Antimalarial potential of kolaviron, a biflavonoid from Garcinia kola seeds, against Plasmodium berghei infection in Swiss albino mice
Adaramoye Oluwatosin, Akinpelu Tolulope, Kosoko Ayokulehin, Okorie Patricia, Kehinde Aderemi, Falade Catherine, Ademowo Olusegun
1Biochemistry Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
2Pharmacology Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
3Medical Microbiology Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
4Pharmacology and IAMRAT Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
Antimalarial potential of kolaviron, a biflavonoid from Garcinia kola seeds, against Plasmodium berghei infection in Swiss albino mice
Adaramoye Oluwatosin1*, Akinpelu Tolulope2, Kosoko Ayokulehin1, Okorie Patricia2, Kehinde Aderemi3, Falade Catherine2, Ademowo Olusegun4
1Biochemistry Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
2Pharmacology Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
3Medical Microbiology Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
4Pharmacology and IAMRAT Department, University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan , Ibadan, Oyo/South-West 20005, Nigeria
Objective: To investigate the antimalarial potential of kolaviron (KV), a biflavonoid fraction from Garcinia kola seeds, against Plasmodium berghei (P. berghei) infection in Swiss albino mice.
Methods: The study consists of seven groups of ten mice each. Groups Ⅰ, Ⅱand Ⅲ were normal mice that received corn oil, KV1 and chloroquine (CQ), respectively. Groups Ⅳ, Ⅴ, Ⅵ and Ⅶwere infected mice that received corn oil, CQ, KV1 and KV2, respectively. CQ, KV1 and KV2 were given at 10-, 100- and 200-mg/kg daily, respectively for three consecutive days. Results: Administration of KV1 and KV2 significantly (P<0.05) suppressed P. berghei-infection in the mice by 85% and 90%, respectively, while CQ produced 87% suppression relative to untreated infected group after the fifth day of treatment. Also, KV2 significantly (P<0.05) increased the mean survival time of the infected mice by 175%. The biflavonoid prevented a drastic reduction in PCV from day 4 of treatment, indicating its efficacy in ameliorating anaemia. Significant (P<0.05) oxidative stress assessed by the elevation of serum and hepatic malondialdehydewere observed in untreated P. berghei-infected mice. Specifically, serum and hepatic malondialdehyde levels increased by 93% and 78%, respectively in the untreated infected mice. Furthermore, antioxidant indices, viz; superoxide dismutase, catalase, glutathione-s-transferase, gluathione peroxidase and reduced gluathione decreased significantly (P<0.05) in the tissues of untreated P. berghei-infected mice. KV significantly (P<0.05) ameliorated the P. berghei-induced decrease in antioxidant status of the infected mice. Conclusions: This study shows that kolaviron, especially at 200 mg/kg, has high antimalarial activities in P. berghei-infected mice, in addition to its known antioxidant properties.
ARTICLE INFO
Article history:
Received 10 October 2013
Received in revised form 15 December 2013
Accepted 15 January 2014
Available online 20 February 2014
Antimalaria
Antioxidant
Biflavonoid
Kolaviron
Plasmodium berghei
1. Introduction
Malaria is a parasitic infection caused byPlasmodiumspecies, and is one of the oldest and greatest health challenges affecting 40% of the world’s population[1]. Malaria deaths peaked at 1.82 million in 2004 and fell to 1.24 million in 2010 (714 000 and 524 000 deaths are children that are less than and greater than 5 years, respectively) and over 80% total deaths occur in sub-Sahara Africa[2]. The disease is a major obstacle to economic advancement of many developing and tropical nations predisposing people to poverty. Chemotherapy remains a major means of malaria control. However, the previously efficacious chloroquine (CQ) is failing both as a prophylactic and therapeuticantimalarial drug in many endemic countries of Africa, due to the emergence of CQ resistantPlasmodium falciparumstrains with mutant alleles for CQ resistance transporter proteins (pfcrtT76) and multidrug resistance glycoprotein-1 (pfmdr-1Y86)[3,4]. Hence, the diminished potency of CQ in many of the endemic countries have paved way for research into discovery and/ or development of new antimalarial drugs. In the last decade, several fundamental researches have been conducted to explore antimalarial activity of many plants, includingCitrus cinensis,Carica papaya,Swertia chirata[5],Bidens pilosa[6],Nigella sativa[7],Piper sarmentosum,Tinospora cordifolia[8] and many others[9].
Garcinia kolaHeckel (family Guttiferae) is a cultivated large forest tree, valued in most parts of West and Central Africa for its edible nuts[10]. The seed, known as bitter kola or false kola, is commonly chewed and serves as an alternative to true kola nuts (Cola nitidaandCola accuminata). Extracts of various parts of the plant are used extensively in traditional African medicine[11], especially for the preparation of remedies for the treatment of laryngitis, cough and liver diseases[12]. Chemical investigations of the seeds have shown that they contain a complex mixture of phenolic compounds, including GB-type biflavonoids, xanthones, benzophenones, cycloartenol and triterpenes[13,14]. Kolaviron (KV) (Figure 1) is a bifavonoid complex isolated from the seeds ofGarcinia kolaand has been reported to possess neuroprotective, antiinfammatory, antimicrobial, antioxidant, antigenotoxic and hepatoprotective activities in model systems via multiple biochemical mechanisms[15-18]. Furthermore, studies by Adaramoyeet al[19] and, Adaramoye and Medeiros[20] showed that KV has anti-atherogenic and vasorelaxant effects in animal model and isolated smooth muscle, respectively. There is limited information on the effect of this biflavonoid on the growth ofPlasmodiumspecies in animal model. This study was therefore designed to investigate thein vivoantimalarial effect of kolaviron inPlasmodium berghei(P. berghei)-infected mice.

Figure 1. Structures of KV and CQ.
2. Materials and methods
2.1. Chemicals
Glutathione, Hydrogen peroxide, 5,5’-dithios-bis-2-nitrobenzoic acid and epinephrine were purchased from Sigma Chemical Co., Saint Louis, MO USA. Absolute ethanol, trichloroacetic acid and thiobarbituric acid were purchased from British Drug House Chemical Ltd., Poole, UK. Other chemicals were of analytical grade and purest quality available.
2.2. Plant material and extraction procedure
Garcinia kolaseeds (Guttiferae heckel) seeds were purchased from a local vendor in Ibadan, Nigeria. Kolaviron was extracted from the fresh seeds of the Kola (3.5 kg) and characterized according to the method of Iwuet al[21]. Briefly, powdered dried seeds were extracted with light petroleum ether (b.p. 40-60 ℃) in a soxhlet extractor for 24 h. The defatted, dried marc was repacked and then extracted with methanol. The extract was concentrated and diluted to twice its volume with distilled water and extracted with ethyl acetate. The concentrated ethyl acetate fraction gave a yellow solid known as kolaviron. The yield of the preparation was 6%.
2.3. Animals
Male adult Swiss albino mice were obtained from the animal house of the Institute for Advanced Medical Research and Training, College of Medicine, University of Ibadan, Nigeria. The animals were housed in well-aerated plastic cages, fed with standard mouse cubes (Ladokun Feeds, Nigeria, Ltd) and supplied with clean drinking waterad libitum.P. bergheiused in this study was a donation to the laboratory of one of us (OGA) by Malaria Research and Reference Reagent Resource Centre (MR4). The parasites were maintained in animals by serial passages of blood collected from a patent donor mouse to a naive recipient. Handling of animals and other protocols conform to the guidelines of the National Institutes of Health and Animal Ethical Committee of University of Ibadan, Nigeria, for care of laboratory animals.
2.4. Course of infection and antimalarial activity
The course of infection ofP. bergheifollowing intraperitoneal inoculation in mice was studied in each experimental mouse that received 107parasitized red bloodcells in 0.2 mL inoculum. Thin blood films were prepared from the tail vein of infected mice, fixed with methanol and stained with 10% Giemsa stain using standard procedure. Parasitemia was monitored daily and blood smears were read using ×100 objective of a light microscope.In vivoantimalarial activity againstP. bergheiinfection in mice was done according to Rane’s test as described by Elufioye and Agbedahunsi[22]. The test relies on the ability of a standard inoculum ofPlasmodium yoellito kill the recipient mouse within 12 days of inoculation. Extension of survival beyond 12 days is regarded as activity.
2.5. Study design
Mice weighing between 18 and 23 g were distributed into seven groups of ten animals each. Group Ⅰ: uninfected normal mice (positive control), group Ⅱ: uninfected normal mice that received KV at a dose of 100 mg/kg (KV1), groupⅢ: uninfected normal mice that received CQ, group Ⅳ: untreated infected mice (Negative control), group Ⅴ: infected mice treated with CQ, group Ⅵ: infected mice that received KV1 and group Ⅶ: infected mice that received KV2 (200 mg/ kg)[23]. CQ and KV were adminstered to infected mice after 72 h of parasite inoculation when the infection was established. CQ and KV were dissolved in distilled water and corn oil, respectively and given daily for three consecutive days (Days 3, 4 and 5 post-infection) to the animals by oral gavage. The control animals received equivolume of corn oil (vehicle), and CQ was given at dose of 10 mg/kg body weight[24]. The levels of parasitemia in the mice were monitored daily untill day 10 before half of the animals (n=5) were sacrificed. The blood and liver of sacrificed animals were obtained for biochemical assay. The remaining five mice per group were monitored to obtain survival time.
2.6. Preparation of samples
Portion of the whole blood from each animal was collected into heparinized bottles, stored at 4 ℃ and the red cells were assayed for antioxidant parameters. The other portion was taken into plain centrifuge tubes and allowed to stand for 2 h before centrifugation to obtain serum. The serum was used to determine the extent of lipid peroxidation and some enzymes markers. Liver was excised after dissection of the animals and rinsed in ice-cold 1.15% KCl, dried and weighed. The liver samples were homogenized in 4 volumes of 50 mM phosphate buffer, pH 7.4 using a Potter Elvehjem homogenizer and centrifuged at 10 000gfor 15 minutes to obtain post-mitochondrial supernatant fraction (PMF).
2.7. Biochemical and physiological assays
2.7.1. Determination of Haematocrit
The haematocrit or packed cell volume (PCV) was determined to predict the effectiveness of the biflavonoid in preventing anaemic conditions in malaria[25]. Blood was drawn from the tail of the mice in the different groups into heparinised capillary tubes. Capillary tubes were filled to mark, sealed at one end and spun for ten minutes in a micro-haematocrit centrifuge. The haematocrit of each animal was subsequently read with haematocrit reader.
2.7.2. Protein
Serum and PMF protein levels were determined according to the method of Lowryet al[26] using bovine serum albumin as standard.
2.7.3. Alanine (ALT) and aspartate aminotransferases (AST)The activities of serum ALT and AST were determined by the combined methods of Mohun and Cook[27], and Reitman and Frankel[28].
2.7.4. Total bilirubin and urea
Serum total bilirubin and urea levels were assayed by the methods of Rutkowski and Debaare[29] and, Talke and Schubert[30], respectively.
2.7.5. Superoxide dismutase (SOD), catalase (CAT) and glutathione-S-transferase (GST)
SOD activity was measured by the nitroblue tetrazolium reduction method of McCord and Fridovich[31]. The GST activity was determined by the method of Habiget al[32], the method is based on the rate of conjugate formation between glutathione and 1-chloro-2,4-dinitrobenzene. The CAT activity was assayed by measuring the rate of decomposition of hydrogen peroxide at 240 nm as described by Aebi[33].
2.7.6. Glutathione Peroxidase (GPx), Reduced glutathione (GSH) and lipid peroxidation
The GPx activity was determined according to the method of Rotrucket al[34]. Reduced GSH level was assayed by measuring the rate of formation of chromophoric product in a reaction between 5,5-dithio-bis (2-nitrobenzoic acid) and free sulfhydryl groups at 412 nm by the method of Moronet al[35]. The extent of lipid peroxidation (LPO) was estimated by the method of Wallset al[36]. The method involved the reaction between malondialdehyde (MDA) and thiobarbituric acid to form a pink precipitate, which was read at 535 nm spectrophotometrically.

Table 1 Effect of KV and CQ on the levels of parasitemia in normal and P. berghei-infected mice.
2.8. Statistical analysis
The results were expressed as mean±standard deviation (SD) of 10 mice per group. Data were analysed using one-way analysis of variance (ANOVA) followed bypost-hocDuncan’s multiple range test for analysis of biochemical data using SPSS (12.0) statistical software. Values were considered statistically significant at P<0.05.
3. Results
3.1. Effects of KV and CQ on parasitemia and body weight of P. berghei infected mice
A progressive increase in average percentage parasitaemia was observed inP. bergheiinfected mice, with a maximum of 81% average parasitaemia by day 10 (post infection). However, the results showed that CQ, KV1 and KV2 were able to suppress parasitaemia considerably by day 6 (post infection), while KV2 had the highest percentage suppression of 92% at day 10 post-infection (Table 1). In addition, KV1 and KV2 extended the mean survival time of the mice to 15.8 and 28.1 days, respectively, when compared with CQ (14.6 days) and untreated infected group (10.2 days) (Table 2). In addition,P. bergheiinfection caused significant (P<0.05) decrease in the body weights-gain in the mice relative to normal. Treatment with CQ and KV significantly (P<0.05) increased the body weight-gain of the infected mice (Table 2).

Table 2 Effect of KV and CQ on the mean survival time and body weights in normal and P. berghei-infected mice.
3.2. Effects of KV and CQ on PCV and serum biochemical indices of P. berghei infected mice
The PCV of untreated, infected mice decreased significantly (P<0.05) as the infection progressess. However, treatment with KV and CQ significantly (P<0.05) ameliorated theP.berghei-induced decrease in PCV at days 6 and 7 postinfection, respectively (Table 3).P. bergheiinfection also caused significant (P<0.05) increase in the activity of serum alanine aminotransferase (ALT) in the mice. Importantly, the serum ALT of untreated, infected mice increased by 107%, relative to normal, while treatment with CQ and KV reversed theP. berghei-induced alterations in the activity of ALT. There were no significant differences (P>0.05) in the levels of serum urea, total bilirubin and activity of serum AST ofP. bergheiinfected mice when compared to others (Table 4).

Table 3 Effect of KV and CQ on the PCV in normal and P. berghei-infected mice.
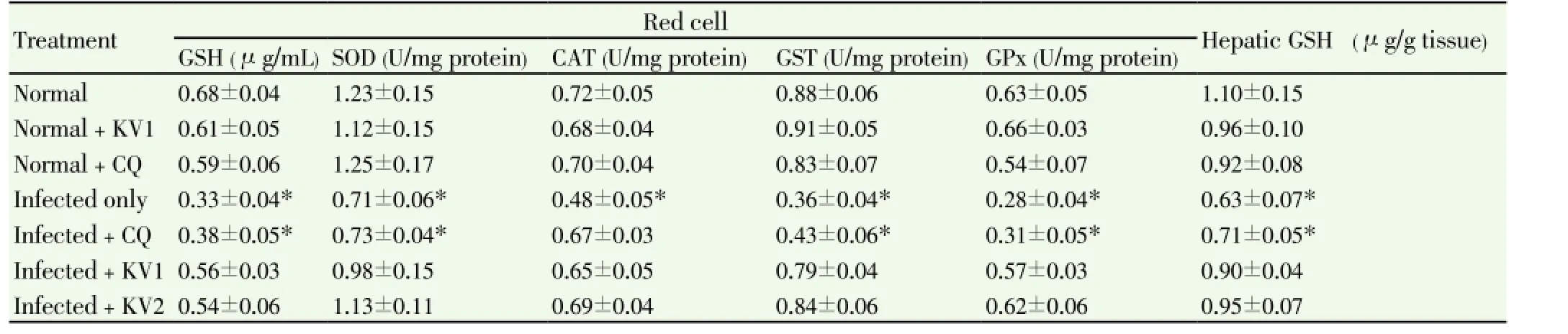
Table 5 Effect of KV and CQ on enzymic and non-enzymic antioxidant profiles of P. berghei-infected mice.
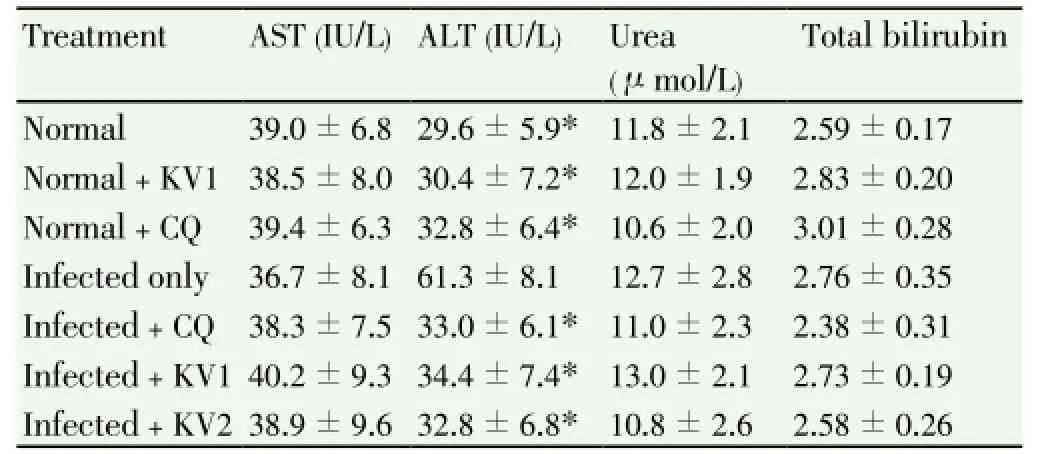
Table 4 Effect of KV and CQ on serum biochemical indices of P. berghei -infected mice.
3.3. Effects of KV and CQ on the antioxidant profiles of P. berghei infected mice
A significant (P<0.05) increase in MDA levels (lipid peroxidation index) was observed inP. bergheiinfected mice as parasitemia increased. Precisely, serum and hepatic MDA levels were increased by 93% and 78%, respectively in infected mice when compared to normal. Administration of KV alone significantly (P<0.05) decreased the MDA values of the untreated, infected mice (Figure 2).P. bergheiinfection caused a significant (P<0.05) decrease in the levels of red cell and hepatic GSH as well as the activities of SOD, CAT, GPx and GST of untreated infected mice relative to normal (Table 5, Figures 3 and 4). However, treatment with KV alone completely attenuatedP. berghei-induced decrease in the GSH levels (Table 5), while administration of CQ and KV significantly (P<0.05) ameliorated the activities of hepatic CAT, GST and GPx of the infected mice (Figures 3 and 4).
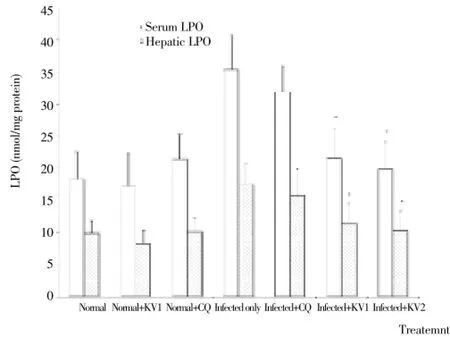
Figure 2. Effects of KV and CQ on levels of serum and hepatic LPO in P. berghei-infected mice.* Significantly different from infected only (P<0.05).

Figure 3. Effects of KV and CQ on activities of hepatic SOD and CAT in P. berghei-infected mice.* Significantly different from infected only (P<0.05).

Figure 4. Effects of KV and CQ on activities of GST and GPx in P. berghei-infected mice.* Significantly different from infected only (P<0.05).
4. Discussion
The spread of resistance in the malaria parasite to safe, affordable and commonly available antimalarial drugs especially the monotherapeutic drugs such as chloroquine and sulfadoxine-pyrimethamine[37], is a major problem in malaria chemotherapy especially in resource poor endemic areas. The emergence of resistance to the artemisinins which form the backbone of the currently efficacious artemisinin-based combination therapy in Cambodia[38] and Myanmar[39] underscore the need to discover and develop new antimalarial drugs. The present study has not only validated the antiplasmodic activity of KV but has also demonstrated its inherent antioxidant properties. In this study, KV suppressed the growth of the establishedP. bergeiparasites by 93%, against 85% obtained in CQ-treated group at day 10. This suggests that the antimalarial efficacy of KV againstP. bergheiis better than CQ. The biflavonoid did not clear the parasites completely, but it exhibited a marked and significant reduction in multiplication of parasites during treatment, indicating that KV may have a direct action on the parasites. It has been reported that several plant constituents,viz; flavonoids, tannins, quinonoid, xanthene, polyphenols, and terpenoids possess protein-binding and enzyme-inhibiting properties[40,41]. The likely mechanism of action of this biflavonoid may be the inhibition of key pathogenic enzymes of the parasite since KV is known to interfere with enzyme systems[42,43]. Anaemia is a consistent feature ofPlasmodiuminfections[44] caused by, among other factors, increased lipid peroxidation as a consequence of oxidative damage to the membrane components of erythrocytes[45]. It was observed that in addition to the suppression of parasitemia, KV prevented a drastic reduction in PCV values in infected mice showing its ability to ameliorate anaemia. The amelioration of theP. bergheiinduced anaemia by KV may be attributable to its scavenging effects towards the generated ROS and thereby reducing the oxidative attack to which the erythrocytes membranes are exposed in the infected mice. The reduction in anaemia was consistent with the marked decrease in parasite load observed in the course of infection in the groups of mice treated with 100- and 200- mg/kg doses of the biflavonoid. This may be a subtle evidence of the efficacy of the antimalarial effect of KV as red blood cell lysis tends to be more severe with sustained parasitemia.
The role of oxidative stress as an important clinical and biochemical mechanism of the disease pathogenesis is increasingly becoming relevant[46,47]. It results from the high metabolic rate of the rapidly growing and multiplying parasite which produces large quantities of toxic redox active by-products. The observed elevation in MDA values of infected mice in this study is in concordance with the findings of Rodrigues and Gamboa[48] and Okeolaet al[7]. Increased MDA implies increase in reactive oxygen species (ROS) levels, which are cellular renegades, and can wreak havoc in biological systems by tissue damage, altering biochemical compounds, corroding cell membranes and killing out rightly[49]. This claim was further supported by decrease in red cell and hepatic activities of SOD, CAT, GPx, GST and levels of GSH in the infected mice, which indicate that excess ROS probably inactivate these antioxidant enzymes. This observation is in line with the findings of Ibrahimet al[50], who linked the reduced activities of SOD and CAT inP. bergheiinfection to excessive generation of ROS. However, administration of KV increased the activities of the antioxidant enzymes. It would appear therefore that the biflavonoid kept the levels of ROS low thereby reducing the extent ofP. berghei-induced lipid peroxidation and/ or increase the levels of substrate (GSH) required for detoxification by GPx and GST. The in vivo antioxidant effects of KV led to the restoration of antioxidant status of infected mice and, this would obviously provide greater protection for cell membrane components as well as other susceptible cellular components and hence significantly retarding theP. bergheiassociated organ pathological effects.P. bergheiinfection has been reported to cause hepatomegaly and splenomegaly in the mice model[51] and was linked to increased phagocytosis of infected cells by macrophages and deposition of malarial pigment as well as activation and hyperplasia of the reticulo-endothelial system during the disease[52]. In this study, elevated levels of serum ALT was observed in infected mice. The ability of KV to reverse the serum ALT values in this study, could suggest that KV may be protective againstP. bergheiinducedhepatomegaly in the mice.
In conclusion, kolaviron, a biflavonoid fromGarcinia kolaseeds, elicited potent antimalarial activity inP. bergheiinfected mice. In addition, kolaviron at the administered doses ameliorated the parasite-induced anaemia and body weight alterations, possibly through interfering with lipid peroxidation process as well as sparing endogenous primary antioxidant enzymes reserves.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Greenwood B, Mutabingwa T. Malaria. Nature 2002; 415: 670-672.
[2] Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012; 379(9814): 413-431.
[3] Happi CT, Gbotosho GO, Folarin OA, Bolaji OM, Sowunmi A, Kyle DE, et al. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. Am J Trop Med Hyg 2006; 75(1): 155-161.
[4] Mubjer RA, Adeel AA, Chance ML, Hassan AA. Molecular markers of anti-malarial drug resistance in Lahj Governorate, Yemen: baseline data and implications. Malar J 2011; 10: 245. doi: 10.1186/1475-2875-10-245.
[5] Saha P, Das S. Highlighting the anti-carcinogenic potential of an ayurvedic medicinal plant, Swertia Chirata. Asian Pac J Cancer Prev 2010; 11(6): 1445-1449.
[6] Lee WC, Peng CC, Chang CH, Huang SH, Chyau CC. Extraction of antioxidant components from Bidens pilosa flowers and their uptake by human intestinal Caco-2 cells. Molecules 2013; 18(2): 1582-1601.
[7] Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO, Ademowo OG. Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoelli nigeriensis. Parasitol Res 2011; 108(6): 1507-1512.
[8] Saha S, Ghosh S. Tinospora cordifolia: One plant, many roles. Anc Sci Life 2012; 31(4): 151-159.
[9] Melillo de Magalhāes P, Dupont I, Hendrickx A, Joly A, Raas T, et al. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem 2012; 134(2): 864-871.
[10] Hutchinson J, Dalziel JM. Cycadaceae: Guttiferae. In: Happer FN (ed.). Flora of West tropical Africa. 2nd edn. London: Her Majesty's Stationary Office; 1956, p. 295.
[11] Xu HX, Mughal S, Taiwo O, Lee SF. Isolation and characterization of an antibacterial biflavonoid from an African chewing stick Garcinia kola Heckel (Clusiaceae). J Ethnopharmacol 2013; 147(2): 497-502.
[12] Farombi EO, Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health 2011; 8(6): 2533-2555.
[13] Seanego CT, Ndip RN. Identification and antibacterial evaluation of bioactive compounds from Garcinia kola (Heckel) seeds. Molecules 2012; 17(6): 6569-6584.
[14] Antia BS, Pansanit A, Ekpa OD, Ekpe UJ, Mahidol C, Kittakoop P. Alpha-glucosidase inhibitory, aromatase inhibitory, and antiplasmodial activities of a biflavonoid GB1 from Garcinia kola stem bark. Planta Med 2010; 76(3): 276-277.
[15] Adaramoye OA, Farombi EO, Adeyemi EO, Emerole GO. Inhibition of human low density lipoprotein oxidation by flavonoids of Garcinia kola seeds. Pak J Med Sci 2005a; 21(3): 331-339.
[16] Igado OO, Olopade JO, Adesida A, Aina OO, Farombi EO. Morphological and biochemical investigation into the possible neuroprotective effects of kolaviron (Garcinia kola bioflavonoid) on the brains of rats exposed to vanadium. Drug Chem Toxicol 2012; 35(4): 371-380.
[17] Olaleye SB, Onasanwo SA, Ige AO, Wu KK, Cho CH. Antiinflammatory activities of a kolaviron-inhibition of nitric oxide, prostaglandin E2and tumor necrosis factor-alpha production in activated macrophage-like cell line. Afr J Med Med Sci 2010; 39(Suppl): 41-46.
[18] Lacmata ST, Kuete V, Dzoyem JP, Tankeo SB, Teke GN, Kuiate JR, Pages JM. Antibacterial activities of selected cameroonian plants and their synergistic effects with antibiotics against bacteria expressing MDR phenotypes. Evid Based Complement Alternat Med 2012; 2012: doi: 10.1155/2012/623723
[19] Adaramoye OA, Nwaneri VO, Anyanwu KC, Farombi EO, Emerole GO. Possible anti- atherogenic effect of kolaviron (A Garcinia kola seed extract) in hypercholesterolemic rats. Clin Exp Pharmacol Physiol 2005b; 32(1-2): 40-46.
[20] Adaramoye OA, Medeiros IA. Endothelium-independent vasodilation induced by kolaviron, a biflavonoid complex from Garcinia kola seeds, in rat superior mesenteric arteries. J Smooth Muscle Res 2009; 45(1): 39-53.
[21] Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Anti-diabetic and aldose reductase activities of biflavanones of Garcinia kola. J Pharm Pharmacol 1990; 42(4): 290-292.
[22] Elufioye TO, Agbedahunsi JM. Antimalarial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J Ethnopharmacol 2004; 93(2-3): 167-171.
[23] Adaramoye OA, Awogbindin I, Okusaga JO. Effect of kolaviron, a biflavonoid complex from Garcinia kola seeds, on ethanolinduced oxidative stress in liver of adult wistar rats. J Med Food2009; 12(3): 584-590.
[24] Ogunbayo OA, Adisa RA, Ademowo OG, Olorunsogo OO. Incidence of chloroquine induced oxidative stress in the blood of rabbit. Intl J Pharmacol 2006; 2(5): 121-125.
[25] WHO. The biology of malaria parasites. Report of a WHO scientific group. Technical Report Series. 2. Geneva: World Health Organization; 1980.
[26] Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193(1): 265-275.
[27] Mohun AF, Cook LJ. Simple method for measuring serum level of glutamate-oxaloacetate and glutamate-pyruvate transaminases in laboratories. J Clin Pathol 1957; 10(2): 394-399.
[28] Reitman S, Frankel S. A colorimetric method for the determination of serum level of glutamate-oxaloacetate and pyruvate transaminases. Am J Clin Pathol 1957; 28(1): 56-63.
[29] Rutkowski RB, Debaare L. An ultra-micro colorimetric method for determination of total and direct serum bilirubin. Clin Chem 1966; 12(7): 432-438.
[30] Talke H, Schubert GE. Enzymatische Harnstoff bestimmung in Blut and serum in Optischen Test nach Warburg. Klin Wochschr 1965; 43(1): 174.
[31] McCord JM, Fridovich I. Superoxide dismutase, an enzymatic function for erythrocuperin. J Biol Chem 1969; 244(22): 6049-6055.
[32] Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249(22): 7130-7139.
[33] Aebi H. Catalase. In: Bergmeyer HV(ed.). Methods of enzymatic analysis. New York: Verlag Chemie; 1974, p. 673-684.
[34] Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973; 179(4073): 588-590.
[35] Moron MS, Depierre JW, Mannervick B. Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lung and liver. Biochim Biophys Acta 1979; 582(1): 67-78.
[36] Walls R, Kumar KS, Hochstein P. Aging human erythrocytes. Differential sensitivity of young and old erythrocytes to hemolysis induced by peroxide in the presence of thyroxine. Arch Biochem Biophys 1976; 174(2): 463-468.
[37] Frevert U, Nacer A. Immunobiology of Plasmodium in liver and brain. Parasite Immunol 2013; doi: 10.1111/pim.12039.
[38] Dondorp MD, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in malaria parasite. N Engl J Med 2009; 361(5): 455-467.
[39] Huang F, Tang L, Yang H, Zhou S, Sun X, Liu H. Therapeutic efficacy of artesunate in the treatment of uncomplicated Plasmodium falciparum malaria and anti-malarial, drugresistance marker polymorphisms in populations near the China-Myanmar border. Malaria J 2012; 11: 278. doi:10.1186/1475-2875-11-278
[40] Falé PL, Ascensāo L, Serralheiro ML, Haris PI. Interaction between Plectranthus barbatus herbal tea components and acetylcholinesterase: binding and activity studies. Food Funct 2012; 3(11): 1176-1184.
[41] Selvanayagam ZE, Gnanavendhan SG, Balakrishna K, Rao RB, Sivaraman J, Subramanian K, et al. Ehretianone, a novel quinonoid xanthene from Ehretia buxifolia with antisnake venom activity. J Nat Prod 1996; 59(7): 664-667.
[42] Okunji C, Komarnytsky S, Fear G, Poulev A, Ribnicky DM, Awachie PI, et al. Preparative isolation and identification of tyrosinase inhibitors from the seeds of Garcinia kola by highspeed counter-current chromatography. J Chromatogr A 2007; 1151(1-2): 45-50.
[43] Penduka D, Okoh OO, Okoh AI. In-vitro antagonistic characteristics of crude aqueous and methanolic extracts of Garcinia kola (Heckel) seeds against some Vibrio bacteria. Molecules 2011; 16(4): 2754-2765.
[44] Mawson AR. The pathogenesis of malaria: a new perspective. Pathog Glob Health 2013; 107(3): 122-129.
[45] Taoufiq Z, Pino P, N'dilimabaka N, Arrouss I, Assi S, Soubrier F, et al. Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malar J 2011; 10: 52-57.
[46] Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol 2004; 34(2): 163-189.
[47] Tjahjani S, Bsa P, Syafruddin D, Agoes R, Hanggono T, Immaculata M. Oxidative stress in Plasmodium falciparum culture incubated with artemisinin. Pro ASEAN Con Trop Med Parasitol 2008; 3(1): 47-50.
[48] Rodrigues JR, Gamboa ND. Effect of dequalinium on the oxidative stress in Plasmodium berghei-infected erythrocytes. Parasitol Res 2009; 104(6): 1491-1496.
[49] Isaksson C, Sepil I, Baramidze V, Sheldon BC. Explaining variance of avian malaria infection in the wild: the importance of host density, habitat, individual life-history and oxidative stress. BMC Ecol 2013; 13. doi: 10.1186/1472-6785-13-15.
[50] Ibrahim MA, Zuwahu MM, Isah MB, Jatau ID, Aliyu AB, Umar IA. Effects of vitamin E administration on Plasmodium berghei induced pathological changes and oxidative stress in mice. Trop Biomed 2012; 29(1): 98-106.
[51] Arinola AG, Onubogu DI, Salimonu LS. Spleen weight, liver weight and levels of circulating immune complexes in vitamin deficient mice infected with Plasmodium berghei. Afr J Clin Exp Microbiol 2005; 6(2): 95-99.
[52] Baird KJ, Maguire JD, Price RN. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol 2012; 80: 203-270.
*Corresponding authors: Adaramoye Oluwatosin, Biochemistry Department,University of Ibadan, University way, Oyo-Ojoo, Road, Ibadan, Ibadan, Oyo/South-West 20005, Nigeria.
E-mail: aoadaramoye@yahoo.com.
 Asian Pacific Journal of Tropical Medicine2014年2期
Asian Pacific Journal of Tropical Medicine2014年2期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Bond strength analysis of the bone cement- stem interface of hip arthroplasties
- Hepatic effect of NAC on sevear acute pancteatise of rats
- Comparative analysis of different cyclosporine A doses on protection after myocardial ischemia/reperfusion injury in rat
- Comparison on serum biomarkers for anovulatory and ovulatory dysfunctional uterine bleeding in Lizu females
- Preparation of novel biodegradable pHEMA hydrogel for a tissue engineering scaffold by microwave-assisted polymerization
- Mathematical modeling for selecting center locations for medical and health supplies reserve in Hainan Province
