Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
Li Zhang, Gen-Jian Zheng, Ya-Tong Guo, Lan Zhou, Jie Du, Hong He
1Department of Orthodontics, Hubei-MOST KLOS & KLOBM, School and Hospital of Stomatology, Wuhan University, Wuhan 430079, China
2Department of Stomatology, The Affiliated Hospital of Hainan Medical College, Hainan Medical University, Haikou 570102, China
3College of Materials and Chemical Engineering, Hainan University, Haikou 571100, China
Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
Li Zhang1#, Gen-Jian Zheng2#*, Ya-Tong Guo3, Lan Zhou2, Jie Du3, Hong He1*
1Department of Orthodontics, Hubei-MOST KLOS & KLOBM, School and Hospital of Stomatology, Wuhan University, Wuhan 430079, China
2Department of Stomatology, The Affiliated Hospital of Hainan Medical College, Hainan Medical University, Haikou 570102, China
3College of Materials and Chemical Engineering, Hainan University, Haikou 571100, China
Objective: To prepare a novel biodegradable poly(2-hydroxyethylmethacrilate) (pHEMA) hydrogel as tissue engineering scaffold. Methods: The pHEMA hydrogel was synthesized by microwaveassisted polymerization using 2-hydroxyethyl methacrylate (HEMA) as the raw material, potassium persulfate as the initiator, and PCLX as the cross-linking additive. The hydrogels was characterized with FTIR and NMR spectroscopy. The physical and chemical properties of the prepared hydrogel were evaluated, and its degradation performance was tested. The cytotoxicity of the optimum composite hydrogel was measured by an MTT assay to confirm the feasibility of its use in tissue engineering. Results: The optimum conditions under which the hydrogel was prepared by microwave-assisted polymerization are as follows: 1.5 g cross-linking additive, 0.3 g initiator, reaction temperature of 80 ℃, and microwave power of 800 W. Degradation studies showed good degradation profiles with 75% in 17 days. Additionally, the hydrogels did not elicit any cytotoxic response in in vitro cytotoxic assays. Conclusion: A biodegradable pHEMA hydrogel was successfully prepared by microwave-assisted polymerization, as confirmed from FTIR and NMR results. The hydrogel shows promising applications in tissue engineering, and its healing ability and biocompatibility will be evaluated in detail in the future.
ARTICLE INFO
Article history:
Received 10 October 2013
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Poly(2-hydroxyethylmethacrilate)
1. Introduction
An ideal scaffold for biomedical applications should be a three-dimensional constitution that is highly porous to guarantee oxygen permeability, sufficient nutrient transport to the cells, and removal of waste products[1]. Additionally, the scaffold should have mechanical properties suitable for thein vivotissue at the site of implantation and should be easily connected to the vascular system of the host[2-4].
Hydrogels are an important three-dimensional class of scaffolds used in tissue engineering, with their primary advantage being cell protection, which is possible because of their aqueous nature. However, various hydrogels which are used widely in medicine today are not biodegradable and cannot be easily excreted from the body[5]. As early as 1960, poly(2-hydroxyethylmethacrylate) (pHEMA) hydrogels had been successfully applied as biomedical materials in plastic surgery, ophthalmology, and drug delivery[6-9]. pHEMA was the preferred choice of scaffold material, because its excellent mechanical properties and high hydrophilicity made it suitable for conversion into different architectures[10]. Unfortunately, similar to other hydrogels, pHEMA hydrogels are not biodegradable and are typicallyinserted into the body by surgical means, limiting their widespread application.
Injectable biodegradable hydrogels are promising candidates for novel biomedical materials, because they can be incorporated into the body by simple injection without the need for any surgical procedure[11]. In addition, biodegradable hydrogels have played a significant role as scaffold material for vocal cord tissue engineering. These hydrogels will provide an ample aqueous environment for cell metabolism, act as efficient transmission channels, form good contact with the cell tissues, and can be injected in situ[5].
In this study, a novel biodegradable pHEMA hydrogel was synthesized by microwave-assisted polymerization using 2-hydroxyethyl methacrylate (HEMA) as the raw material, potassium persulfate as the initiator, and polycaprolactone cross-linking agent (PCLX) as the cross-linking additive. The hydrogel was characterized by Fourier transform infrared (FTIR) and1H nuclear magnetic resonance (NMR) spectroscopy. Further, the degradation behavior of the hydrogel in PBS was determined by the weighing method and the cytotoxicity was measured by an MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay, in order to evaluate the feasibility of using the hydrogel as a tissue engineering scaffold.
2. Materials and methods
2.1. Chemicals
The following chemicals were purchased from engineering and technology research centers in Guangdong Province of China: polycaprolactonediol, methylene chloride, triethylamine,n-hexane, tetrahydrofuran, methyl alcohol, dimethylformamide, ethyl alcohol, ethylene glycol, acetone, ethyl 2-bromoisobutyrate, sodium bicarbonate, HEMA (99% minimum purity), magnesium sulfate, acryloyl chloride, and potassium persulfate. MTT was purchased from Sigma. All the chemicals were used without further purification.
2.2. PCLX synthesis
Polycaprolactone diol (95 g, 0.047 mol) was dissolved in 80 mL methylene chloride, to which triethylamine (40 mL, 0.14 mol) was added. A solution of acryloyl chloride (50 mL, 0.14 mol) in methylene chloride (40 mL) in a dropping funnel was added dropwise, and the mixture was allowed to react for 12 h at 0 ℃ and then for 24 h at room temperature. The resulting chloride salt was removed by filtration to obtain an oily liquid. Methylene chloride was removed by distillation, after which coldn-hexane was added to the reaction mixture. After the solution was agitated and dissolved, the solution delaminates. The oily lower layer was removed with a separation funnel. The reaction scheme is shown in Figure 1.
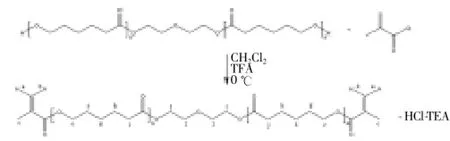
Figure 1. PCLX cross-linking agent synthesis reaction.
2.3. Synthesis of biodegradable pHEMA hydrogel
Potassium persulfate (0.3 g) and PCLX (1.5 g) were dissolved in dimethylformamide (1.5 mL). HEMA (10 mL) was added to the reaction mixture, and the solution was subjected to microwave heating (temperature: 80 ℃; power: 800 W). After 100 seconds, the pHEMA hydrogel was obtained. The hydrogel was removed and flushed with acetone/water (90:10, v/v) several times in order to remove any unreacted monomer.
2.4. FTIR
The chemical structure of the synthesized pHEMA hydrogel was examined by FTIR using a TENSOR spectrometer with a resolution of 2 cm-1, measuring 20 scans. The pure hydrogel was crushed into a powder, which was used to prepare KBr pellets (5 mg of sample per 500 mg of KBr). Spectra were recorded over the range 4 000 to 400 cm-1with a data acquisition rate of 4 cm-1per point by transmission sampling.
2.5. NMR measurements
NMR spectra were recorded at room temperature using a Bruker AV 400 spectrometer operating at 400 MHz with a multinuclear broadband NP 5 mm probe. Chemical shifts were recorded in part per million (ppm).
2.6. In vitro degradation assay
Thein vitrodegradation rate and profile of the hydrogels in PBS were measured by the percent weight loss and weight swelling ratio. All the samples were lyophilized and weighed before the degradation studies to confirm the original dryweight. The prepared freeze-dried samples were placed in 50 mL PBS at physiological temperature (37 ℃) for a set schedule, after which they were removed from the solution, freeze-dried, and weighed again. The percentage weight loss and swelling ratio were evaluated by using the following equations:
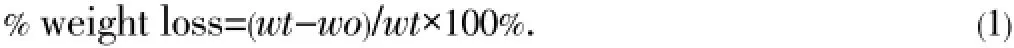
where,wtis the initial dry weight of the sample andwois the dry weight of the sample after a specific degradation period.
2.7. Evaluation of in vitro elution cytotoxicity
The cytotoxicity of the synthesized hydrogels was evaluated at time points of 12, 24, and 48 h after incubation by the MTT assay. The results of the cytotoxicity experiment was illustrated by the ratio (%) of the OD values for the extracttreated cells and control cells. High density polyethylene and 5% (v/v) DMSO were used as negative and positive controls, respectively.
3. Results
3.1. Characterization of pHEMA scaffolds
The FTIR spectra obtained for pHEMA are shown in Figure 2. Briefly, the –OH stretching vibration band was observed at 3 438.14 cm-1; the antisymmetric stretching vibration band of the methyl group was observed at 2 960.28 cm-1; the stretching vibration band of C=O was observed at 1 727.86 cm-1; the characteristic absorption band of C-O was observed at 1 276.26 cm-1; the antisymmetric stretching the vibration band of C-O-C was observed at 1 161.89 cm-1; the distortion vibration bands of methyl and methylene were observed at 1 454.90, 947.12, and 851.78 cm-1(Figure 2). The characteristic absorption band of the vinyl group was not observed, which indicated that the HEMA monomer was successfully converted to the pHEMA polymer through the free radical polymerization reaction.

Figure 2. FTIR spectra of pHEMA.
The NMR spectrum of the pHEMA hydrogel is shown in Figure 3. Briefly, the multiplet at 1.35 ppm indicated the protons of the methyl group on an alpha-carbon. Signals due to the protons of the methylene group linked to the hydroxyl moiety of the cross-linking agent were observed at 2.25 and 3.90 ppm. The –OH proton signal appeared at 3.60 ppm, while peaks due to the methylene group of the side chain ester were seen at 3.85 ppm. The peaks due to the protons of the methylene connected to the hydroxyl group on the side chain appeared at 4.10 ppm. Based on the characteristics of the NMR peaks, the sample was confirmed to contain RCOOR’, R–O–R, and C=C functional groups.
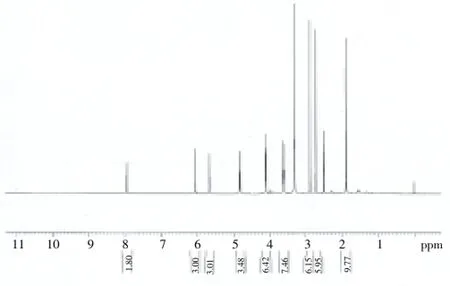
Figure 3. NMR spectra of pHEMA.
3.2. In vitro degradation
Degradation studies showed good degradation profiles with 75% in 17 days (Figure 4).

Figure 4. Weight loss as a function of time for pHEMA hydrogel in PBS.
3.3. In vitro elution cytotoxicity
The results of the cytotoxicity experiment (Figure 5) showed that the OD values of all groups increased gradually with the prolonged cultivation time, except that of the positivecontrol. The time points in each group could be used to compare the effect of different materials on cell growth.

Figure 5. Cytotoxicity of pHEMA hydrogel against L-929 cells by MTT assay.
The relative growth rate of all the groups was grade 0. No statistical difference was observed over the chosen time period between the test groups and negative control, indicating that the hydrogels and their degradation products would have good biocompatibility and meet the requirements for use in medical applications.
As shown in Figure 6, the L-929 cells treated with pHEMA hydrogel had a low density, were sparsely distributed, and were almost completely attached to the bottom of the culture plate at 24 h. After 48 h, the cells rapidly proliferated in pairs, and after 72 h, the number of cells increased significantly and grew on the bottom of the culture plate. Microscopic observations of cells in different groups revealed the cells were in the long-stripped fusiform or irregular multi-deformation shape; the cells grew readily on the wall of the culture plate and were vacuole-free; discrete grains were visible in the cytoplasm; and the cell number increased normally. The growth morphology of the L-929 cells are not different between the test groups and negative control.

Figure 6. Microscopic observation of L-929 cells.A, B and C represent the control cells after culture for 24, 48 and 72 h respectively, while D, E and F represent the cells treated with pHEMA hydrogel for 24, 48 and 72 h respectively (100×).
4. Discussion
pHEMA and its functionalized derivatives have been used in a wide range of biomedical applications[12]. These hydrogel polymers have physical properties similar to those of natural gel-like extracellular matrices and may contain pores large enough to accommodate living cells, or they may be designed to dissolve or degrade away, releasing growth factors and creating pores into which living cells may penetrate and proliferate[13]. However, many pHEMA hydrogels are not degradable, limiting their usefulness in bone repair and replacement[14]. Lopezet alverified that pHEMA inherently exhibited low protein adsorption and cell adhesion properties[15]. In an attempt to promote degradation and cell adhesion, scaffolds were prepared by microwaveassisted polymerization using HEMA as monomer, potassium persulfate as initiator, and PCLX as cross-linking additive. The addition of degradable polycaprolactone segments in the initiator and cross-linker as well as specific control over the pHEMA molecular weight under microwave-assisted polymerization made the hydrogel bioresorbable. Our characterization of pHEMA indicates that the experimental method is feasible by which a gel network structure can be prepared successfully. The finding from our study, which is consistent with those represented by Franket al[16], is that microwave irradiation assists rapid and uniform heating of a reaction solution. Thereby, microwave irradiation was proved to be advantageous over conventional heating methods that result in slow and uneven distribution of heat. In addition, the torsional vibration frequency of microwaves is similar to that of various chemical groups, and microwave-assisted polymerization can change the molecule conformation, activate functional groups without damaging the macromolecular chain, and increase the reaction rate greatly[16].
The results of degradation of the synthesized hydrogels in PBS provide an evidence that degradation performance is excellent. Atzetet al.reported that rupture of a sufficient number of PCL bonds from a pHEMA backbone segment would allow for dissolution of the chain and cause a consequent decrease in the mass of the hydrogel[17], which is supported by our data. Our data also suggest that the hydrogels were subjected to bulk degradation, as demonstrated by measurable initial changes in properties for all degradable samples and 75% degradation in 17 days. Degradation was found to depend strongly on several factors. The increase in the content of the cross-linker leads to a reduction in weight loss regardless of the type of the comonomer, which is also supported by Huanget al., who stated that incorporation of a co-monomer enhanced the degradation dramatically at a similar crosslink density[18,19]. Prior toin vivoexperiments, the cytotoxicity of the hydrogels and their degradation products must be evaluated to assesstheir biocompatibility. The MTT assay, initially introduced by Mosmann[20] in 1983, was regarded as a sensitive method for the evaluation of the cytotoxicity of dental materials[21,22]. The present MTT analysis also demonstrates that the hydrogels tested are non-cytotoxic to fibroblasts over the concentration range examined.
In conclusion, a novel biodegradable pHEMA hydrogel was synthesized and characterized. Characterization by FTIR and NMR confirmed the expected identity and purity of the hydrogel. Biodegradation studies showed 75% uniform bulk degradation of the hydrogel over a period of 17 days. In addition, the biodegradable hydrogel had no observable cytotoxicity toward L-929 fibroblast cells. Therefore, this novel bioresorbable pHEMA can play an important role as a scaffold for tissue engineering applications.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of Hainan Province (Grant No. 812200). The authors wish to acknowledge Qing-Song Zhang, Professor of School of Materials Science and Engineering, Tianjin Polytechnic University, for his valuable suggestions.
[1] Hillel A, Shah P, Elisseeff J. Hydrogels in cell encapsulation and tissue engineering. In: Jenkins M, editor. Biomedical polymers. Cambridge, England: Woodhead Publishing Ltd.; 2007, p. 57-75.
[2] Lee JB, Park HN, Ko WK, Bae MS, Heo DN, Yang DH, et al. Poly(L-lactic acid)/hydroxyapatite nanocylinders as nanofibrous structure for bone tissue engineering scaffolds. J Biomed Nanotechnol 2013; 9(3): 424-429.
[3] Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJ. Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng 2007; 13(8): 1905-1925.
[4] Wagoner Johnson AJ, Herschler BA. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater 2011; 7(1): 16-30.
[5] Atzet S, Curtin S, Trinh P, Bryant S, Ratne B. Degradable poly(2-hydroxyethyl methacrylate)-co-polycaprolactone hydrogels for tissue engineering scaffolds. Biomacromolecules 2008; 9(12): 3370-3377.
[6] Matters AT, Anseth KS, Bowman CN. A statistical kinetic model for the bulk degradation of PLA-b-PEG-b-PLA hydrogel networks: Incorporating network non-idealities. J Phys Chem B 2001; 105(34): 8069-8076.
[7] Bayramoğlu G, Yalçin E, Arica MY. Adsorption of serum albumin and γ-globulin from single and binary mixture and characterization of pHEMA-based affinity membrane surface by contact angle measurements. Biochem Eng J 2005; 26(1): 12-21.
[8] Casimiroa MH, Lealb JP, Gil MH. Characterisation of gamma irradiated chitosan/pHEMA membranes for biomedical purposes. Nucl Instrum Meth B 2005; 236(1-4): 482-487.
[9] Arica MY, Yilmaz M, Yalçın E, Bayramoğlu G. Surface properties of reactive yellow 2 immobilised pHEMA and HEMA/chitosan membranes: Characterisation of their selectivity to different proteins. J Membrane Sci 2004; 240(1-2): 169-178.
[10] Meng X, Stout DA, Sun L, Beingessner RL, Fenniri H, Webster TJ. Novel injectable biomimetic hydrogels with carbon nanofibers and self assembled rosette nanotubes for myocardial applications. J Biomed Mater Res A 2013; 101(4): 1095-1102.
[11] Nguyen MK, Lee DS. Injectable biodegradable hydrogels. Macromol Biosci 2010; 10(6): 563-579.
[12] Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev 2001; 101: 1869-1879.
[13] Ye ZY, Zhou Y, Cai HB, Tan WS. Myocardial regeneration: Roles of stem cells and hydrogels. Adv Drug Deliv Rev 2011; 63: 688-697.
[14] Wichterle O, Lim D. Hydrophilic gels for biological use. Nature 1960; 185(4706): 117-118.
[15] Lopez GP, Ratner BD, Rapoza RJ, Horbett TA. Plasma deposition of ultrathin films of poly(2-hydroxyethyl methacrylate): Surface analysis and protein adsorption measurements. Macromolecules 1993; 13: 3247-3253.
[16] Wiesbrock F, Hoogenboom R, Schubert US. Microwave-assisted polymer synthesis: State-of-the-art and future perspectives. Macromol Rapid Commun 2004; 25: 1739-1764.
[17] Atzet S, Curtin S, Trinh P, Bryant S, Ratner B. Degradable poly(2-hydroxyethyl methacrylate)-co-polycaprolactone hydrogels for tissue engineering scaffolds. Biomacromolecules 2008; 9: 3370-3377.
[18] Huang JJ, Zhao DC, Dangaria SJ, Luan XH, Diekwisch TGH, Jiang GQ, et al. Combinatorial design of hydrolytically degradable, bonelike biocomposites based on PHEMA and hydroxyapatite. Polymer 2013; 54(2): 909-919.
[19] Huang JJ, Ten E, Liu G, Finzen M, Yu WL, Lee JS, et al. Biocomposites of pHEMA with HA/b-TCP (60/40) for bone tissue engineering: Swelling, hydrolytic degradation, and in vitro behavior. Polymer 2013; 54(3): 1197-1207.
[20] Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Method 1983; 65: 55-63.
[21] Schweikl H, Schmalz G. Toxicity parameters for cytotoxicity testing dental materials in two different mammalian cell lines. Cur J Oral Sci 1996; 104: 292-299.
[22] Osorio RM, Hefti A, Vertucci FJ, Shawley AU. Cytotoxicity of endodontic materials. J Endodon 1998; 24: 91-95.
*Co-corresponding author: Dr. Hong He, Department of Orthodontics, Hubei-MOST KLOS & KLOBM, School and Hospital of Stomatology, Wuhan University, Wuhan 430079, China.
Tel: +86-27-87686224
E-mail: drhehong@hotmail.com
Dr. Gen-Jian Zheng, Department of Stomatology, The Affiliated Hospital of Hainan Medical College, Hainan Medical University, Haikou 570102, China.
Tel: +86-898-66774287
E-mail: airforcezhgj@163.com
#These authors contributed equally to this work.
Foundation project: Project supported by the National Natural Science Foundation of Hainan Province (Grant No. 812200).
Biodegradable hydrogel
Microwave-assisted polymerization
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Diagnosis and multi-modality treatment of adult pulmonary plastoma: Analysis of 18 cases and review of literature
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Hepatic effect of NAC on sevear acute pancteatise of rats
