Exercise training and antioxidant supplementation independently improve cognitive function in adult male and female GFAP-APOE m ice
Kiran Chaudhari,Jessica M.Wong,Philip H.Vann,Nathalie Sum ien
Department of Pharmacology and Neuroscience and Institute for Aging and Alzheimer’s Disease Research, University of North Texas Health Science Center at Fort Worth,Fort Worth,TX 76107,USA
Exercise training and antioxidant supplementation independently improve cognitive function in adult male and female GFAP-APOE m ice
Kiran Chaudhari,Jessica M.Wong,Philip H.Vann,Nathalie Sum ien*
Department of Pharmacology and Neuroscience and Institute for Aging and Alzheimer’s Disease Research, University of North Texas Health Science Center at Fort Worth,Fort Worth,TX 76107,USA
Purpose:The purpose of this study was to determ ine if antioxidantsupplementation,moderate exercise,and the combination of both treatments could ameliorate cognitive performance in adult mice and whether the apolipoprotein E(APOE)genotype as well as sex could influence the functional outcomes of the treatments.
Methods:For a period of 16 weeks,separate groups of male and female m ice expressing either the human APOE3 or APOE4 isoforms were fed either a control diet(NIH-31)or the control diet supplemented w ith vitamins E and C(1.12 IU/g dietα-tocopheryl acetate and 1.65 mg/g ascorbic acid).The mice were further separated into a sedentary group or a group that followed a daily exercise regimen.After 8 weeks on the treatments,the m ice were adm inistered a battery of functional tests including tests to measure cognitive and affective function.
Results:There was no effectof genotype or treatmenton the learning performance in the Morris watermaze.In the discriminated avoidance task, APOE4 m ice performed better in learning the discrim ination component of the task.Overall,exercise improved performance of APOE4 and APOE3 m ice on various aspects of the active avoidance task.Antioxidantsupplementation improved performance only in the APOE4 m ice.On the test for anxiety,APOE4 mice spent more time in the open arms and supplementation w ith antioxidant reversed that effect.
Conclusion:Exercise was the mosteffective treatmentat improving cognitive function in both genotypes and sex,while antioxidants seemed to be effective only in the APOE4.In young adult m ice only non-spatial learning and memory were improved.The combination of the two treatments did not yield further improvement in cognition,and there was no antagonistic action of the antioxidant supplementation on the beneficial effects of exercise.
CopyrightⒸ2014,Shanghai University of Sport.Production and hosting by Elsevier B.V.A ll rights reserved.
A lzheimer’s disease;Antioxidant;Apolipoprotein E;Cognition;Exercise;Vitam in C;Vitamin E;Water maze
1.Introduction
Apolipoprotein E(APOE)is a soluble protein and an integral part of the lipid transport and distribution system.1In humans,there exist three alleles coding for the three major isoforms ofAPOE:E2,E3,and E4.In the central nervous system,APOE has an important role in neurogenesis and neuroprotection.2The most commonly found isoform is theAPOE3,present in 79%of the population,while theAPOE2andE4are lower w ith 14%and 7%presence,respectively. Although not a determinant of the disease,theAPOE4presence has been established as a major genetic risk factor for development of late-onset sporadic Alzheimer’s disease (AD).3—5APOE4has also been associated w ith exacerbated cognitive declines during non-pathological non-AD dementia.6—8
While there remains some controversy,it is believed that women expressingAPOE4have an increased risk ofdeveloping AD when compared to men.9,10Furthermore,APOE4presence has been associated w ith cognitive declines during normalaging in women only.11Many studies in rodents expressing humanAPOEgenes have supported an interaction of sex andAPOEgenotype.Using different rodent models, females expressing humanAPOE4had impaired spatial learning and memory,which seemed to be exacerbated when compared to males.2,12
In m iddle-aged individuals,APOE4has been associated w ith cognitive deterioration and memory loss,13,14however reports on differences w ithin younger population are confl icted.15—18While some studies have reported a lack of difference betweenAPOE4carriers and non-carriers,17,18others have described a worsening associated w ithAPOE4.16More interestingly others have related an improved cognitive performance associated w ith the presence ofAPOE4.19—22Studies in humanAPOE-expressing mouse models also remain inconclusive regarding the effect ofAPOE4on cognitive function in young m ice,howevermoststudies hinted at a better performance in youngAPOE4mice compared toAPOE3.12,23,24This type of antagonistic pleiotropy(agedependent shift from beneficial to deleterious outcomes) associated w ith theAPOEisoforms may also impact the outcome of interventions.
Human and animal studies have suggested that lifestyle factors may play an important role in preventing cognitive deterioration and dementia.25—34APOE4has been associated w ith an increase in oxidative stress levels,35,36and oxidative stress has been associated w ith brain dysfunction.37Therefore antioxidant intake should decreaseAPOE4-associated oxidative stress and improve cognitive function,an interaction that has been demonstrated in several studies.25,38—40As another factor,physical activity has been shown to reduce the risk of AD,41—43delay onset,30and improve AD symptoms in an activity intensity-(dose)and duration-dependent manner.44Recent studies have established the existence of a potential interaction betweenAPOEgenotype and exercise on cognition.Most studies have reported that the beneficial effects of exercise are more pronounced inAPOE4carriers when compared to non-carriers,45,46however one study reported the opposite.47Exercise training has also been shown to lower oxidative stress while improving cognition.34,48Based on these studies and the potential existence of a common mechanism of action,it can be hypothesized that combining antioxidant w ith exercise training w ill lead to a synergistic or additive beneficial effect,49—52a therapeutic approach employed by many health conscious individuals and recommended by healthcare professionals.However,the occurrence of such outcome has not been fully established in relation to age,sex/gender,and genetic make-up.For example,a recent study demonstrated an antagonistic action of antioxidant supplementation on beneficial effects of exercise.53
Even though,antioxidant intake and exercise training have been previously studied,there are no data available evaluating the effect of these factors in both sexes,in two genotypes in young adult m ice w ithin the same study.The goals of the currentstudy were 1)to characterize the cognitive and anxiety phenotypes of the adult glial fibrillary acidic protein(GFAP)-APOE3andAPOE4m ice(humanAPOEexpressed under a GFAP promoter);2)to determ ine whether antioxidant intake and exercise training led to beneficial improvements in these young m ice,same as previously reported in older ones;3)to determ ine whether the combination of antioxidant and exercise yield a synergistic or additive beneficialeffect;and lastly 4)to determ ine whether the beneficialoutcomes are genotypedependent.
2.M ethods
2.1.Animals
A ll animal protocols were approved by the Institutional Animal Care and Use Comm ittee at the University of North Texas Health Science Centerat FortWorth.Separate groups of male and female GFAP-APOE*3(B6.Cg-Tg(GFAP-APOE*3) 37Hol Apoetm1Unc/J)and GFAP-APOE*4(B6.Cg-Tg(GFAPAPOE*4)1HolApoetm1Unc/J)m ice were obtained from Jackson Laboratories(catalog numbers 004633 and 004631;totalnof 180)at the age of 2 months and subsequently maintained in the UNT Health Science Center vivarium.The m ice were housed in groups of 3 or 4 in standard polycarbonate cages (28×17×12.5 cm)w ith corncob bedding andad libitumaccess to food and water,and were maintained at ambient temperature(23±1°C),undera 12-h light/dark cycle starting at 06:00.The m ice were weighed weekly,and survival was monitored throughout the study.A group of young(2 months,n=12)male and female C57BL/6 m ice(w ild-type)was used as a control to compare theAPOE3andE4controls to determ ine whether the behavioral differences betweenAPOE3andE4were due to an altered phenotype of the transgenic m ice.
2.2.Treatment
The mice were fed,ad libitum,either a control diet(Lab-DietⓇR&M 5LG6 4F,cat#:5S84)or the control diet supplemented w ith vitam ins E and C (modified 5LG6 w ith1.65 mg/g diet of ascorbic acid and 1.12 IU/g diet ofαtocopheryl acetate,cat#:5SH0).Furthermore,the m ice were either sedentary or follow ing a moderate exercise regimen. Based on this,the mice were random ly assigned to one of four experimental groups:(1)sedentary fed the control diet(Sed-Con),(2)sedentary fed the vitam ins E and C supplemented diet(SedEC),(3)forced exercise fed the control diet(ExCon), (4)forced exercise fed the vitam ins E and C supplemented diet (ExEC).Each experimentalgroup was balanced for sex of the mice.The moderate exercise regimen was introduced progressively using treadm ills(AccuPacer Treadm ill;Omnitech Electronics Inc.,Columbus,OH,USA).Over a 12-day period, the training was gradually incremented in time and speed to reach a maximal exercise of 1 h(6,8,10,and 12 m/m in for 5 m in each,and then at 14 m/m in for 40 min).The training protocol used was a modification of previously published exercise protocols.54Forced exercise was implemented viatransient0.29 m A electric footshock to the feet.Each exercise mouse was paired w ith a control which received the same number of shock for each training day.
The m ice were on their respective treatments for 8 weeks prior to and throughout behavioral assessments for a total of 16 weeks.The mice received a series of behavioral tests and the results of the cognitive tests are presented in this manuscript.Morris water maze(MWM)and discrim inated avoidance were used to measure different aspects of cognitive function.The mice were about 5—6 months old when tested for cognitive function.
2.3.MWM
Spatial learning and memory were measured using an MWM test slightly modified from described previously.55On a given trial,the mouse was allowed to sw im in a tank fi lled w ith opacified water and maintained at 24±1°C.The m ice were able to escape the water by means of a hidden platform (1.5 cm below the surface of the water).A computerized tracking system recorded various measures such as path length and sw imm ing speed(Any-maze;Stoelting Co.,Wood Dale, IL,USA).
The testconsisted of fourphases:(1)pre-training phase:the tank was covered by a black curtain to hide surrounding visual cues.The m ice learned the components of sw imm ing and climbing onto a platform using a straight alley that had a platform atone end.The m ice were allowed to swim until they reached the platform or a maximum of 60 s had elapsed.The m ice received two sessions consisting of five trials w ith an intertrial interval of 5 m in;(2)acquisition phase:the black curtain was removed and the m ice were tested for their ability to locate a hidden platform using spatial cues around the room. Each daily session consisted of five trials,at 2-m in intervals, during which the mouse had to sw im to the platform from one of four different starting points in the tank.The m ice were allowed to sw im until they reached the platform or a maximum of 90 s had elapsed.Testing was conducted over nine sessions(Tuesday—Friday and Monday—Friday).On sessions 2,4,5,7,and 9,a probe trial was conducted as the fi fth trial during which the platform was submerged to a depth that prevented the m ice from climbing onto it.The platform was raised after 30 s,and the trial was ended when the mouse successfully located it;(3)retention phase:one 60-s probe trial session was conducted 1 week after the ninth session of the previous phase;(4)visible platform phase:the mice were given a total of eight sessions(2/day separated by 2 h),each consisting of five trials w ith a 10-m in inter-trial interval.The platform was identified by a triangular flag that was raised above the surface of the water.On each trial the mouse had to sw im to the platform from a different starting point and the platform was moved to a different location before each trial. Thus,the mouse had to learn to associate the location of the flag w ith location of the platform.
Path length(distance taken to reach the platform)over sessions was used as the primary measure of performance.The path-independent sw im speed was calculated by dividing distance by the latency to reach the platform.On probe trial, spatialbias for the platform location was evaluated in terms of the percentage of time spentw ithin a 40-cm diameter annulus surrounding the platform location.
2.4.Discriminated avoidance
A T-maze constructed of acrylic(black for the sides and clear for the top)was utilized for the discrim inated avoidance task.The maze was divided into three compartments:a start box(10×6.3×6 cm),a stem(17.5×6.3×6 cm),and two goalarms(14.5×6.3×6 cm),each separated by clearacrylic doors.The maze rested on a grid floor wired to deliver 0.69-mA scrambled shock to the feet.
The test consisted of three sessions separated by 1 h.On each training trial,the mouse was placed in the start box,and the startdoorwas removed to signal the beginning of the trial. On the fi rst trial of the fi rst session(information trial),the mouse received shock in the fi rstarm entered(preference arm) and was permitted to escape shock by running to the opposite arm,which was then designated the correct arm for the remainder of the session.On subsequent trials,shock was initiated 5 s after the opening of the startdoor if the mouse had not entered the correct goal arm or immediately upon entry into the incorrectarm.In either case,the shock continued until the correct goal arm was entered or a maximum of 60 s had elapsed.Upon the mouse’s entry into the correctarm,the door was closed(to prevent departure),and,after 10 s,the mouse was removed(by detaching the goalarm)and allowed to enter a holding cage for1 m in.Training in this fashion continued at 1-min intervals until the mouse had met the criterion of a correct avoidance(defined as running directly to the correct arm w ithin 5 s)on four of the last five training trials of which the last two must be w ithin 5 s.The second session of avoidance training was a reversal such that the m ice were required to run to the goalarm opposite that to which they had been trained on the previous session.Two measures were considered to show the ability of the m ice to learn the discrim ination and avoidance components of the task.Their ability to learn was considered inversely proportional to the number of trials required to reach the avoidance criterion aforementioned and the number of trials required to reach the discrim ination criterion(4 out5 correct turns regardless of the time taken).
2.5.Elevated plus maze
To measure anxiety,an elevated plus maze test was conducted using a plus maze elevated three feetabove the floor in a dim ly lit test room(60 W)consisting of two arms opened to the room and two arms enclosed such that the floor is not visible.A computerized tracking system was used to monitor the position of the mice in the maze(Any-maze).The mice were positioned in the center of the plus facing an open arm and were given 5 min to explore the maze.The amountof time spent in the closedvs.open arms was recorded.
2.6.Statistical analysis
Functional performance of the m ice on the behavioral tests was assessed using two-way analyses of variance(ANOVA) w ith Genotype and Treatment as between-group factors. Planned individual comparisons between different genotype groups(E3 vs.E4)and treatment groups(SedConvs.SedECvs.ExConvs.ExEC)were performed using a single degree-offreedom F tests involving the error term from the overall ANOVA.Performances were also considered in three-way w ith Session as the repeated measure.The effects of strain w ithin the SedCon groups were analyzed using a one-way ANOVA w ith Strain(w ild-typevs.E3 vs.E4)as a factor. Planned individual comparisons were performed using a single degree-of-freedom F tests involving the error term from the overall ANOVA.Pooling male and female data was not responsible for driving any of the main results.Theαlevel was set at 0.05 for all analyses.The software used for the analyses was Systat 13(Systat Software Inc.,San Jose,CA, USA).
3.Results
3.1.MWM
The performance of the m ice as measured by path length and sw imming speed is presented in Fig.1.Path length of all w ild-type,E3,andE4m ice decreased as a function of sessions (Fig.1A).The effect of testing session on path length was confi rmed by an analysis of variance w ith Session as repeated measure(p<0.05).There was no effect of Strain or Treatmenton the performance of the m ice as supported by a lack of significantmain effects or interaction of Strain and Treatment (allp>0.259).The w ild-type C57BL/6 SedCon group took shorterpath length than theE3orE4m ice,especially between sessions 3 and 7.This was supported by an ANOVA revealing a main effect of Strain(p<0.05).
Overall,theE4m ice swam faster than theE3ones,which was supported by a main effect of Strain(p<0.05).The SedEC and ExEC mice seemed to swim slower than the controls throughout the sessions,however this was not supported by the analysis of the path-independent sw imm ing speed yielding no main effect of Treatment(p=0.057)or interaction of Strain and Treatment(p=0.359).The w ildtype andE4mice swam faster than theE3m ice,which was supported by a main effect of Strain(p<0.01)follow ing a one-way ANOVA.
Accuracy for spatial memory was measured by conducting a probe trial as the last trial of sessions 2,4,5,7,and 9 (Fig.2).All the m ice tested developed a strong bias for the platform location(p<0.05),however there was no difference between the performance of theE3andE4strains(p=0.052) and no effect of Treatment(p=0.067).W ithin the SedCon groups,the w ild-type m ice seemed to develop a strong bias on session 5 which remained for the rest of the sessions;however the difference betweenE3andE4was only seen on session 5. This observation was supported by a significant interaction between Session and Strain(p<0.01),and no main effectof Strain was detected(p=0.346).
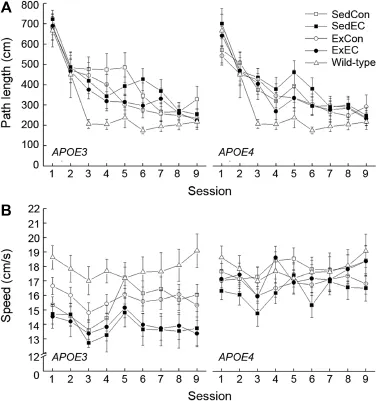
Fig.1.Effects of genotype,antioxidant intake,and moderated exercise on Morris water maze performance as measured by path length(A)and sw imming speed(B)in adult GFAP-APOE3,GFAP-APOE4,and C57BL/6(w ildtype)m ice.Each value represents the mean±SEM.ForAPOE3,sedentary w ith control diet(SedCon)n=20,sedentary w ith diet rich in vitamins E and C(SedEC)n=21,exercise w ith control diet(ExCon)n=21,exercise w ith diet rich in vitamins E and C(ExEC)n=21;forAPOE4,SedConn=20, SedECn=21,ExConn=20,ExECn=19;for w ild-type SedConn=12.
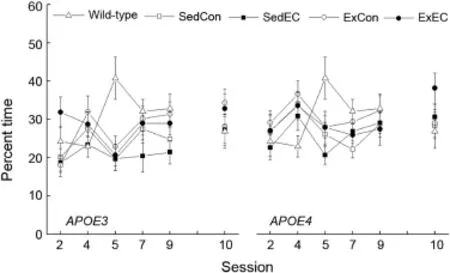
Fig.2.Effects of genotype,antioxidant intake,and moderated exercise on Morris watermaze performance as measured by percent time spent in a 40-cm area around the hidden platform location in adult GFAP-APOE3,GFAPAPOE4,and C57BL/6 (w ild-type)m ice.Each value represents the mean±SEM.ForAPOE3,sedentary w ith control diet(SedCon)n=20, sedentary w ith diet rich in vitamins E and C(SedEC)n=21,exercise w ith control diet(ExCon)n=21,exercise with diet rich in vitam ins E and C (ExEC)n=21;forAPOE4,SedConn=20,SedECn=21,ExConn=20, ExECn=19;for w ild-type SedConn=12.
Spatial retention was also tested 1 week after the last session in a single probe trial(session 10).A ll the m ice retainedthe previously learned information well.In comparing genotypes w ithin the SedCon groups,there was no effect of Strain (p=0.97)on the performance of the m ice.There was no difference between the performance of theE3andE4mice and no effect of Treatment(allp>0.221).
The mice were also tested on a visible platform test to determ ine whether their vision may have affected their performance in the MWM.A composite measure,learning index,was calculated by averaging the path length taken by the m ice to the flagged platform during sessions 2,3,and 4 (Fig.3).There was no discernable effect of Strain or Treatment on the performance of the m ice,which was supported by a lack of main effect or interaction between Strain and Treatment(allp>0.164).Sw imm ing speed on sessions 2,3, and 4 was also averaged and considered for analysis.The speed of the SedConE4mice was 25%faster than the Sed-ConE3ones,and there was no effectof the Treatmenton the speed of theE3orE4m ice.These observations were supported by a significant main effect of Strain(p<0.05)and a lack of main effect of Treatment or an interaction(allp>0.386).There were no differences in performance between the w ild-type,E3,andE4mice when analyzing the learning index(p=0.989).The speed of the w ild-type was comparable to the one of theE4m ice,which was significantly higher than the sw imm ing speed of theE3m ice.This was supported by a significant effect of Strain(p< 0.05) follow ing a one-way ANOVA.
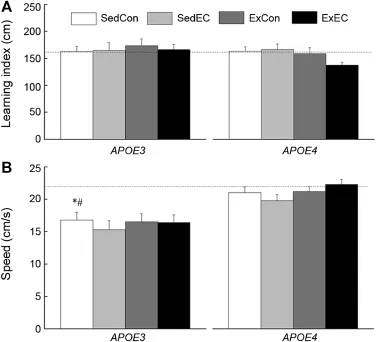
Fig.3.Effects of genotype,antioxidant intake,and moderated exercise on visible platform performance as measured by learning index(A)and sw imming speed(B)in adult GFAP-APOE3,GFAP-APOE4,and C57BL/6(w ildtype)m ice.The dotted line represents the performance of the SedCon w ildtype m ice.Each value represents the mean±SEM.ForAPOE3,sedentary w ith control diet(SedCon)n=20,sedentary w ith diet rich in vitam ins E and C(SedEC)n=19,exercise w ith control diet(ExCon)n=21,exercise with diet rich in vitam ins E and C(ExEC)n=21;forAPOE4,SedConn=20, SedECn=20,ExConn=19,ExECn=18;for w ild-type SedConn=12. *p<0.05,compared with w ild-type;#p<0.05,compared w ith SedConE4.
3.2.Discriminated avoidance test
Components of the discrim inated avoidance learning were considered for effects of Strain and Treatment during the acquisition and reversal sessions.Learning of the preemptive response is shown in Fig.4,whereas the discriminative component is shown in Fig.5.During acquisition,the SedConE4mice took 13%more trials than theirE3counterparts.The ExCon and ExECE3m ice took 27%and 22.5%less trials to reach the avoidance criterion compared to the SedConE3m ice.Number of trials taken to make a correct avoidance response was reduced by 18%—20%in the SedEC,ExCon, and ExECE4mice in comparison to their genotype-matched control(SedCon).Analysis of the trials to avoidance criterion for session 1 yielded a significant main effects of Strain and Treatment(allp<0.021)butno interaction of Strain and Treatment(p=0.63).In the reversal session,there was no difference between the SedConE3and SedConE4.Similar improved performances were observed w ith the m ice on antioxidantsupplementation and/or exercise regimen,however they were not as large in theE4groups(11%—16%)while they remained in the same range for theE3(26%).Analysis of the data from session 2 indicated only a significantmain effect of Treatment(p=0.002),and did not yield a significantStrain× Treatment interaction(p=0.38).There were no significant differences between the SedCon groups from each genotype in acquisition(p=0.390)and reversal(p=0.371).
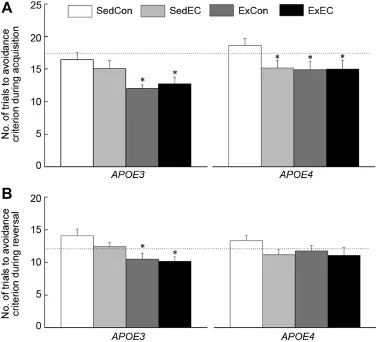
Fig.4.Effects of genotype,antioxidant intake,and moderated exercise on discrim inated avoidance performance as measured by the number of total trial taken to reach discrim inative criterion during acquisition(A)and reversal(B) sessions in adult GFAP-APOE3,GFAP-APOE4,and C57BL/6(w ild-type) m ice.The dotted line represents the performance of the SedCon w ild-type m ice.Each value represents the mean±SEM.ForAPOE3,sedentary w ith control(SedCon)n=20,sedentary w ith diet rich in vitam ins E and C(SedEC)n=20,exercise w ith control diet(ExCon)n=20,exercise w ith diet rich in vitam ins E and C(ExEC)n=20;forAPOE4,SedConn=20,SedECn=20, ExConn=15,ExECn=17;for w ild-type SedConn=12.*p<0.05, compared w ith genotype-matched SedCon.
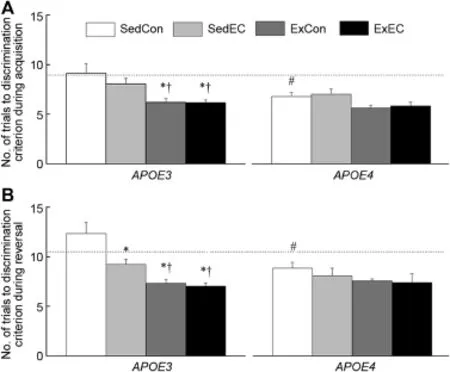
Fig.5.Effects of genotype,antioxidant intake,and moderated exercise on discrim inated avoidance performance as measured by the number of total trial taken to two consecutive correct turns during acquisition(A)and reversal(B) sessions in adult glial GFAP-APOE3,GFAP-APOE4,and C57BL/6(w ildtype)m ice.The dotted line represents the performance of the SedCon w ildtype m ice.Each value represents the mean±SEM.ForAPOE3,sedentary w ith control diet(SedCon)n=20,sedentary w ith diet rich in vitam ins E and C(SedEC)n=20,exercise w ith control diet(ExCon)n=20,exercise w ith diet rich in vitam ins E and C(ExEC)n=20;forAPOE4,SedConn=20, SedECn=20,ExConn=15,ExECn=17;for w ild-type SedConn=12. *p<0.05,compared w ith genotype-matched SedCon;#p<0.05,compared w ith SedConE3;†p<0.05,compared w ith genotype-matched SedEC.
Perusal of the discrim inative component(Fig.5)during acquisition and reversal revealed a strain-related difference in performance.Interestingly,the SedConE4m ice learned the discrim inative component of the active avoidance task taking less trials that the SedConE3ones.Furthermore,significant effects of Treatmentwere only observed in theE3m ice.In the acquisition session,the ExCon and ExEC mice took 32%less trials to reach the criterion compared to the SedConE3m ice while it was only about 15%less trials for theE4m ice. Analysis of the trials to the discrim inative component for session 1 yielded main effects of Strain and Treatment(allp<0.008)butdid not reveala significant interaction between Strain and Treatment(p=0.23).In the reversal session,the all treatedE3m ice took 25%—42%less trials than the SedCon m ice while theE4treated m ice improved only by 8%—16%. An analysis of the data during session 2 indicated significant main effects of Strain and Treatmentas well as an interaction between Strain and Treatment(allp< 0.036).For the discrim inative component of the active avoidance,a one-way ANOVA yielded only a main effect during the reversal phase(p=0.039),however this main effectwas solely driven by the significant difference betweenE3andE4.There were no significant differences between the genotypes in both phases.
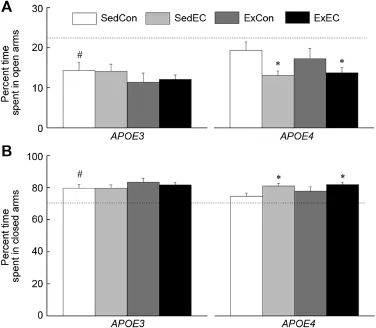
Fig.6.Effects of genotype,antioxidant intake,and moderated exercise on elevated plus maze performance as measured by the percent time spent in the open arms(A)and closed arms(B)sessions in adult GFAP-APOE3,GFAPAPOE4,and C57BL/6(w ild-type)m ice.The dotted line represents the performance of the SedCon wild-type m ice.Each value represents the mean±SEM.ForAPOE3,sedentary w ith control diet(SedCon)n=20, sedentary w ith diet rich in vitamins E and C(SedEC)n=20,exercise w ith control diet(ExCon)n=21,exercise with diet rich in vitamins E and C (ExEC)n=21;forAPOE4,SedConn=20,SedECn=20,ExConn=17, ExECn=18;for w ild-type SedConn=12.*p<0.05,compared w ith genotype-matched SedCon;#p<0.05,compared w ith w ild-type.
3.3.Elevated plus maze
The effect of Strain and Treatment were analyzed in terms of percent time spent in the closed arms and open arms of the plus maze(Fig.6).In theE3group,there was no effect of Treatment on either measure;however it seems that the supplementation w ith EC diet reduced the amountof time spent in the open arms by theE4m ice.Furthermore,overall theE4m ice spent more time in the open arms compared to theE3ones.Analyses of the data revealed a significantmain effectof Strain for percent time in open arms(p<0.05),however no effect of Treatment or an interaction between Strain and Treatment were found(allp>0.109).When comparing the SedCon treatments groups across w ild-type,E3,andE4genotype there was no difference in their time spent in open arms (p=0.071)and closed arms(p=0.052).
4.Discussion
The main findings of this study were(1)APOEgenotypes showed differentialbehavioralphenotype on the discriminative avoidance task,however there was no interaction of sex;(2)APOE4m ice exhibited higher sw imm ing speeds and lower anxiety levels;(3)APOE3andAPOE4m ice performance was different from w ild-type controls in the MWM,butnoton the non-spatialcognitive task;(4)supplementation w ith vitamins E and C decreased sw imm ing speed and anxiety;(5)exercisetraining improved cognitive function in both genotypes,while antioxidantsupplementation primarily improved function in theAPOE4m ice;and(6)no synergism/additive or antagonism effectwas detected between antioxidantand exercise treatments.
Our study aimed at cognitively phenotyping the GFAPAPOE3andAPOE4m ice using two different tests including spatial and non-spatial tasks.WhileAPOE4have been associated w ith accelerated cognitive declines6,7,56and neurodegenerative diseases,24,57,58reports regarding cognitive outcomes in youngAPOE4population have remained inconclusive.In humans,APOE4has been associated w ith better performance in young individuals which then shifts to a negative outcome in older individuals.20—22This antagonistic pleiotropy has notbeen well studied and has remained elusive. Studies in animal models have led to confl icting results w ith some studies show ing early signs of deleterious effects withAPOE4,16and others show ing improvements.12,19,23,24Some of the differences may be due to the mouse model chosen: targeted replacement modelvs.hAPP-Yac/APOE-TR model, as wellas the differentbehavioral tests conducted.In ourstudy we opted to use the GFAP-APOEmice,in which the expression of the humanAPOEisoforms is under glial promoter control.56Our findings suggested thatAPOE4performed better on the discrim inative component of the active avoidance but not on the avoidance component,which is more difficult to learn and achieve.Furthermore,even though there was no main effectof Sex on any of the measures,itis noteworthy that on the MWM,femaleAPOE4in the SedCon group seemed to perform better than theAPOE3SedCon ones.Our data suggested that indeedAPOE4may confer some type of beneficial effect at a younger age.Our m ice were about 5—6 months when tested for cognitive function,and it is possible that theAPOEeffectwould have been larger if tested ata youngerage.
Interestingly,in the current study,theAPOE4m ice exhibited a behavioral profi le that seemed to match the one of the w ild-type m ice on activity-and affective-related tasks.The speed measured in the water maze task and the anxiety levels of theAPOE4m ice were sim ilar to the w ild-type ones,while theAPOE3mice were less active in the water and seemed more anxious.Studies of older m ice showed thatE3andE4m ice were more anxious than the w ild-type.56Furthermore, while ourstudy yielded a better performance on the MWM for the w ild-type compared toAPOE3andE4m ice,other studies have indicated a lack of effect of genotype on this particular task.56While the methodology was different,it is noteworthy thatE3andE4m ice did notdiffer in their performance in both studies.Interestingly,both studies showed differences in working memory w ith Hartman et al.56show ing impairments associated w ithAPOE4while our study yielded a better performance associated w ithE4when compared toE3.These differences may be due to the age of the m ice used in the study (young adult in the present onevs.older m ice in Hartman’s study),which are in supportof the antagonistic pleiotropy that has been associated w ithAPOE4expression.
While our analyses did not reveal any interactions of Sex and Treatment on any of the measures presented,we conducted full analyses including Sex as a factor.The resulting analyses did not show any interaction of Sex w ith Strain, supporting that the performance of each strain on the behavioral tests was not influenced by the sex of the m ice.This was in contradiction w ith previous reports that clearly indicated a further impairment inAPOE4females when compared toAPOE4males.12It is noteworthy that these studies show ing impairments were done in a differentmodelexpressing humanAPOEisoforms12or in m ice that were relatively older.2Furthermore,epidemiological studies looking at the association betweenAPOE4and AD risk or cognitive declines have been done in relatively old populations and have also demonstrated that age is an influencing factor.For example,a study by Qiu et al.59has identified a strong association betweenAPOE4and AD risk that was stronger in men than in women.Despite a lack of sex interaction in these young adult m ice,it is noteworthy that the females and males responded to the same extent to the Treatmentand thatsex was nota driving factor of the observed treatment effects.
TheAPOE4m ice also exhibited another interesting and unexpected behavior in this study.Interestingly,theAPOE4mice had higher swimming speed in the hidden and visible platform tests.Even though this observation could be a sign of higher motivation,it did not translate to an improvement in spatial learning and memory.Furthermore,the lack ofan effectofStrain or Treatmenton the visible platform phase indicated that motivation was nota factor influencing the performance of the mice. While otherstudiesin humanshave reported hyperactivity being associated w ith the presence ofAPOE4,60the m ice in our study did notexhibit increased locomotion orexploration during open field testor elevated plus maze(data notshown).Other studies have actually reported decreased locomotoractivity inAPOE4-TRm ice61and even slowerswimming speed in the MWM.62TheAPOE4sw imm ing speed was also higher during the visible platform phase of the sw im maze.Vitam in E is transported via the same transporterasAPOEwhich isdefective inAPOE4m ice, therefore vitamin E levels should be lower inAPOE4mice compared toAPOE3ones.Antioxidant intake has been associated in some instances w ith increased sw imming speed and spontaneous activity,63and w ith hyperactivity.64Though the mechanisms by which antioxidants may affect hyperactivity remain unknown,there seemed to be a definite influence.
Though this increase in activity was only observed on the swim maze task,a test for anxiety was performed to determine whether it could be linked to an increase in anxiety-related behavior as noted in previous studies.65,66Overall,theAPOE4m ice spent more time in the open arms than theirAPOE3counterpart,results that are in contradiction w ith previous studies.65Furthermore,supplementation w ith vitamins influenced in a genotype-dependentmanner the behavior of the m ice on this task.In theAPOE4m ice,the supplementation w ith antioxidants increased anxiety.This was in definite contrastw ith several studies relating that vitamin E depletion increases anxiety.67Furthermore,other studies have demonstrated a decrease in anxiety in rats supplemented with vitamins E and C.68
In our active avoidance paradigm,the results differed depending on whether we analyzed the discrim inative component or the avoidance component of the task.In thediscrim inative component,which is the component of active avoidance that the m ice learned fi rst,there was a definite improvement in performance follow ing exercise and antioxidant treatment in theAPOE3mice.The lack of significant improvement in theAPOE4mice could be due to a maximum plateau of performance due to the set criterion.The criterion was setas the numberof trials to reach fouroutof five correct turns,therefore four trials would be the m inimum number of trials than a mouse could take.On average the SedConAPOE4m ice took between six and eight trials,thereby making it difficult to detecta significanteffectof Treatment.The effects of Treatment were mostly due to exercise Treatment as the performance of theAPOE3m ice remained largely unaffected by supplementation w ith antioxidants.
In the avoidance component of the task,exercise training improved performance of theAPOEm ice,irrespective of genotype in the acquisition phase.Interestingly,supplementation w ith antioxidants was only effective in theAPOE4m ice.This is most likely due to the transporter protein being dysfunctional69andAPOE4mice having lower vitam in E levels,70therefore more responsive to antioxidant supplementation.Physical activity has been shown to reduce AD risk,41—43to improve cognitive function and to have a positive impact on functional plasticity.44Interestingly,APOE4allele carriers with a sedentary life style have been shown to be more vulnerable to excessive amyloid deposition in brain.45,71Physical activity levels have been strongly positively associated w ith cognitive function in individuals carryingAPOE472,73supported by transgenic mouse model carrying humanAPOE4.33These studies focused on individuals or m ice in which cognitive dysfunction was present,while our study demonstrated that improvement can also be attained w ithoutapparent cognitive dysfunction and did notseem to be dependent upon theAPOEgenotype.
Studies on combination of antioxidant and exercise have led to confl icting results.In aged rodents,studies have reported an additive effectcombining vitamin E w ith sw imm ing exercise on markers of oxidative stress in various brain regions.50,52In young diabetic patients,antioxidant intake abolished the activation of molecular regulators ofendogenous antioxidant enzymes by a moderate exercise regimen.53APOE4has been associated w ith lower antioxidant activity,74decreased capacity to remove by-products of oxidative stress75and increased oxidative stress.76Therefore,a combination of antioxidants to lower oxidative stress and exercise to boost antioxidant defenses should lead to a further improvement than each intervention independently.Our study did not reveal such a beneficial additive interaction;in fact most effects observed w ith the combined Treatment m im icked the effects seen w ith exercise.The lack of an additive/synergistic effect on cognitive function may have been due to reaching a maximum ceiling of performance.While each intervention independently improved the performance of the m ice,it may have improved to a maximal levelof performance and further improvements by combining Treatments cannot be detected. Further studies w ill be needed to determ ine whether the combination had an additive/synergistic effectat the molecular levelwhich did not translate to further improvements due to a ceiling effect being reached.
5.Conclusion
Even though the effects were m inor and in select domains of cognition,our study supported previous reports ofAPOE4m ice perform ing better thanAPOE3m ice at a young age. While the beneficialeffectof exercise training on learning and cognitive flexibility was found in both genotype and in both males and females,the beneficial effect of antioxidant supplementation seemed to be genotype dependent.Lastly,in young adult m ice the combination of exercise and antioxidant did not lead to additive or antagonistic effects.
Acknow ledgment
This research was supported by grant NIRG-10-173988 and donation from the Pine Fam ily Foundation.
1.Arendt T.Alzheimer’s disease as a disorder of mechanisms underlying structural brain self-organization.Neuroscience2001;102:723—65.
2.van Meer P,Acevedo S,Raber J.Impairments in spatialmemory retention of GFAP-apoE4 female m ice.Behav Brain Res2007;176:372—5.
3.Genin E,Hannequin D,Wallon D,Sleegers K,Hiltunen M,Combarros O, et al.APOE and A lzheimer disease:a major gene w ith sem i-dom inant inheritance.Mol Psychiatry2011;16:903—7.
4.Kim J,Basak JM,Holtzman DM.The role of apolipoprotein E in A lzheimer’s disease.Neuron2009;63:287—303.
5.Blacker D,Haines JL,Rodes L,Terwedow H,Go RC,Harrell LE,et al. ApoE-4 and age at onset of A lzheimer’s disease:the NIMH genetics initiative.Neurology1997;48:139—47.
6.Corder EH,Saunders AM,Strittmatter WJ,Schmechel DE,Gaskell PC, Small GW,etal.Gene dose of apolipoprotein E type 4 allele and the risk of A lzheimer’s disease in late onset fam ilies.Science1993;261:921—3.
7.CraftS,TeriL,Edland SD,KukullWA,Schellenberg G,M cCorm ick WC, etal.Accelerated decline in apolipoprotein E-epsilon4 homozygotes w ith Alzheimer’s disease.Neurology1998;51:149—53.
8.Martins CA,Oulhaj A,de Jager CA,W illiams JH.APOEalleles predict the rate of cognitive decline in Alzheimer disease:a nonlinear model.Neurology2005;65:1888—93.
9.Farrer LA,Cupples LA,Haines JL,Hyman B,KukullWA,Mayeux R,etal. Effectsofage,sex,and ethnicity on the association between apolipoprotein E genotype and Alzheimerdisease.Ameta-analysis.APOE and Alzheimer Disease Meta Analysis Consortium.JAMA1997;278:1349—56.
10.Molero AE,Pino-Ramirez G,Maestre GE.Modulation by age and gender of risk for A lzheimer’s disease and vascular dementia associated w ith the apolipoprotein E-epsilon4 allele in Latin Americans:findings from the Maracaibo Aging Study.Neurosci Lett2001;307:5—8.
11.Mortensen EL,Hogh P.A genderdifferencein theassociation betweenAPOEgenotype and age-related cognitive decline.Neurology2001;57:89—95.
12.Raber J,Wong D,Buttini M,Orth M,Bellosta S,Pitas RE,etal.Isoformspecifi c effects of human apolipoprotein E on brain function revealed inApoEknockoutmice:increased susceptibility of females.Proc Natl Acad Sci USA1998;95:10914—9.
13.Reitz C,Mayeux R.Endophenotypes in normal brain morphology and A lzheimer’s disease:a review.Neuroscience2009;164:174—90.
14.Bour A,Grootendorst J,Vogel E,Kelche C,Dodart JC,Bales K,et al. M iddle-aged human apoE4 targeted-replacement m ice show retention defi cits on a w ide range of spatial memory tasks.Behav Brain Res2008;193:174—82.
15.Acevedo SF,Piper BJ,Craytor MJ,Benice TS,Raber J.Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children.Pediatr Res2010;67:293—9.
16.A lexander DM,W illiams LM,Gatt JM,Dobson-Stone C,Kuan SA, Todd EG,et al.The contribution of apolipoprotein E alleles on cognitive performance and dynam ic neural activity over six decades.Biol Psychol2007;75:229—38.
17.Dennis NA,Browndyke JN,Stokes J,Need A,Burke JR,Welsh-BohmerKA, et al.Temporal lobe functional activity and connectivity in young adultAPOEε4 carriers.Alzheimers Dement2010;6:303—11.
18.Reiman EM,Chen K,A lexander GE,Caselli RJ,Bandy D,Osborne D, et al.Functional brain abnormalities in young adults at genetic risk for late-onset A lzheimer’s dementia.ProcNatlAcadSciUSA2004;101:284—9.
19.Moreau PH,Bott JB,Zerbinatti C,Renger JJ,Kelche C,Cassel JC,etal. ApoE4 confers better spatial memory than apoE3 in young adult hAPPYac/apoE-TR m ice.Behav Brain Res2013;243:1—5.
20.Jochemsen HM,Muller M,van der Graaf Y,Geerlings M I.APOE epsilon4 differentially infl uences change in memory performance depending on age.The SMART-MR study.Neurobiol Aging2012;33:832. e15—22.
21.Puttonen S,Elovainio M,Kivimaki M,Lehtimaki T,Keltikangas-Jarvinen L.The combined effects of apolipoprotein E polymorphism and low-density lipoprotein cholesterol on cognitive performance in young adults.Neuropsychobiology2003;48:35—40.
22.Yu YW,Lin CH,Chen SP,Hong CJ,Tsai SJ.Intelligence and eventrelated potentials for young female human volunteer apolipoprotein E epsilon4 and non-epsilon4 carriers.Neurosci Lett2000;294:179—81.
23.Grootendorst J,Bour A,Vogel E,Kelche C,Sullivan PM,Dodart JC,etal. Human apoE targeted replacement mouse lines:h-apoE4 and h-apoE3 m ice differ on spatial memory performance and avoidance behavior.Behav Brain Res2005;159:1—14.
24.Belinson H,Lev D,Masliah E,M ichaelson DM.Activation of the amyloid cascade in apolipoprotein E4 transgenic m ice induces lysosomal activation and neurodegeneration resulting in marked cognitive defi cits.J Neurosci2008;28:4690—701.
25.Fotuhi M,Zandi PP,Hayden KM,Khachaturian AS,Szekely CA, Wengreen H,et al.Better cognitive performance in elderly taking antioxidant vitam ins E and C supplements in combination w ith nonsteroidal anti-infl ammatory drugs:the Cache County Study.Alzheimers Dement2008;4:223—7.
26.Harrison FE,Allard J,Bixler R,Usoh C,LiL,May JM,etal.Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 m ice.Nutr Neurosci2009;12:203—18.
27.Maxwell CJ,Hicks MS,Hogan DB,Basran J,Ebly EM.Supplementaluse of antioxidant vitam ins and subsequent risk of cognitive decline and dementia.Dement Geriatr Cogn Disord2005;20:45—51.
28.Head E,Nukala VN,Fenoglio KA,Muggenburg BA,Cotman CW, Sullivan PG.Effects of age,dietary,and behavioral enrichment on brain m itochondria in a canine model of human aging.ExpNeurol2009;220:171—6.
29.Adlard PA,Perreau VM,Pop V,Cotman CW.Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease.J Neurosci2005;25:4217—21.
30.Bugg JM,Head D.Exercise moderates age-related atrophy of the medial temporal lobe.Neurobiol Aging2011;32:506—14.
31.Erickson KI,Voss MW,Prakash RS,Basak C,Szabo A,Chaddock L,etal. Exercise training increases size of hippocampus and improves memory.Proc Natl Acad Sci USA2011;108:3017—22.
32.Garcia-Mesa Y,Lo´pez-Ramos JC,Gime´nez-Llort L,Revilla S,Guerra R, Gruart A,et al.Physical exercise protects against A lzheimer’s disease in 3xTg-AD m ice.J Alzheimers Dis2011;24:421—54.
33.Nichol K,Deeny SP,Seif J,Camaclang K,Cotman CW.Exercise improves cognition and hippocampal plasticity inAPOEepsilon4 m ice.Alzheimers Dement2009;5:287—94.
34.Marosi K,Bori Z,Hart N,Sa´rga L,Koltai E,Rada´k Z,et al.Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats.Neuroscience2012;226:21—8.
35.Tayler H,Fraser T,M iners JS,Kehoe PG,Love S.Oxidative balance in A lzheimer’s disease:relationship to APOE,Braak tangle stage,and the concentrations of soluble and insoluble amyloid-β.J Alzheimers Dis2010;22:1363—73.
36.Zito G,Polimanti R,Panetta V,Ventriglia M,Salustri C,Siotto MC,etal. Antioxidant status andAPOEgenotype as susceptibility factors for neurodegeneration in A lzheimer’s disease and vascular dementia.Rejuvenation Res2013;16:51—6.
37.Markesbery WR.Oxidative stress hypothesis in A lzheimer’s disease.Free Radic Biol Med1997;23:134—47.
38.Yasuno F,Tanimukai S,Sasaki M,Ikejima C,Yamashita F,Kodama C, et al.Combination of antioxidant supplements improved cognitive function in the elderly.J Alzheimers Dis2012;32:895—903.
39.Corrigan FM,Van Rhijn A,Horrobin DF.Essential fatty acids in A lzheimer’s disease.Ann N Y Acad Sci1991;640:250—2.
40.Sano M,Ernesto C,Thomas RG,Klauber MR,Schafer K,Grundman M, et al.A controlled trial of selegiline,alpha-tocopherol,or both as treatment for Alzheimer’s disease.The Alzheimer’s Disease Cooperative Study.N Engl J Med1997;336:1216—22.
41.Scarmeas N,Luchsinger JA,Schupf N,Brickman AM,Cosentino S, Tang MX,et al.Physical activity,diet,and risk of A lzheimer disease.JAMA2009;302:627—37.
42.Larson EB,Wang L,Bowen JD,M cCorm ick WC,Teri L,Crane P, et al.Exercise is associated w ith reduced risk for incident dementia among persons 65 years of age and older.AnnInternMed2006;144:73—81.
43.Buchman AS,Boyle PA,Yu L,Shah RC,Wilson RS,Bennett DA.Total daily physical activity and the risk of AD and cognitive decline in older adults.Neurology2012;78:1323—9.
44.Liu-Ambrose T,Nagamatsu LS,Graf P,Beattie BL,Ashe MC,Handy TC. Resistance training and executive functions:a 12-month random ized controlled trial.Arch Intern Med2010;170:170—8.
45.Head D,Bugg JM,Goate AM,Fagan AM,M intun MA,Benzinger T,etal. Exercise engagement as a moderator of the effects of apoe genotype on amyloid deposition.Arch Neurol2012;69:636—43.
46.Brown BM,Peiffer JJ,Martins RN.Multiple effects of physical activity on molecular and cognitive signs of brain aging:can exercise slow neurodegeneration and delay A lzheimer’s disease?Mol Psychiatry2013;18:864—74.
47.Obisesan TO,Umar N,Paluvoi N,Gillum RF.Association of leisure-time physical activity w ith cognition by apolipoprotein-E genotype in persons aged 60 years and over:the National Health and Nutrition Exam ination Survey(NHANES-III).Clin Interv Aging2012;7:35—43.
48.Radak Z,Kaneko T,Tahara S,Nakamoto H,Pucsok J,Sasva´ri M,et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain.Neurochem Int2001;38:17—23.
49.Cetin E,Top EC,Sahin G,Ozkaya YG,Aydin H,Toraman F.Effect of vitam in E supplementation with exercise on cognitive functions and total antioxidant capacity in older people.JNutrHealthAging2010;14:763—9.
50.Devi SA,Kiran TR.Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain.Neurobiol Aging2004;25:501—8.
51.Jolitha AB,Subramanyam MV,Asha Devi S.Modification by vitam in E and exercise of oxidative stress in regions of aging rat brain:studies on superoxide dismutase isoenzymes and protein oxidation status.Exp Gerontol2006;41:753—63.
52.Wu A,Ying Z,Gomez-Pinilla F.Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition.Neuroscience2008;155:751—9.
53.Ristow M,Zarse K,Oberbach A,K lo¨ting N,Birringer M,Kiehntopf M, etal.Antioxidantspreventhealth-promoting effectsof physicalexercise in humans.Proc Natl Acad Sci USA2009;106:8665—70.
54.Su SH,Chen H,Jen CJ.C57BL/6 and BALB/c bronchoalveolar macrophages respond differently to exercise.J Immunol2001;167:5084—91.
55.Sumien N,Sims MN,Taylor HJ,Forster MJ.Profi ling psychomotor and cognitive aging in four-way cross m ice.Age(Dordr)2006;28:265—82.
56.Hartman RE,Wozniak DF,NardiA,Olney JW,Sartorius L,Holtzman DM. Behavioral phenotyping of GFAP-ApoE3 and-ApoE4 transgenic m ice: ApoE4 m ice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology.Exp Neurol2001;170:326—44.
57.Huang Y.Roles of apolipoprotein E4(ApoE4)in the pathogenesis of A lzheimer’s disease:lessons from ApoE mouse models.Biochem Soc Trans2011;39:924—32.
58.Aboud O,M rak RE,Boop FA,Griffin WS.Epilepsy:neuroinflammation, neurodegeneration,andAPOEgenotype.Acta Neuropathol Commun2013;1:41.
59.Qiu C,Kivipelto M,Aguero-Torres H,W inblad B,Fratiglioni L.Risk and protective effects of the APOE gene towards Alzheimer’s disease in the Kungsholmen project:variation by age and sex.J Neurol Neurosurg Psychiatry2004;75:828—33.
60.Keltikangas-Jarvinen L,Raikkonen K,Lehtimaki T.Dependence between apolipoprotein E phenotypes and temperament in children,adolescents, and young adults.Psychosom Med1993;55:155—63.
61.Kornecook TJ,McKinney AP,Ferguson MT,Dodart JC.Isoform-specific effectsofapolipoprotein E on cognitive performance in targeted-replacement m ice overexpressing human APP.Genes Brain Behav2010;9:182—92.
62.Reverte I,K lein AB,Ratner C,Dom ingo JL,Colom ina MT.Behavioral phenotype and BDNF differences related to apoE isoforms and sex in young transgenic m ice.Exp Neurol2012;237:116—25.
63.Sumien N,Heinrich KR,Shetty RA,Sohal RS,Forster MJ.Prolonged intake of coenzyme Q10 impairs cognitive functions in m ice.J Nutr2009;139:1926—32.
64.Joshi K,Lad S,Kale M,Patwardhan B,Mahadik SP,Patni B,et al. Supplementation with flax oil and vitamin C improves the outcome of attention deficit hyperactivity disorder(ADHD).Prostaglandins Leukot Essent Fatty Acids2006;74:17—21.
65.Siegel JA,Haley GE,Raber J.Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR m ice.Neurobiol Aging2012;33:345—58.
66.Robertson J,Curley J,Kaye J,Quinn J,Pfankuch T,Raber J.apoE isoforms and measures of anxiety in probable AD patients andApoe-/-m ice.Neurobiol Aging2005;26:637—43.
67.Desrumaux C,Risold PY,Schroeder H,Deckert V,Masson D,Athias A, et al.Phospholipid transfer protein(PLTP)deficiency reduces brain vitam in E content and increases anxiety in m ice.FASEBJ2005;19:296—7.
68.Hughes RN,Low ther CL,van Nobelen M.Prolonged treatment with vitam ins C and E separately and together decreases anxiety-related openfield behavior and acoustic startle in hooded rats.Pharmacol Biochem Behav2011;97:494—9.
69.Huebbe P,Jofre-Monseny L,Rimbach G.A lpha-tocopherol transport in the lung is affected by the apoE genotype—studies in transgenic apoE3 and apoE4 m ice.IUBMB Life2009;61:453—6.
70.Huebbe P,Lodge JK,Rimbach G.Implications of apolipoprotein E genotype on infl ammation and vitam in E status.Mol Nutr Food Res2010;54:623—30.
71.Brown BM,Peiffer JJ,Taddei K,Lui JK,Laws SM,Gupta VB,et al. Physical activity and amyloid-beta plasma and brain levels:results from the Australian imaging,biomarkers and lifestyle study of ageing.Mol Psychiatry2013;18:875—81.
72.Schuit AJ,Feskens EJ,Launer LJ,Kromhout D.Physical activity and cognitive decline,the role of the apolipoprotein e4 allele.Med Sci Sports Exerc2001;33:772—7.
73.Etnier JL,Caselli RJ,Reiman EM,Alexander GE,Sibley BA, Tessier D,et al.Cognitive performance in older women relative to ApoE-ε4 genotype and aerobic fi tness.MedSci Sports Exerc2007;39:199—207.
74.Tamaoka A,M iyatake F,Matsuno S,Ishii K,Nagase S,Sahara N,et al. Apolipoprotein E allele-dependent antioxidant activity in brains w ith Alzheimer’s disease.Neurology2000;54:2319—21.
75.Pedersen WA,Chan SL,Mattson MP.A mechanism for the neuroprotective effectof apolipoprotein E:isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal.JNeurochem2000;74:1426—33.
76.Ramassamy C,Averill D,Beffert U,Theroux L,Lussier-Cacan S, Cohn JS,et al.Oxidative insults are associated w ith apolipoprotein E genotype in A lzheimer’s disease brain.NeurobiolDis2000;7:23—37.
Received 11 February 2014;revised 4 April 2014;accepted 14 April 2014
*Corresponding author.
E-mail address:nathalie.sumien@unthsc.edu(N.Sumien)
Peer review under responsibility of Shanghai University of Sport
2095-2546/$-see front matter CopyrightⒸ2014,Shanghai University of Sport.Production and hosting by Elsevier B.V.A ll rights reserved. http://dx.doi.org/10.1016/j.jshs.2014.04.004
 Journal of Sport and Health Science2014年3期
Journal of Sport and Health Science2014年3期
- Journal of Sport and Health Science的其它文章
- Women’s health in exercise and aging:What do we know?
- Why women see differently from the way men see?A review of sex differences in cognition and sports
- Sex differences in exercise and drug addiction:A m ini review of animal studies
- Women and exercise in aging
- Effects of carbohydrate supplements on exercise-induced menstrual dysfunction and ovarian subcellular structural changes in rats
- Surgical menopause enhances hippocampal amyloidogenesis follow ing global cerebral ischem ia
