statistically significant.
3. Results
3.1. Comparison of CK-MB and cTnI between groups
The CK-MB mass was significantly lower in the T2, T3, and T4 groups than in the T1 group (P<0.05). The CK-MB mass was significantly lower in the T3 and T4 groups than in the T1 group (P<0.05). The T3 and T4 groups differed in the CK-MB mass, but the difference did not reach statistical significance (Table 1). The cTnI value was significantly lower in the T2, T3, and T4 groups than in the T1 group (P<0.05). Albeit the T2, T3, and T4 groups differed regarding cTnⅠ, the differences did not reach statistical significance (Table 1).
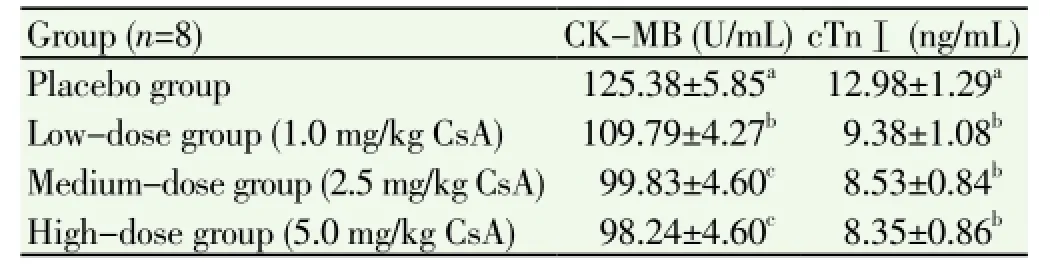
Table 1 Effect of CsA on CK-MB and cTnⅠ in the rat model of myocardial ischemia/reperfusion injury.
3.2. Comparison of HR between groups
Prior to the ligation of the coronary artery, the HR did not differ between the groups. After ligation, the HR declined to various extents in all groups. There was no statistical significance between the different groups (Table 2).
3.3. Comparison of MAP groups
Prior to ligation, no significant differences were noted in the baseline MAP, whereas the values decreased to different degrees at the beginning of the reperfusion, and 30, 60, 90, and 120 min later. No statistical significance was seen in terms of MAP at any time point between the T3 and T4 groups. Pair-comparisons between other groups revealed statistically significant differences (P<0.05). The MAP declined to a lesser extent in the T3 and T4 groups as compared to that in the T1 and T2 groups (P<0.05), and the extent of MAP decrease in the T2 group was significantly smaller than that in the T1 group (P<0.05) (Table 2).
3.4. Comparison of LVSP between groups
No significant differences were noted before ligation in the baseline LVSP, whereas the MAP decreased to different degrees at the beginning of reperfusion and 30, 60, 90, and 120 min later. No statistically significant changes in LVSP were visualized at any time point in the T3 and T4 groups. Pair-comparisons among other groups revealed a statistically significant difference in the LVSP (P<0.05). The LVSP values declined to significantly lower levels in the T3 and T4 groups as compared to that in the T1 and T2 groups (P<0.05), and the extent of LVSP decrease in the T2 group was significantly smaller than that in the T1 group (P<0.05)(Table 2).

Table 2 Effect of CsA on the dynamics of blood flow in the rat model of myocardial ischemia/reperfusion injury.
3.5. Comparison of the extent of myocardial infarction between groups
The extent of myocardial ischemic/reperfusion injury in all groups was 45.00%±2.07%, 35.29%±1.52%, 29.05%±2.08%, and 26.90%±1.86%, respectively. The values in the T2, T3, and T4 groups were significantly smaller than that in the T1 group (P<0.05), and the extent of myocardial ischemia/reperfusion injury in the T3 and T4 groups was significantly lower than that in the T2 group (P<0.05). The T3 and T4 groups differed in terms of this parameter, but the difference did not reach statistical significance, as illustrated in Figure 1.

Figure 1. Effect of CsA on the extent of myocardial infarction in the rat model of myocardial ischemia/reperfusion injury.T1, T2, T3 and T4 represent the placebo group, low-dose group (1.0 mg/kg CsA), medium-dose group (2.5 mg/kg CsA) and high-dose group (5.0 mg/kg CsA), respectively. Means marked by differnt letters are significantly different at the 0.05 level by the LSD test.
4. Discussion
Worldwide, acute myocardial infarction has dramatically high mortality rates. The extent of the infarcted cardiac area is closely correlated with the mortality that occurs as a result of this condition. Therefore, reducing the extent of myocardial infarction is a key task and therapeutically important goal[9]. Coronary artery intervention can restore the blood flow in ischemic tissues and alleviate the severity of acute myocardial infarction[10]. However, a more serious consequence, reperfusion injury, may be induced when the blood flow through the coronary arteries is subsequently restored[11]. Previous studies using animal models of acute myocardial infarction suggested that reperfusion injury is likely to cause 50% of the myocardial infarction[12,13]. Consequently, the development of approaches that could alleviate reperfusion injury following interventional therapy, to reduce the extent of myocardial infarction and to improve prognosis, becomes a new and important direction for clinicians.
Previous studies also proposed new strategies to target ischemic/reperfusion injury. Self-adaptation, the ability of heart for endogenous protection, has been deemed to decrease the incidence of coronary heart disease, mitigate the severity of myocardial infarction, improve myocardial contraction, and enhance clinical prognosis. At present, it is suggested that pre- and post-ischemia adaptation plays a role in myocardial reperfusion via a common signaling pathway. The use of therapeutic approaches that act on related elements of this signaling pathway were shown to protect the myocytes similarly to the way pre- and postischemic adaptation do[14]. Therefore, the concept of postmedication adaptation was proposed. According to this concept, therapeutic drugs could alleviate the extent of myocardial infarction during myocardial reperfusion by activating the reperfusion injury salvage kinase (RISK) pathway, consisting of a variety of kinases involved in protecting the heart from reperfusion injury[15]. At the same time, mPTP was discovered as another vital target of the downstream pathway. An additional evidence supported the idea that the protective effects of pre- and post-ischemia adaptation on the heart are mediated by RISK-mPTP pathway[15]. Consequently, mPTP is viewed as a vital target of pharmacological therapies. Moreover, post-ischemic adaptation avoids the need for invasive interventions, and it can therefore easily be applied during the treatment of patients with acute myocardial infarction undergoing reperfusion therapy and, additionally, it is easy to be performed.
mPTP is a non-specific channel whose changes in permeability may facilitate the entry of protons and that of certain molecules and solutes under 1 500 Da through the inner mitochondrial membrane. This reduces the mitochondrial membrane potential and, coupled with the decrease in oxidative phosphorylation, leads to ATP deficiency, swollen mitochondria, and cellular apoptosis. CsA, as a specific mPTP inhibitor, is able to inhibit the opening of mPTP and plays a vital role in preventing and alleviating the incidence of ischemia/reperfusion injury. Although it has been convincingly proven that CsA protects the myocytes by blocking the opening of mPTP at the risk of ischemic/ reperfusion injury, there have been no studies to validate the dose-effect relationship and to identify the optimal CsA dose. It has been merely confirmed byin vitromodels that low-dose cyclosporin (0.5-2.0 μmol/L) exerts protective effects and that this protection is lost at doses >5 μmol/L[16]. Skyschallyet al. conducted ischemia/reperfusion injury experiments in mammalian models using a dose of 5 mg/kgand showed that the extent of myocardial infarction in the CsA and control groups was approximately 25% and 45%, respectively[17]. Pagel and Krolikowski delivered a 5 mg/kg dose of cyclosporin in rabbit models and observed findings that are inconsistent with those from previous studies[18]. A number of investigations utilized different doses and yielded disparate conclusions. CsA, as an immunosuppressant, poses potential harm after its long-term use, including renal and liver toxicities, and also increases the susceptibility for infections and carcinomas. Intravenously delivered CsA has been mainly used for organ transplant recipients to prevent rejection, and the dose is generally less than 5 mg/kg. Gomezet alreviewed previous studies and found that a dose of <5 mg/kg of CsA did not hurt the dynamics of blood flow or cause alternative toxicities[19].
This study demonstrated that various CsA doses exert different protective effects on ischemia/reperfusion injury. The protective effect of CsA peaks at a certain dose, and we found this optimal dose, at which the best protective effects are observed, to be approximately 2.5 mg/kg.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This study was financially supported by the Key Science and Technology Project of Haikou City, with grant number 2011-0142.
[1] Aronow WS. Epidemiology, pathophysiology, prognosis, and treatment of systolic and diastolic heart failure. Cardiol Rev 2006; 14(3): 108-124.
[2] Li Q, Zhou LY, Gao GF, Jiao JQ, Li PF. Mitochondrial network in the heart. Protein Cell 2012; 3(6): 410-418.
[3] Pott C, Eckardt L, Goldhaber JI. Triple threat: the N+/Ca2+exchanger in the pathophysiology of cardiac arrhythmia, ischemia and heart failure. Curr Drug Targets 2011; 12(5): 737-747.
[4] Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 2009; 46: 821-831.
[5] Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol 2012; 8(6): 863-884.
[6] Yang XM, Philipp S, Downey JM, Cohen MV. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol 2006; 101(4): 311-318.
[7] Li J, Zhang HF, Wu F, Nan Y, Ma H, Guo W, et al. Insulin inhibits tumor necrosis factor-α induction in myocardial ischemia/ reperfusion: Role of Akt and endothelial nitric oxide synthase phosphorylation. Crit Care Med 2008; 36(5): 1551-1558.
[8] Zhou XW, Liao ZF, Chen J, Liu LM. Establishment and evaluation of myocardial ischemia reperfusion injury in rats. Lab Anim Comp Med 2009; 29(4): 223-232.
[9] Rodríguez-Sinovas A, Abdallah Y, Piper HM, Garcia-Dorado D. Reperfusion injury as a therapeutic challenge in patients with acute myocardial infarction. Heart Fail Rev 2007; 2(3-4): 207-216.
[10] van Domburg RT, Sonnenschein K, Nieuwlaat R, Kamp O, Storm CJ, Bax JJ, et al. Sustained benefit 20 years after reperfusion therapy in acute myocardial infarction. J Am Coll Cardiol 2005; 46(1): 15-20.
[11] Volpi A, De Vita C, Franzosi MG, Geraci E, Maggioni AP, Mauri F, et al. Determinants of 6-month mortality in survivors of myocardial infarction after thrombolysis. Results of the GISSI-2 data base. The Ad hoc Working Group of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-2 Data Base. Circulation 1993; 88(2): 416-429.
[12] Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003; 361(9351): 13-20.
[13] Braunwald E, Kloner RA. Myocardial reperfusion: A double-edged sword? J Clin Invest 1985; 769(5): 1713-1719.
[14] Vinten-Johansen J. Postconditionig: A mechanical maneuver that triggers biological and molecular cardioprotective responses to reperfusion. Heart Fail Rev 2007; 12(3-4): 235-244.
[15] Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, et al. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res 2011; 91: 108-115.
[16] Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol 1991; 23(12): 1351-1354.
[17] Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarc size in pigs. Cardiovasc Drugs Ther 2010; 24(1): 85-87.
[18] Pagel PS, Krolikowski JG. Transient metabolic alkalosis during early reperfusion abolishes helium preconditioning against myocardial infarction: Restoration of cardioprotection by cyclosporin A in rabbits. Anesth Analg 2009; 108(4): 1076-1082.
[19] Gomez L, Li B, Mewton N, Sanchez I, Piot C, Elbaz M, et al. Inhibition of mitochondrial permeability transition pore opening: translation to patients. Cardiovas Res 2009; 83(2): 226-233.
*Corresponding author: Dr. Shi-Juan Lu, Department of Cardiology, Haikou People’s Hospital, Haikou 570208, China.
Tel: +86-898-66189795
Fax: +86-898-66189795
E-mail: 125769612@qq.com
Foundation project: This study was financially supported by Key Science and Technology Project of Haikou City, with grant number 2011-0142.
Myocardial ischemia/reperfusion injury
Mitochondrial permeability transition pore
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Hepatic effect of NAC on sevear acute pancteatise of rats
- Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
