Sex differences in exercise and drug addiction:A m ini review of animal studies
Yuehui Zhou,Chenglin Zhou,*,Ren Li,b
aDepartment of Sport Psychology,School of Sport Science,Shanghai University of Sport,Shanghai 200438,China
bCenter for Hormone Advanced Science and Education,Roskamp Institute,Sarasota,FL 34243,USA
Sex differences in exercise and drug addiction:A m ini review of animal studies
Yuehui Zhoua,Chenglin Zhoua,*,Rena Lia,b
aDepartment of Sport Psychology,School of Sport Science,Shanghai University of Sport,Shanghai 200438,China
bCenter for Hormone Advanced Science and Education,Roskamp Institute,Sarasota,FL 34243,USA
Grow ing literature has demonstrated that exercise may be an effective prevention and treatment option for drug addiction.In the past few years,many studies have suggested that there were sex differences in all phases of drug addiction.However,very limited research has investigated sex differences in the effectiveness of exercise intervention in drug addiction and rehabilitation.In this mini review,we summarize the effectof sex on the results of using exercise to preventand treatdrug addiction.The studies we considerspan various animalmodels and use multiple types of exercise to examine the effectiveness of exercise on the neurobiologicalmechanism of exercise rehabilitation.We believe that exercise as an adjuvant intervention strategy can be applied better in drug addiction prevention and recovery.
Animal studies;Drug addiction;Exercise;Sex difference
1.Introduction
Drug addiction,also known as substance dependence,is a chronic disorder characterized by the compulsion to seek and take a drug,loss of control in lim iting intake,and emergence of a negative emotional state when access to a drug is prohibited.The neurobiology of drug addiction involves specific neuronal pathway dysfunctions and pathological neuropsychological dysfunctions.1Recent research has found that there are significant sex differences in many aspects of drug addiction,including its neurobiology mechanism.2-5In general,males are more likely to engage in risky behavior that includes experimenting with drugs of abuse compared to females,while females are more likely to begin taking drugs as self-medication to reduce stress or alleviate depression.6In addition,sex differences in patterns of drug-cue exposure, severity,and outcomes of drug addiction have also been reported.7,8Clinical studies also demonstrated that female subjects w ith substance dependence showed higher scores of approaching tendencies and more motor impulsivity than male individuals w ith drug dependence,9and female addicts are more unw illing to take part in detoxification treatment.10Moreover,studies of brain activation and subjective craving behavior showed that female cocaine users had a positive correlation between craving behavior and brain activation in the m idbrain,hippocampus,ventrolateral prefrontal cortex, and thalamus,whereas male cocaine users showed the correlations between craving and activation in the dorsolateral, dorsomedial,temporal,and parietal cortices as well as in the hippocampus.7In addition,studies also showed that female drug users are more likely to develop depression and anxiety than male subjects with drug addiction.11,12The sex differences in drug addiction are also confi rmed in animal studies. For example,female rats have higher levels of morphine and heroin intake than male rats,while female rats are morevulnerable and sensitive than males to the reinstatement of cocaine-seeking behavior.6,13,14Both human and animal studies demonstrated that circulating levels of ovarian steroid hormones account for these sex differences,and that progesterone and allopregnanalone counteract the effects of estrogen and reduce drug seeking behavior in females.15
Recently,an increasing evidence indicates that exercise leads to positive results in drug addiction prevention and recovery.16But few studies can elaborate on this phenomenon in more detail.We hypothesize that exercise may affect neuroplasticity and regulate the positive reinforcement of the drug through influencing the neurotransm itters system,cellsignaling molecules and its gene expression,epigenetics, neuroplasticity,and neurogenesis.In this review,we discuss the sex differences of addiction models,exercise intervention in drug addiction recovery and its underlying neurobiological mechanism.We believe that a better understanding of sex differences in exercise intervention in drug addiction prevention and recovery w ill provide a stronger theoretical basis for novel sex-specific rehabilitations.
2.Sex differences in animalmodel of drug addiction
2.1.Self-administration(SA)paradigm
The traditional animal models of drug abuse are framed by the behaviorist view that emphasizes the action of drugs as positive reinforcer,much like food,water,and other“natural”reinforcers.Studies showed that female rats go into stable SA behaviors more rapidly at a lower dose and are more sensitive to the positive reinforcement of drugs compare to male rats.17The female animals are also likely maintaining higher drug intake throughout the SA extinction than males.18In general, female animals learn to self-administervarious drugs(cocaine, methylphenidates,and amphetam ine)faster,and are more sensitive to the rewarding effects than males.19Further research indicated thatovariectom ized female rats showed the same craving behavior as males when reinstated by drug, slower acquisition,lower drug intake,and longer extinction in SA compared to intact female rats.17,20,21Together,these studies suggested thatovary hormones,such as estrogens,play critical roles in the sex differences in drug addiction behaviors, such as acquisition,maintenance,craving,extinction,and reinstatementof SA in animals.
2.2.Conditioned place preference(CPP)paradigm
In addition to SA,CPP experiments provide additional information on the rewarding effects of drug abuse.As reported in SA,female rats required shorter training cycle and lower doses of the drug to acquire CPP compared to male rats.22,23This sex difference in CPP between female and male rats was observed in both adolescent and adulthood.24However,some studies showed controversial results in the gender effect on CPP.For example,studies reported no gender difference in CPP acquisition at a low or high dose of cocaine(3 or 25 mg/kg),except that female rats were more reinstated than male rats.25At doses of morphine from 0.2 to 10.0 mg/kg, male and female rats showed the same level of preference for the drug-associated chamber,butwhen the dose was increased from 10.0 to 17.5 mg/kg,morphine lost positive reinforcer in males while female rats maintained a strong preference for the morphine-associated chamber at doses up to 30 mg/kg.26The controversial results in gender effects on CPP behavior are also associated w ith specific drugs and strain of animals. Studies reported that there was no sex difference in amphetamine induced CPP.27,28Furthermore,studies of nicotine addiction showed a dose dependentCPP only in male rats,not in female rats.29On the other hand,there is a significant gender difference in morphine induced CPP in W istar rats,30but not in SD rats.26In accordance w ith SA,the rewarding effect of drugs in CPP is also closely associated w ith ovary hormones.For example,ovariectom ized female rats showed a reduction of cocaine induced CPP behavior compared to intact females.31
There were few studies about the effect of exercise only on CPP,butenough data suggest that rats find long term voluntary wheel running rewarding,32,33which can develop and sustain significant CPP to brief periods or nightly,34,35and also produce plasticity in the mesolimbic reward pathway like repeated exposure to drug or natural rewards.33Therefore, there may be sex differences in exercise’s effecton drug based upon these animal models of drug addiction.
3.Sex differences in various types of exercises’effects on drug rehabilitation
In the animal experiments on drug addiction through exercise intervention,voluntary running wheel and forced treadmill running are the main modes of exercise.Running wheel is an active exercise and is w idely used,while forced treadm ill running is passive and less used.
A lthough exercising has been investigated as an intervention fordrug addiction and rehabilitation,few studieshave been done on the sex differences in the effectiveness of exercise on drug rehabilitation in animals.Sex differences in both wheel and treadmill running behaviors have been documented.For instance,female rats w ith drug addiction often run more laps (longer distance)in wheelexercise than males w ithin the same time frame.36-39In a 10-day forced treadm ill running training, male rats developed small reduction of serum corticosteroidbinding globulin,which was not found in female rats,40suggesting a differentphysiological response induced by treadm ill exercise in female and male rats.There were little studies about the psychological response between the two exercise models. Wheel running tended to attract individuals who are highly motivated to engage in frequent,sustained exercise,which reflected a voluntary,active physical and mental state,while treadm ill running attracts those that are forced to exercise, which reflects an attitude towardsexercise.41Only very recently have there been studies demonstrating the sex differences in exercise intervention for drug addiction and rehabilitation.In one such study,Sanchez and colleagues42found that 10-day wheel running after the formation of rat SA attenuatednicotine-seeking in male ratsonly under the conditionsofaccess to unlocked wheels.However,access to either locked or unlocked wheels was sufficient to suppress nicotine-seeking during extinction in females.Also 14-day wheel running during abstinence on subsequent cocaine-seeking of rat’s SA can effectively reduce relapse vulnerability in a dose dependent manner,and this effectdiffers by sex and estrous cycle.43
Since females perform wheel and treadm ill running differently than males,it is important to include the consideration of sex-specific effectiveness of exercise on drug rehabilitation in animal experiments.
In addition to wheel and treadm ill running,forced sw imm ing has also been used for studies of exercise rehabilitation. Forced sw imm ing exercises could be evaluated for exercise performance,psychological status (anxiety and stress), cognition(learning and memory),etc.44,45It also showed the same effectiveness as displayed in voluntary wheel running in alcohol conditioned place aversion(CPA),46,47suggesting that forced sw imm ing may be one mode for exercise intervention for drug addiction.Again,compared to male rats,female rats were more active in water48and swam faster in speed with less sign of fatigue.49,50
4.Sex differences in effectiveness of exercise in drugaddiction
Exercises attenuate drug-seeking behaviors during drug initiation,escalation,extinction,abstinence,and relapse.51,52It is crucial to decrease the susceptibility(preventive effect)atan early stage of drug addiction and reduce the drug craving (therapeutic effect)at later stages in order to prevent relapse.
4.1.Sex differences in preventive effects of exercise on drug addiction
As most of animal studies in biology research show,researches about exercise as a means of prevention on drug abuse are limited by investigating male animals only,53-68w ith a few studies on female animals,69-74while the others did notspecify the gender.75,76In regard to the sex differences as shown in Table 1,the results of these studies have been inconsistent.One study found that chronic forced treadm ill exercise for 6 weeks induced reduction of drug craving behavior in adolescent female and male rats,but only male rats showed inhibited CPP.77Another study reported that female rats,after 13-day unlim ited voluntary wheel exercise, showed decreased alcohol consummation(ethanol/total liquid) than non-exercised females,while no difference was found in males.78However,other studies of voluntary wheel running for 6 weeks after cocaine exposure demonstrated that exercise weakens cocaine SA under extended-access conditions in both sexes.Although the escalating intake was more in females than males,it was not statistically significant.38,39Together, evidence suggests a sex difference in the preventive effects of exercise on drug addiction.
4.2.Sex differences in therapeutic effects of exercise on drug addiction
Sim ilarly,in animal studies of therapeutic effects of exercise on drug abuse,most are investigated in male animals,47,79-96w ith very few studies in females.97-99In very lim ited literature as shown in Table 1,one study pointed outthat although voluntary wheel running was able to decrease drug intake in both male and female rats,wheel-running activity had a greater suppressant effect on cocaine SA in females than in males,and in females,wheel-running and cocaine SA are substitutable as reinforcers.100This was supported by the discoveries that female rats were more sensitive to the rewarding effects of the drug.101Sim ilarly,some research revealed that wheel running during abstinence differentially weakens subsequent nicotine-seeking in males and females that had extended access to nicotine SA of adolescent rats.42Peterson and his colleagues43also found wheel running dose-dependently decreased cocaine-seeking in both gendered rats,but males showed a greater attenuation of cocaine-seeking w ith longer access to wheel running than that in females,which might be related to interference of the estrous cycle phase in females.
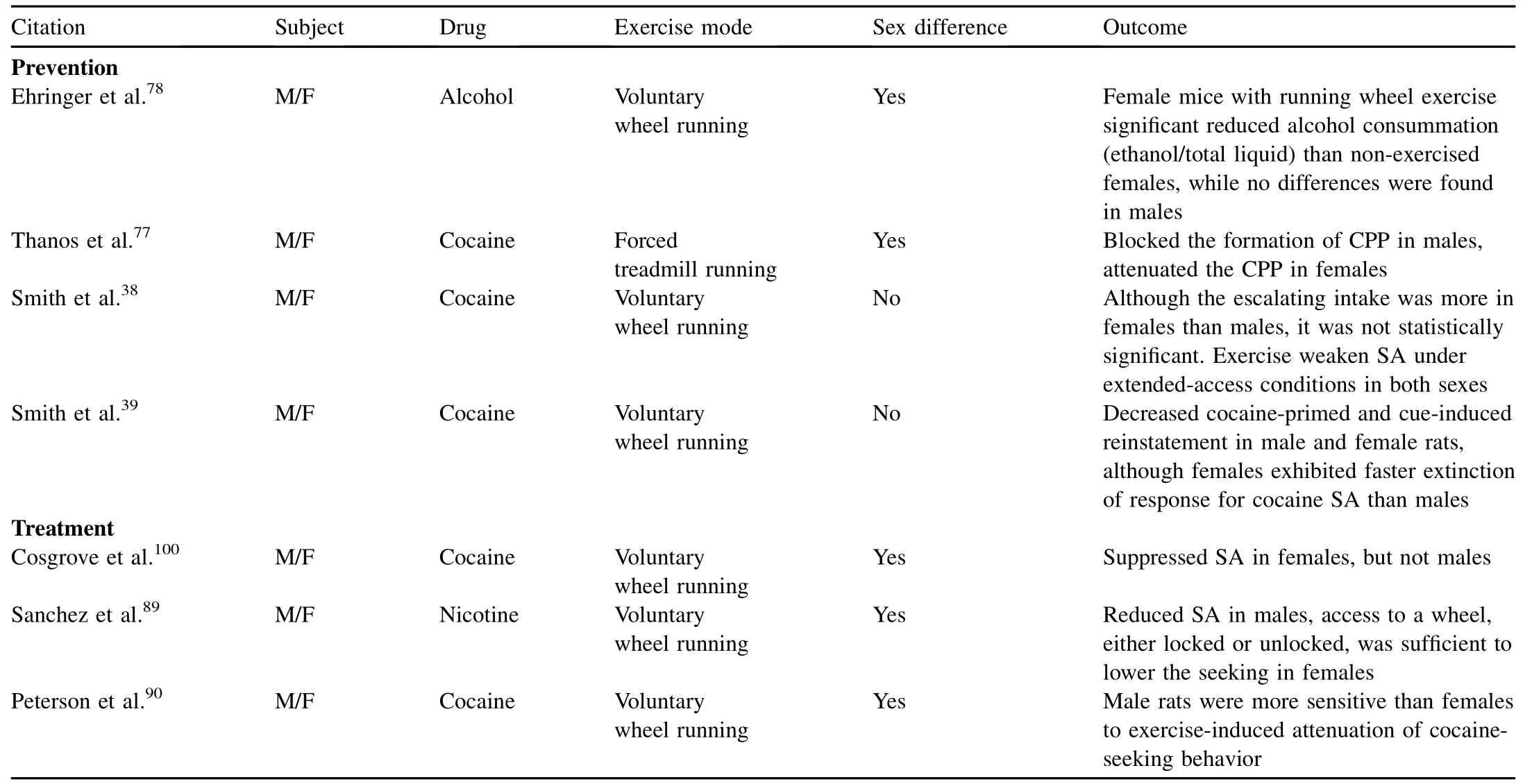
Table 1 Sex differences in effects of exercise on drug addiction.
5.Sex differences in neurobiological mechanism of exercise intervention in drug addiction
Long-term drug abuse led to corresponding compensatory changes in the mesolimbic dopam ine reward system,including a series of molecular events induced by multiple kinds of neurotransmitters and their receptors in ventral tegmentalarea, nucleus accumbens and prefrontal cortex.102Exercise may affect neruoplasticity and regulate the positive reinforcement of drugs through influencing certain signaling molecules and neuroanatomy structure, depending on gonadal hormones.95,103
Firstly,the neurotransm itters system seem to play a crucial role in the process of the formation and development of drug addiction.104There was sexual dimorphism of dopam ine, noradrenalin,5-HT,and endocannabinoid in the hypothalamus of adult or new born rodents,which responded to steroid hormone differently.105,106Addicted males and females have different responses to dopam ine transport and the activity or inhibitory of the D1 and D2 dopam ine receptor.107,108On the otherhand,compared to male m ice w ith hypoactivity,females w ith higher exercise performance often show a reduced function of the D1 and D5 dopamine receptors.109In turn,an inhibition of dopam ine transporter w ill decrease the wheel running level of m ice that are high and active.110Lightfoot111pointed out that drug abuse,neurotransmitters,and exercise may be regulated by sex hormones and its receptors.Secondly, cell-signaling molecules and their gene expression to drug abuse and exercise were also different between males and females.Some studies reported that the brain regional basal levelof protein kinase A(PKA)and phosphorylated DARPP-32 in nucleus accumbens were higher in females than that of males before or after drug addiction,but not in the caudate nucleus.112,113Furthermore,cocaine-induced PKA would facilitate phosphorylation of cAMP response element binding protein(CREB),102which is also regulated by gonadal hormone.114Others reported that there was a sex-specific neuroimmunoendocrine response associated with signaling pathways and the transcription factor CREB to exercise in m ice.115Thirdly,the changes in epigenetics were considered to be the underlying mechanism by drug.116Sex differences in epigenetic processes such as acetylation and methylation(at least four related parameters:DNA methyltransferase 3,DNA methylation patterns,MeCP2,and nuclear co-repressors)may confer sexually dimorphic risks and a resilience to developing neurological and mental health disorders later in life.117Fourthly,drug addiction is a pathology of staged neuroplasticity,118which is also highly different between males and females.For example,the spine density of medium spiny neurons in nucleus accumbens is higher in female cocaine addiction rats during abstinence,as well as the spiculate protuberance compared to males.The magnitude of the cocaineinduced increase in spine density also appeared greater in females than that in males.Moreover,the changes of dendritic spine plasticity were associated w ith addicted behaviors in females only,and females showed greater locomotor activity and higher behavioral sensitization to cocaine than males.119Lastly,the sex differences in hippocampal neurogenesis would account for the susceptibility of drug addiction,and repeated drug abuse further inhibited the neurogenesis in certain brain regions,which caused a reinforcement of drug rewarding effect.120Studies demonstrated that male rats w ith drug experiences at adolescence showed greater reduction of hippocampus dentate gyrus neurogenesis compared to female rats.121Furthermore,aerobic exercise improved the spatial memory in normal or addicted individuals,which was dependent on hippocampus neurogenesis.This positive correlation w ith newborn cells in the hippocampus was more prom inent in female rats than in males.122In conclusion,the sex differences in neurobiological mechanisms of exercise intervention in drug addiction may be related to the sexspecific actions in neurotransmitters systems,cell-signaling molecules and their gene expression,epigenetics,neuroplasticity,and neurogenesis.
6.Conclusion and future directions
As briefl y reviewed above,it is clear that there are sex differences in exercise intervention in drug addiction prevention and recovery.The sex differences are found in various animal models,the types of exercise,and the effectiveness of exercise on drug addiction and rehabilitation.A lthough exercise intervention in treating drug addiction has been w idely recognized and used in human rehabilitation,the sex differences in exercise intervention’s effect on drug addiction and rehabilitation are understudied.One of the main reasons is that much of the animal studies were performed on one gender, particularly male.As a recent article published inNatureby Pollitzer123indicated,sex differences exist not only in basic cell biology,but also in clinical research including drug effectiveness and side effects.While the majority of animal studies used male subjects exclusively,the outcome from those animal studies may influence the future translational approaches in human studies since the gender differences were notspecified.In this review,we fi rstdiscussed sex differences in various drug addictions in two major animal models:SA and CPP paradigms.Then,we discussed the different effectsof active and passive exercises on drug rehabilitation on male and female animals.Lastly,we specifically summarized the preventive and therapeutic effects of exercise on drug addiction in male and female animals.Indeed,to furtherunderstand the sex differences in drug addiction and exercise intervention, more studies on the neurobiological mechanisms of exercise and its roles in preventing and treating drug addiction are needed.
Acknow ledgment
This work was supported by grants from the Shanghai Science and Technology Comm ission(NO.13490503600)and National Natural Science Foundation of China (NO.31171004).
1.Levy N.Addiction is not a brain disease(and it matters).Front Psychiatry2013;4:1—7.
2.Lynch WJ,Roth ME,Carroll ME.Biological basis of sex differences in drug abuse:preclinical and clinical studies.Psychopharmacology(Berl)2002;164:121—37.
3.Lynch WJ.Sex differences in vulnerability to drug self-adm inistration.Exp Clin Psychopharmacol2006;14:34—41.
4.Hu M,Crombag HS,Robinson TE,Becker JB.Biological basis of sex differences in the propensity to self-adm inister cocaine.Neuropsychopharmacology2004;29:81—5.
5.Bobzean SA,Denobrega AK,Perrotti LI.Sex differences in the neurobiology of drug addiction.Exp Neurol2014;259:64—74.
6.Becker JB,Perry AN,Westenbroek C.Sex differences in the neural mechanisms mediating addiction:a new synthesis and hypothesis.Biol Sex Differ2012;3:1—35.
7.Moeller FG.Sex,stress,and drug cues in addiction.Am J Psychiatry2012;169:351—3.
8.Kennedy AP,Epstein DH,Phillips KA,Preston KL.Sex differences in cocaine/heroin users:drug-use triggers and craving in daily life.Drug Alcohol Depend2013;132:29—37.
9.Perry RI,Krmpotich T,Thompson LL,M ikulich-Gilbertson SK, Banich MT,Tanabe J.Sex modulates approach systems and impulsivity in substance dependence.Drug Alcohol Depend2013;133:222—7.
10.Kissin WB,Tang Z,Campbell KM,Claus RE,Orwin RG.Gender-sensitive substance abuse treatmentand arrestoutcomes forwomen.J Subst Abuse Treat2014;46:332—9.
11.Fox HC,Sinha R.Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women.Harv Rev Psychiatry2009;17:103—19.
12.Doran N.Sex differences in smoking cue reactivity:craving,negative affect,and preference for immediate smoking.AmJAddict2013;23:211—7.
13.Becker JB,Hu M.Sex differences in drug abuse.Front Neuroendocrinol2008;29:36—47.
14.Roth ME,Cosgrove KP,Carroll ME.Sex differences in the vulnerability to drug abuse:a review of preclinical studies.Neurosci Biobehav Rev2004;28:533—46.
15.Carroll ME,Anker JJ.Sex differences and ovarian hormones in animal models of drug dependence.Horm Behav2010;58:44—56.
16.Zschucke E,Gaudlitz K,Stro¨hle A.Exercise and physical activity in mental disorders:clinical and experimental evidence.J Prev Med Public Health2013;46(Suppl.1):S12—21.
17.Kerstetter KA,Ballis MA,Duffin-Lutgen S,Carr AE,Behrens AM, Kippin TE.Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen.Neuropsychopharmacology2012;37:2605—14.
18.Fattore L,Fadda P,Fratta W.Sex differences in the self-adm inistration of cannabinoids and other drugs of abuse.Psychoneuroendocrinology2009;34(Suppl.1):S227—36.
19.Van Swearingen AE,Walker QD,Kuhn CM.Sex differences in noveltyand psychostimulant-induced behaviors of C57BL/6 m ice.Psychopharmacology(Berl)2013;225:707—18.
20.Fattore L,A ltea S,Fratta W.Sex differences in drug addiction:a review of animal and human studies.Womens Health(LondEngl)2008;4:51—65.
21.Fattore L,Spano MS,A ltea S,Angius F,Fadda P,Fratta W.Cannabinoid self-adm inistration in rats:sex differences and the influence of ovarian function.Br J Pharmacol2007;152:795—804.
22.Russo SJ,Jenab S,Fabian SJ,Festa ED,Kemen LM,Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine.Brain Res2003;970:214—20.
23.Randall CK,Kraemer PJ,Bardo MT.Morphine-induced conditioned place preference in preweanling and adult rats.Pharmacol Biochem Behav1998;60:217—22.
24.Zakharova E,Wade D,Izenwasser S.Sensitivity to cocaine conditioned reward depends on sex and age.Pharmacol BiochemBehav2009;92:131—4.
25.Bobzean SA,Dennis TS,Addison BD,Perrotti LI.Influence of sex on reinstatement of cocaine-conditioned place preference.Brain Res Bull2010;83:331—6.
26.Cicero TJ,Ennis T,Ogden J,Meyer ER.Gender differences in the reinforcing properties of morphine.Pharmacol BiochemBehav2000;65:91—6.
27.Mathews IZ,M cCormick CM.Female and male rats in late adolescence differ from adults in amphetam ine-induced locomotoractivity,butnot in conditioned place preference for amphetamine.Behav Pharmacol2007;18:641—50.
28.Schindler CW,Bross JG,Thorndike EB.Gender differences in the behavioraleffectsofmethamphetam ine.Eur JPharmacol2002;44:231—5.
29.Yararbas G,Keser A,Kanit L,Pogun S.Nicotine-induced conditioned place preference in rats:sex differences and the role of mGluR5 receptors.Neuropharmacology2010;58:374—82.
30.Karam i M,Zarrindast MR.Morphine sex-dependently induced place conditioning in adult W istar rats.Eur J Pharmacol2008;582:78—87.
31.Russo SJ,Festa ED,Fabian SJ,Gazi FM,Kraish M,Jenab S,et al. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats.Neuroscience2003;120:523—33.
32.Brene´S,Bjørnebekk A,Aberg E,Mathe´AA,Olson L,Werme M.Running is rewarding and antidepressive.Physiol Behav2007;92:136—40.
33.Greenwood BN,Foley TE,Le TV,Strong PV,Loughridge AB,Day HE, et al.Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway.Behav BrainRes2011;217:354—62.
34.Belke TW,Wagner JP.The reinforcing property and the rewarding aftereffect of wheel running in rats:a combination of two paradigms.Behav Process2005;68:165—72.
35.Lett BT,Grant VL,Gaborko LL.Wheel running simultaneously produces conditioned taste aversion and conditioned place preference in rats.Learn Motiv2001;32:129—36.
36.Eikelboom R,M ills R.A microanalysis of wheel running in male and female rats.Physiol Behav1988;43:625—30.
37.Kanarek RB,D’Anci KE,Jurdak N,Mathes WF.Running and addiction: precipitated w ithdrawal in a ratmodel of activity-based anorexia.Behav Neurosci2009;123:905—12.
38.Sm ith MA,Walker KL,Cole KT,Lang KC.The effects of aerobic exercise on cocaine self-adm inistration in male and female rats.Psychopharmacology(Berl)2011;218:357—69.
39.Smith MA,Pennock MM,Walker KL,Lang KC.Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats.Drug Alcohol Depend2012;121:54—61.
40.Brown DA,Johnson MS,Armstrong CJ,Lynch JM,Caruso NM, Ehlers LB,et al.Short-term treadmill running in the rat:what kind of stressor is it?J Appl Physiol2007;103:1979—85.
41.Leasure JL,Jones M.Forced and voluntary exercise differentially affect brain and behavior.Neuroscience2008;156:456—65.
42.Sanchez V,Moore CF,Brunzell DH,Lynch WJ.Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-adm inistration model.Psychopharmacology(Berl)2014;23:1753—62.
43.Peterson AB,Hivick DP,Lynch WJ.Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking:impact of sex and estrous cycle in rats.Psychopharmacology(Berl)2014;231:2661—70.
44.Petit-Demouliere B,Chenu F,Bourin M.Forced swimm ing test in m ice: a review of antidepressant activity.Psychopharmacology2005;177:245—55.
45.Chen WC,Huang WC,Chiu CC,Chang YK,Huang CC.Whey protein improves exercise performance and biochem icalprofi les in trained m ice.Med Sci Sports Exerc2014;46:1517—24.
46.Masaki T,Nakajima S.Further evidence for conditioned taste aversion induced by forced sw imm ing.Physiol Behav2005;84:9—15.
47.Nakajima S.Conditioned ethanol aversion in rats induced by voluntary wheel running,forced sw imm ing,and electric shock:an implication for aversion therapy of alcoholism.IntegrPhysiolBehavSci2004;39:95—104.
48.Brotto LA,Barr AM,Gorzalka BB.Sex differences in forced-sw im and open-field testbehaviours after chronic adm inistration of melatonin.Eur J Pharmacol2000;402:87—93.
49.Birren JE,Kay H.Sw imming speed of the albino rat:I.Age and sex differences.J Gerontol1958;13:374—7.
50.Kay H,Birren JE.Sw imm ing speed of the albino rat:II.Fatigue,practice and drug effects on age and sex differences.J Gerontol1958;13:378—85.
51.Lynch WJ,Peterson AB,Sanchez V,Abel J,Sm ith MA.Exercise as a novel treatment for drug addiction:a neurobiological and stagedependent hypothesis.Neurosci Biobehav Rev2013;37:1622—44.
52.Sm ith MA,Lynch WJ.Exercise as a potential treatment for drug abuse: evidence from preclinical studies.Front Psychiatry2011;2:1—10.
53.Lett BT,Grant VL,Koh MT,Flynn G.Prior experience w ith wheel running produces cross-tolerance to the rewarding effect of morphine.Pharmacol Biochem Behav2002;72:101—5.
54.Eisenstein SA,Holmes PV.Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats.Pharmacol Biochem Behav2007;86:607—15.
55.Xu Z,Hou B,Gao Y,He F,Zhang C.Effects of enriched environmenton morphine-induced reward in m ice.Exp Neurol2007;204:714—9.
56.El Rawas R,Thiriet N,Lardeux V,Jaber M,Solinas M.Environmental enrichment decreases the rewarding but not the activating effects of heroin.Psychopharmacology(Berl)2009;203:561—70.
57.Solinas M,Thiriet N,El Rawas R,Lardeux V,Jaber M.Environmental enrichment during early stages of life reduces the behavioral,neurochem ical,and molecular effects of cocaine.Neuropsychopharmacology2009;34:1102—11.
58.M iladi-Gorji H,Rashidy-Pour A,Fathollahi Y,Akhavan MM, Semnanian S,Safari M.Voluntary exercise ameliorates cognitive deficits in morphine dependent rats:the role of hippocampal brain-derived neurotrophic factor.Neurobiol Learn Mem2011;96:479—91.
59.Smith MA,Pitts EG.Access to a running wheel inhibits the acquisition of cocaine self-adm inistration.PharmacolBiochemBehav2011;100:237—43.
60.Mustroph ML,Stobaugh DJ,M iller DS,DeYoung EK,Rhodes JS.Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J m ice,depending on tim ing of wheel access.Eur J Neurosci2011;34:1161—9.
61.O’Dell SJ,Galvez BA,Ball AJ,Marshall JF.Running wheel exercise ameliorates methamphetam ine-induced damage to dopam ine and serotonin term inals.Synapse2012;66:71—80.
62.M iller ML,Vaillancourt BD,W right Jr MJ,Aarde SM,Vandewater SA, Creehan KM,et al.Reciprocal inhibitory effects of intravenousdmethamphetamine self-administration and wheel activity in rats.Drug Alcohol Depend2012;121:90—6.
63.Smith MA,Pitts EG.Wheel running decreases the positive reinforcing effects of heroin.Pharmacol Rep2012;64:960—4.
64.Engelmann AJ,Aparicio MB,Kim A,Sobieraj JC,Yuan CJ,GrantY,etal. Chronic wheelrunning reducesmaladaptive patternsofmethamphetam ine intake:regulation by attenuation of methamphetam ine-induced neuronal nitric oxide synthase.Brain Struct Funct2013;219:657—72.
65.Chen HI,Kuo YM,Liao CH,Jen CJ,Huang AM,Cherng CG,et al. Long-term compulsive exercise reduces the rewarding effi cacy of 3,4-methylenedioxymethamphetam ine.Behav Brain Res2008;187:185—9.
66.Hosseini M,A laei HA,Naderi A,Sharifi MR,Zahed R.Treadm ill exercise reduces self-adm inistration of morphine in male rats.Pathophysiology2009;16:3—7.
67.Fontes-Ribeiro CA,Marques E,Pereira FC,Silva AP,Macedo TR.May exercise preventaddiction?Curr Neuropharmacol2011;9:45—8.
68.Geuzaine A,Tirelli E.Wheel-running m itigates psychomotor sensitization initiation but not post-sensitization conditioned activity and conditioned place preference induced by cocaine inm ice.Behav Brain Res2014;262:57—67.
69.Sm ith MA,Gergans SR,Iordanou JC,Lyle MA.Chronic exercise increases sensitivity to the conditioned rewarding effects of cocaine.Pharmacol Rep2008;60:561—5.
70.Sm ith MA,Schm idt KT,Iordanou JC,Mustroph ML.Aerobic exercise decreases the positive-reinforcing effects of cocaine.Drug Alcohol Depend2008;98:129—35.
71.Leasure JL,Nixon K.Exercise neuroprotection in a rat model of binge alcohol consumption.Alcohol Clin Exp Res2010;34:404—14.
72.Sm ith MA,Witt MA.The effects of exercise on cocaine selfadministration,food-maintained responding,and locomotor activity in female rats:importance of the temporal relationship between physical activity and initial drug exposure.ExpClinPsychopharmacol2012;20:437—46.
73.Darlington TM,M cCarthy RD,Cox RJ,Ehringer MA.Mesolimbic transcriptional response to hedonic substitution of voluntary exercise and voluntary ethanol consumption.Behav Brain Res2014;259:313—20.
74.Zlebnik NE,Hedges VL,CarrollME,Meisel RL.Chronic wheel running affects cocaine-inducedc-Fosexpression in brain reward areas in rats.Behav Brain Res2014;261:71—8.
75.Rozeske RR,Greenwood BN,Fleshner M,Watkins LR,Maier SF. Voluntary wheel running produces resistance to inescapable stressinduced potentiation of morphine conditioned place preference.Behav Brain Res2011;219:378—81.
76.Toborek M,Seelbach M J,Rashid CS,Andra´s IE,Chen L,Park M,etal. Voluntary exercise protects againstmethamphetam ine-induced oxidative stress in brain m icrovasculature and disruption of the blood-brain barrier.Mol Neurodegener2013;8:1—11.
77.Thanos PK,TucciA,Stamos J,Robison L,Wang GJ,Anderson BJ,etal. Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lew is rats.Behav Brain Res2010;215:77—82.
78.Ehringer MA,Hoft NR,Zunhammer M.Reduced alcohol consumption in m ice w ith access to a running wheel.Alcohol2009;43:443—52.
79.Werme M,Lindholm S,Thore´n P,Franck J,Brene´S.Running increases ethanol preference.Behav Brain Res2002;133:301—8.
80.Solinas M,Chauvet C,Thiriet N,El Rawas R,Jaber M.Reversal of cocaine addiction by environmentalenrichment.Proc Natl Acad Sci U S A2008;105:17145—50.
81.Lynch WJ,Piehl KB,Acosta G,Peterson AB,Hemby SE.Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex.Biol Psychiatry2010;68:774—7.
82.Thiel KJ,Pentkowski NS,Peartree NA,Painter MR,Neisewander JL. Environmental living conditions introduced during forced abstinence alter cocaine-seeking behavior and Fos protein expression.Neuroscience2010;171:1187—96.
83.Thiel KJ,Engelhardt B,Hood LE,Peartree NA,Neisewander JL.The interactive effects of environmental enrichment and extinction interventions in attenuating cue-elicited cocaine-seeking behavior in rats.Pharmacol Biochem Behav2011;97:595—602.
84.Hammer SB,Ruby CL,Brager AJ,Prosser RA,Glass JD.Environmental modulation of alcohol intake in hamsters:effects of wheel running and constant light exposure.Alcohol Clin Exp Res2010;34:1651—8.
85.Chauvet C,Lardeux V,Jaber M,Solinas M.Brain regions associated w ith the reversal of cocaine conditioned place preference by environmental enrichment.Neuroscience2011;184:88—96.
86.Chauvet C,Goldberg SR,Jaber M,Solinas M.Effects of environmental enrichment on the incubation of cocaine craving.Neuropharmacology2012;63:635—41.
87.M iladi-Gorji H,Rashidy-Pour A,Fathollahi Y.Anxiety profi le in morphine-dependent and w ithdrawn rats:effect of voluntary exercise.Physiol Behav2012;105:195—202.
88.Brager AJ,Hammer SB.Impact of wheel running on chronic ethanol intake in aged Syrian hamsters.Physiol Behav2012;107:418—23.
89.Sanchez V,Moore CF,Brunzell DH,Lynch WJ.Effectofwheel-running during abstinence on subsequent nicotine-seeking in rats.Psychopharmacology(Berl)2013;227:403—11.
90.Peterson AB,Abel JM,Lynch WJ.Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex Bdnf exon IV expression in rats.Psychopharmacology(Berl)2014;231:1305—14.
91.Alaei H,Borjeian L,Azizi M,Orian S,Pourshanazari A,Hanninen O. Treadm ill running reverses retention deficit induced by morphine.Eur J Pharmacol2006;536:138—41.
92.Gorton LM,Vuckovic MG,Vertelkina N,Petzinger GM,Jakowec MW, Wood RI.Exercise effects on motor and affective behavior and catecholam ine neurochem istry in the MPTP-lesioned mouse.Behav Brain Res2010;213:253—62.
93.Thanos PK,Stamos J,Robison LS,Heyman G,Tucci A,Wang GJ,etal. Daily treadm ill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement.Behav Brain Res2013;239:8—14.
94.Hashemi Nosrat Abadi T,Vaghef L,Babri S,Mahmood-A lilo M, Beiram i M.Effects of different exercise protocols on ethanol-induced spatialmemory impairment in adultmale rats.Alcohol2013;47:309—16.
95.M iladi-Gorji H,Rashidy-Pour A,Fathollahi Y,Semnanian S,Jadidi M. Effects of voluntary exercise on hippocampal long-term potentiation in morphine-dependent rats.Neuroscience2014;256:83—90.
96.Segat HJ,Kronbauer M,Roversi K,Schuster AJ,Vey LT,Roversi K, et al.Exercise modifies amphetam ine relapse:behavioral and oxidative markers in rats.Behav Brain Res2014;262:94—100.
97.Larson EB,Carroll ME.Wheel running as a predictor of cocaine selfadm inistration and reinstatement in female rats.Pharmacol Biochem Behav2005;82:590—600.
98.Zlebnik NE,Anker JJ,Carroll ME.Exercise to reduce the escalation of cocaine self-adm inistration in adolescent and adult rats.Psychopharmacology(Berl)2012;224:387—400.
99.Zlebnik NE,Anker JJ,Gliddon LA,Carroll ME.Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats.Psychopharmacology(Berl)2010;209:113—25.
100.Cosgrove KP,Hunter RG,Carroll ME.Wheel-running attenuates intravenous cocaine self-adm inistration in rats:sex differences.Pharmacol Biochem Behav2002;73:663—71.
101.Anker JJ,Zlebnik NE,Navin SF,Carroll ME.Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences.Psychopharmacology(Berl)2011;215:785—99.
102.Nestler EJ.Molecular mechanisms of drug addiction.Neuropharmacology2004;47(Suppl.1):24—32.
103.Berchtold NC,Kesslak JP,Pike CJ,Adlard PA,Cotman CW.Estrogen and exercise interact to regulate brain-derived neurotrophic factormRNA and protein expression in the hippocampus.EurJNeurosci2001;14:1992—2002.
104.Tomkins DM,Sellers EM.Addiction and the brain:the role of neurotransm itters in the cause and treatment of drug dependence.CMAJ2001;164:817—21.
105.Connell S,Karikari C,Hohmann CF.Hohmann,sex-specific development of cortical monoam ine levels in mouse.Brain Res Dev Brain Res2004;151:187—91.
106.Siddiqui A,Shah BH.Neonatal androgen manipulation differentially affects the development of monoam ine systems in rat cerebral cortex, amygdala and hypothalamus.Brain Res Dev Brain Res1997;98:247—52.
107.Festa ED,Russo SJ,Gazi FM,Niyomchai T,Kemen LM,Lin SN,et al. Sex differences in cocaine-induced behavioral responses,pharmacokinetics,and monoam ine levels.Neuropharmacology2004;46:672—87.
108.Walker QD,Ray R,Kuhn CM.Sex differences in neurochem ical effects of dopam inergic drugs in rat striatum.Neuropsychopharmacology2006;31:1193—202.
109.Rhodes JS,Garland T.Differential sensitivity to acute adm inistration of Ritalin,apormorphine,SCH 23390,butnot raclopride in m ice selectively bred for hyperactive wheel-running behavior.Psychopharmacology2003;167:242—50.
110.Rhodes JS,Hosack GR,Girard I,Kelley AE,M itchell GS,Garland Jr T. Differential sensitivity to acute administration of cocaine,GBR 12909, and fluoxetine in m ice selectively bred for hyperactive wheel-running behavior.Psychopharmacology2001;158:120—31.
111.Lightfoot JT.Sex hormones’regulation of rodent physical activity:a review.Int J Biol Sci2008;4:126—32.
112.Nazarian A,Sun WL,Zhou L,Kemen LM,Jenab S,Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens.Psychopharmacology(Berl)2009;203:641—50.
113.Lynch WJ,Kiraly DD,Caldarone BJ,Picciotto MR,Taylor JR.Effectof cocaine self-administration on striatal PKA-regulated signaling in male and female rats.Psychopharmacology(Berl)2007;191:263—71.
114.Lee SJ,Campomanes CR,Sikat PT,Greenfield AT,A llen PB, M cEwen BS.Estrogen induces phosphorylation of cyclic AMP response element binding(pCREB)in primary hippocampal cells in a timedependent manner.Neuroscience2004;124:549—60.
115.Gime´nez-Llort L,Garcı´a Y,Buccieri K,Revilla S,Sun˜ol C,Cristofol R, et al.Gender-specific neuroimmunoendocrine response to treadm ill exercise in 3×Tg-AD mice.Int J Alzheimers Dis2010.128354,http://dx. doi.org/10.4061/2010/128354.
116.Renthal W,Nestler EJ.Epigenetic mechanisms in drug addiction.Trends Mol Med2008;14:341—50.
117.Jessen HM,Auger AP.Sex differences in epigenetic mechanisms may underlie risk and resilience for mental health disorders.Epigenetics2011;6:857—61.
118.Kalivas PW,O’Brien C.Drug addiction as a pathology of staged neuroplasticity.Neuropsychopharmacology2008;33:166—80.
119.W issman AM,M cCollum AF,Huang GZ,Nikrodhanond AA, Woolley CS.Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens.Neuropharmacology2011;61:217—27.
120.Noonan MA,Bulin SE,Fuller DC,Eisch AJ.Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction.J Neurosci2010;30:304—15.
121.Lee TT,Wainw right SR,Hill MN,Galea LA,Gorzalka BB.Sex, drugs,and adult neurogenesis:sex-dependent effects of escalating adolescent cannabinoid exposure on adult hippocampal neurogenesis, stress reactivity,and amphetam ine sensitization.Hippocampus2014;24:280—92.
122.Chow C,Epp JR,Lieblich SE,Barha CK,Galea LA.Sex differences in neurogenesis and activation of new neurons in response to spatial learning and memory.Psychoneuroendocrinology2013;38:1236—50.
123.Pollitzer E.Biology:cell sex matters.Nature2013;500:23—4.
Received 25 February 2014;revised 23 March 2014;accepted 23 April 2014
*Corresponding author.
E-mail address:Chenglin_600@126.com(C.Zhou)
Peer review under responsibility of Shanghai University of Sport
2095-2546/$-see front matter CopyrightⒸ2014,Shanghai University of Sport.Production and hosting by Elsevier B.V.A ll rights reserved. http://dx.doi.org/10.1016/j.jshs.2014.04.005
CopyrightⒸ2014,Shanghai University of Sport.Production and hosting by Elsevier B.V.All rights reserved.
 Journal of Sport and Health Science2014年3期
Journal of Sport and Health Science2014年3期
- Journal of Sport and Health Science的其它文章
- Women’s health in exercise and aging:What do we know?
- Why women see differently from the way men see?A review of sex differences in cognition and sports
- Women and exercise in aging
- Effects of carbohydrate supplements on exercise-induced menstrual dysfunction and ovarian subcellular structural changes in rats
- Exercise training and antioxidant supplementation independently improve cognitive function in adult male and female GFAP-APOE m ice
- Surgical menopause enhances hippocampal amyloidogenesis follow ing global cerebral ischem ia
