Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
Yun-Xi Wang, Jing Zhang*, Xiang-Yang Chu, Yi Liu, Fang Li, Zhan-Bo Wang, Li-Xin Wei
1Department of Thoracic Surgery, General Hospital of PLA, Beijing 100853, China
2Department of Oncology, General Hospital of PLA, Beijing 100853, China
3Department of Pathology, General Hospital of PLA, Beijing 100853, China
Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
Yun-Xi Wang1, Jing Zhang1*, Xiang-Yang Chu1, Yi Liu1, Fang Li2, Zhan-Bo Wang3, Li-Xin Wei3
1Department of Thoracic Surgery, General Hospital of PLA, Beijing 100853, China
2Department of Oncology, General Hospital of PLA, Beijing 100853, China
3Department of Pathology, General Hospital of PLA, Beijing 100853, China
The clinical data of 18 patients with PB from April 1989 to April 2013 was analyzed retrospectively, including 11 men and 7 women, aged 45 and 76 years old (mean 53 years). There were 12 cases of PB occurring in right lung and other cases in left lung. Among them, 3 patients had no symptoms, and 15 patients displayed symptoms of cough, chest pain, asthenia or minor haemoptysis. Overall, 11 patients had a preoperative diagnosis of lung cancer, 7 patients were preoperatively diagnosed as the other diseases, which included lung benign tumor (n=5) and mediastinal mass (n=2). All patients received a radical resection. Six patients received postoperative cisplatinbased chemotherapy, and two patients received postoperative irradiation with the dose of 55 Gy. Histologically, 14 cases of 18 patients had biphasic pulmonary blastoma and four cases had well differentiated fetal adenocarcinoma. A total of 12 patients died in a period of 6-36 months after operation, and 1 case was lost after 2 years of follow up. The median survival time was 19 months. PB is a rare primary lung malignant embryonal neoplasm. Despite its assumed embyonal origin, the tumor has a predilection for adults. A preoperative correct diagnosis is very difficult in spite of modern diagnostic imaging and biopsy techniques. Surgical resection is the main method for diagnosis and treatment. Postoperative chemotherapy or irradiation can help eliminate tumor remnants. Its prognosis is very poor, especially for the biphasic type.
ARTICLE INFO
Article history:
Received 10 September 2013
Received in revised form 15 October 2013
Accepted 15 December 2013
Available online 20 Februany 2014
Pulmonary blastoma
1. Introduction
Pulmonary blastoma (PB) is a very rare pulmonary neoplasm, accounting for 0.25% to 0.5% of primitive malignant tumors of the lung. Since 1945, only over 200 cases of PB have been reported worldwide[1]. PB is classified into two types, adult type and child type. From April 1989 to April 2013, 18 adult cases were admitted in our hospital, accounting for 0.21% (18/8 531) of patients with malignant pulmonary tumors. We retrospectively reviewed the clinic features of these 18 PB cases.
2. Case report
2.1. Demographics
A total of 18 patients including 11 men and 7 women were selected, aged 45 to 76 years, with a median of 53 years. All male patients and one female patient had smoking history. Fifteen PB patients had cough, bloody phlegm, chest pain, and chest tightness. Other 3 patients showed no clinical manifestation, all lesions were found during physical examination.
2.2. Diagnosis
Initial chest radiographs and CT scans of all 18 PB patients showed round, oval or lobulated mass (Figure 1-4). Lesions of 15 PB patients were located in the peripheral lung, while it was located close to hilum in the rest three patients. Six PB patients had tumors with smooth margin, and the other12 with lobulated margin. Sixteen lesions were manifested as solitary mass, and the other lesion as multiple nodules. The widest diameter of the tumors ranged from 2 cm to 12 cm. In our study, PET-CT examination was conducted on three patients, showing abnormal radioactive enrichment on lesions with standard uptake value over 4.0. Five cases had enlarged hilar lymph node. Five cases had tumors in the right upper lobe, one in the right middle lobe, five in the right lower lobe, three in the left upper lobe, two in the left lower lobe, one in the right posterior inferior mediastinum and one in the left anterior mediastinum. Eleven cases were preoperatively diagnosed as lung cancers, among which three cases had undifferentiated carcinoma confirmed by CT-guided biopsy, two case had adenocarcinoma confirmed by bronchofibroscopy, five cases were preoperatively diagnosed as benign lung tumor, and two cases were preoperatively diagnosed as mediastinal tumor. Patients who were preoperatively diagnosed as right posterior inferior mediastinal tumor also received ultrasound endogastroscopy. It showed that the tumor was 25 cm to 30 cm away from incisor. The regional esophageal mucosa was smooth and complete, and the lumen was slightly narrowed. Outside the esophageal wall, there was a hyperechoic mass with uneven echo density and unclear border to local lung tissue.

Figure 1. Chest film of Biphasic PB in left upper lobe.
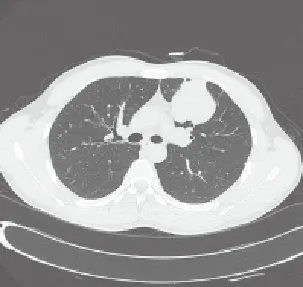
Figure 2. CT scan of Biphasic PB in left upper lobe.

Figure 3. Chest film of WDFA in right lower lobe.
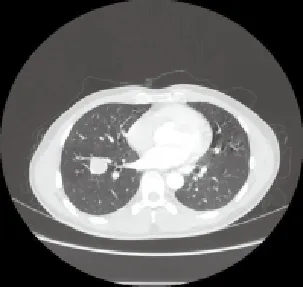
Figure 4. CT scan of WDFA in right lower lobe.
2.3. Treatment
All patients underwent surgery. A total of 15 cases had pulmonary lobectomy combined with hilar and mediastinal lymphadenectomy; one case had partial wedge resection of pulmonary lesions additional to residual lobectomy combined with hilar and mediastinal lymphadenectomy half year later due to reoccurrence; two cases had left pneumonectomy combined with hilar and mediastinal lymphadenectomy considering invasion of the left lower lobe tumor to the left upper lobe causing multiple nodules formation. Six patients received four to six cycles of adjuvant chemotherapy after surgery, of which three took vinorelbine/ cisplatin, two had vinorelbine/ifosfamide, and one had ifosfamide/cisplatin. Two cases underwent 55 Gy radiation therapy due to brain metastases. All cases had successful surgery. The average hospitalization time was 12 days. Twelve patients with pulmonary blastoma died 6-36 months after the surgery, and one case was lost after two years of follow up. The remaining five patients were still alive. Median overall survival was 19 months.
2.4. Pathological analysis
A total of 15 cases had tumors in the peripheral lung near visceral pleura, unconnected to bronchus. Three cases had tumor adjacent to hilum, which did not infiltrate into bronchus. Fourteen cases had tumor without capsule, and 4 cases with pseudocapsule. The diameters of all tumors ranged from 2 cm to 12 cm (mean, 7.3 cm). The sectional surface of the tumors was greyish and fish-like in texture. Three cases had hemorrhage and necrosis inside the tumors, and five cases had hilar lymph node enlargement.
Microscope showed biphasic pulmonary blastoma composed of immature epithelium and mesenchyme. A monolayer of cuboidal epithelial cells was lined up into a small duct-like structure, dispersing among the immature mesenchyme cells that were in round or shortspindle shape with possible mucoid degeneration (Figure 5). Immunohistochemical assays for biphasic pulmonary blastoma were positive for both cytokeratin and vimentin (Figure 6).

Figure 5. Morphology of biphasic PB by HE (10×10).
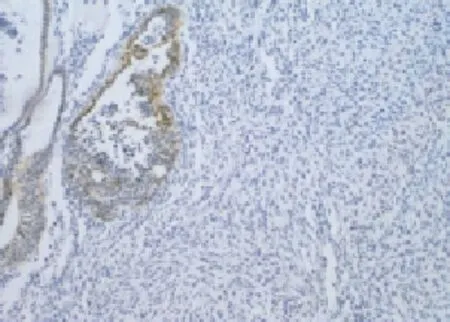
Figure 6. Morphology of biphasic PB by IHC (10×10).
Well differentiated fetal adenocarcinoma was observed, composed of pseudo stratified tall-columnar adenoid epithelial tissue, in duct-like structure similar to fetal lung tubules. The tumor cytoplasm was enriched with glycogen and also dotted with several vacuoles (Figure 7). Immunohistochemical assays for embryotic adenocarcinoma were positive for both cytokeratin and vimentin (Figure 8).
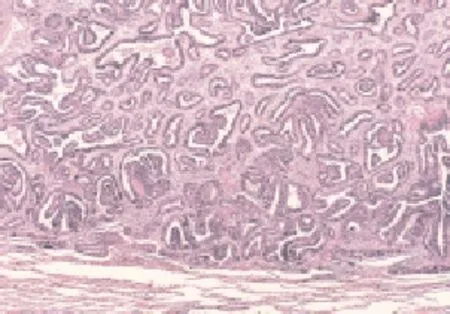
Figure 7. Morphology of WDFA by HE (10×10).

Figure 8. Morphology of WDFA by IHC (10×10).
A total of 14 patients were diagnosed as biphasic pulmonary blastoma, and 4 patients as well differentiated fetal adenocarcinoma.
3. Discussion
3.1. Clinical features of PB
3.1.1. Etiology
Until now, there is still no agreement on the histological origin of pulmonary blastoma[1]. Spencer[2] suggested that the tumor stemmed from the primitive multifunctional stromal lung blastema (pulmonary blast cell). The genesis of adult PB is probably correlated to smoking. Macher-Goppinger et al[3] proved that P53 and β-catenin mutation play an important role in the genesis of adult PB. Childhood pulmonary blastoma (CPB) is immunologically and cytogenetically similar to embryonal rhabdomyosarcoma, indicating that CPB may be associated with partial chromosome deletion[4]. Therefore, it is still unknown as to the causes of PB, which requires further studies and researches.
3.1.2 Clinical manifestations:
The clinical manifestations of adult PB were not distinctive, mostly being cough, chest pain, hemoptysis, and dyspnea. 40% of the patients with adult PB were asymptomatic[5]. Adult PB thus could not easily been differentiated from other lung tumors, either benign or malignant.
3.1.3. Imaging features
For PB patients, chest radiograph usually shows some isolated nodules shadow in the peripheral lung, and CT scans reveal solitary parenchymal mass which could be enhanced by contrast-enhanced CT scans, with possible necrosis. The incidence of PB in the left lung and that in the right lung was insignificantly different[1,6]. 66.7% (12/18) of our patients had their tumors on the right lung, which was not statistical significant because of the small amount of cases. It was possible for patients with pulmonary blastoma to experience hilum or mediastinal lymph node metastatic enlargement, and there was only five such cases in our study. This result might further indicate that the major way of reoccurring or metastasizing was direct invasion or blood metastasis for adult pulmonary plastoma.
3.1.3. Auxiliary examination
A few PB patients could be pathologically diagnosed by preoperative biopsy with either bronchoscopy or CT-guided tumor puncture. However, the pathological results of preoperative biopsy were often misinterpreted as adenocarcinoma or sarcoma of the lung due to the diversity of tumor tissue and the scarcity of sample tissue. Even with advanced imaging and biopsy techniques, it was still hardly possible to make confirmed preoperative diagnosis concerning PB[7].
3.1.4. Pathological classification of PB
Barrett and Barnard[8] firstly named PB as pulmonary embryoma in 1945, because of its pathological resemblance to embryonic lung. In 1961, Spenceret al[2] found that PB was similar to nephroblastoma under the microscope, and first used the term pulmonary blastoma, which is still in use up to now.
PB was classified into two types, adult type and child type. Child PB was commonly seen in children less than 10 years old averaged three years, and composed of malignant mesenchymal tissue[9]. Adult PB mostly happened in adults in their 18 to 70 years averaged 40 years[10,11]. The tumor had no clear boundaries to other lung tissues usually with complication of hemorrhage and necrosis, and the tumor’s sectional surface was often greyish and fish-like in texture. Adult PB was either biphasic or epithelial. Biphasic PB was constituted of malignant primitive epithelial component and primitive mesenchymal component. Epithelial PB was made up of primitive components resembling well differentiated embryotic adenocarcinoma, and thus was also referred to as well differentiated fetal adenocarcinoma[11,12].
3.2. Treatment for PB
3.2.1.Surgery
PB was comprehensively treated with surgery. Since PB was unsusceptible to radiation and chemotherapy, the extent to which PB was radically excised was decisive to prognosis[1,5,13]. Main surgery included lobectomy and complete pneumonectomy. To reduce postoperative reoccurrence rate, local tumor resection should be avoided, unless cardiopulmonary function was inadequate and thus could not tolerate lobectomy and complete pneumonectomy. Pleura, nerve, pericardium or chest wall adjacent to tumors, if infiltrated, should also be resected. When the tumor infiltrated into big arteries or veins, vascular replacement surgery should be performed with the help of specialist to achieve radical excision.
One patient was preoperatively diagnosed as left upper lobe peripheral lung cancer. During the surgery, the frozen section procedure showed inflammatory granuloma, so we also performed partial wedge resection. However, the postoperative pathological report read biphasic pulmonary blastoma. To avoid reoccurrence, the patient received four cycles of chemotherapy, but still half a year later local reoccurrence in left upper lobe was found on CT scan. The second surgery was followed, including left upper lobectomy and hilar and mediastinal lymphadenectomy, without other adjuvant therapies. Two years of follow-up did not find any sign of reoccurrence.
There was a probable chance that PB would metastasize to lymph nodes, and thus hilar and mediastinal lymphadenectomy was absolutely necessary, which could also provide scientific evidences for postoperative adjuvant therapy. For PB patients, then the same surgery as to nonsmall cell lung cancer should be chosen, i.e. lobectomy or pneumonectomy together with hilar and mediastinal lymphadenectomy[1,6].
3.2.2. Radiation and chemotherapy
There was mostly no literature on choosing single chemotherapy or radiation for PB. Meanwhile, no agreement was reached on adjuvant chemotherapy or radiation schema. Many researchers believed that adjuvant chemotherapy or radiation was unbeneficial to PB patients’ overall survival rate[1,6,12]. For PB patients with brain metastasis, radiation therapy might relieve unpleasant symptoms, but be of no long-term efficacious benefit[14].
In our study, six cases, five biphasic PB and one WDFA, received four to six cycles of adjuvant chemotherapy. The six patients died of lung or bone metastasis between 11 and 36 months. Two cases with biphasic PB underwent 55 Gy radiation therapy due to postoperative brain metastasis and died of brain metastasis 6 months and 27 months later respectively. For other patients without chemotherapy or radiation, one patient had well differentiated fetal adenocarcinoma, and the tumor free survival time had been 155 months. Due to the low incidence and thus inadequate numbers of PB patients, there was still no standard comprehensive treatment schema for PB.
3.2.3. Prognosis
The prognosis was not encouraging since two the third of patients were dead two years after diagnosis and the five year survival rate was only 16.5% to 55.6%, even though there were some cases reported with a quite long survival. Clinically, there is still no consensus on factors influencing on PB patients’ prognosis. Overwhelming majority of researchers claimed that the factors might be themorphology of PB cell, its pathological type and stage, and the extent to which the tumor has been surgically resected[1,15,16].
In our study, median overall survival rate was 19 months, but individual difference was huge with the shortest being half a year and the longest being 155 months. Due to the small number of cases, statistical analysis was unfeasible for our study. Primarily, we would conclude that PB patient’s prognosis was partially influenced by the pathological type and stage, and determined by the radical resection surgery. In short, with little cases of PB reported both home and abroad and no authoritative reviews, PB’s morphology and factors influencing its prognosis still require further studies.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Jethava A, Dasanu CA. Adult biphasic pulmonary blastoma. Conn Med 2013; 71(1): 19-22.
[2] Spencer H. Pulmonary blastoma. J Pathol Bacteriol 1961; 82: 161-165.
[3] Macher-Goeppinger S, Penzel R, Roth W, Dienemann H, Thomas M, Schnabel PA, et al. Expression and mutation analysis of EGFR, C-KIT and β-catenin in pulmonary blastoma. J Clin Pathol 2011; 64(4): 349-353.
[4] Dommange-Romero F, Collardeau-Frachou S. Child pleuropulmonary blastoma. Bull Cancer 2010; 97(9): 1047-1052.
[5] Adluri RKP, Boddu SR, Martin-Ucar A, Duffy JP, Beggs FD, Morgan WE. Pulmonary blastoma: a rare tumor with variable presentation. Eur J Cardiothorac Surg 2006; 29: 236-239.
[6] Van Looa S, Boeykensb E, Stappaertsb I, Rutsaert R. Classic biphasic pulmonary blastoma: A case report and review of the literature. Lung Cancer 2011; 73: 127-132.
[7] Robert J, Pache JC, Seium Y. Pulmonary blastoma: report of five cases and identification of clinical features suggestive of the disease. Eur J Cardiothorac Surg 2002; 22: 708-711.
[8] Barrett NR, Barnard WG. Some unusual thoracic tumors. Br J Surg 1945; 32: 447-457.
[9] Fingeret A, Garcia A, Borczuk AC. Rothenberg SS, Aspelund G. Thoracoscopic lobectomy for type 1 pleuropulmonary blastoma in an infant. Pediatr Surg Int 2013; 4(4): 474-477.
[10] Agrowal D, Lahiri TK, Lakhotia S. Pulmonary blastoma in a young adult. Indian J Chest Dis Allied Sci 2012; 54(3): 189-192.
[11] Nakayama T, Ohtsuka T, Kazama A. Classic pulmonary blastoma: a subtype biphasic pulmonary blastoma. Ann Thorac Cardiovasc Surg 2012; 18(2): 125-127.
[12] Walker RI, Suvarna K, Matthews S. Pulmonary blastoma: presentation of two atypical cases and review of the literature. Br J Radiol 2005; 78: 437-440.
[13] Liu ZF, Li F, Jiao SC. Clinical analysis of adult type pulmonary blastoma. China J Modern Med 2009; 19(8): 1208-1210.
[14] Kouvaris JR, Gogou PV, Papacharalampous XN, Kostara HJ, Balafouta MJ, Vlahos LJ. Solitary brain metastasis from classic biphasic pulmonary blastoma: a case report and review of the literature. Onkologie 2006; 29: 568-570.
[15] Liman S, Altinok T, Topcu S, Tastepe AI, Uzar A, Demircan S, et al. Survival of biphasic pulmonary blastoma. Respir Med 2006; 100: 1174-1179.
[16] Zaidi A, Zamvar V, Macbeth F, Gibbs AR, Kulatilake N, Butchart EG. Pulmonary blastoma: medium-term results from a regional center. Ann Thorac Surg 2002; 73: 1572-1575.
*Corresponding author: Jing Zhang, Department of Thoracic Surgery, General Hospital of PLA, Beijing 100853, China.
E-mail: zhangj0125@sina.com
Diagnosis
Treatment
Prognosis
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Hepatic effect of NAC on sevear acute pancteatise of rats
- Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
