Effects of carbohydrate supplements on exercise-induced menstrual dysfunction and ovarian subcellular structural changes in rats
Cn Zho,Xio-Li Liu,Run-Xio Hong,He Li,Ren Li,d,Ren-Wei Wng,*
aSchool of Kinesiology,Shanghai University of Sport,Shanghai 200438,China
bDepartment of Sport,Hubei M inzu University,Enshi 445000,China
cCollege of Physical Education,Shanghai Normal University,Shanghai 201418,China
dCenter for Advance Hormone Science and Education,Roskamp Institute,Sarasota,FL 34243,USA
Original article
Effects of carbohydrate supplements on exercise-induced menstrual dysfunction and ovarian subcellular structural changes in rats
Can Zhaoa,Xiao-Li Liub,Run-Xiao Honga,He Lic,Rena Lia,d,Ren-Wei Wanga,*
aSchool of Kinesiology,Shanghai University of Sport,Shanghai 200438,China
bDepartment of Sport,Hubei M inzu University,Enshi 445000,China
cCollege of Physical Education,Shanghai Normal University,Shanghai 201418,China
dCenter for Advance Hormone Science and Education,Roskamp Institute,Sarasota,FL 34243,USA
Background:Exercise-associated menstrualdysfunction(EAMD)is a common health problem in female athletes as a partof female athlete triad (FAT),a condition related to low energy availability.In this study,we explored the possibility that carbohydrate supplements can improve the status of EAMD and prevent exercise-induced ovarian injury in a FAT ratmodel.This research aimed to provide experimental evidence w ith regard to the relationship of energy intervention and EAMD.
Methods:Forty-five female Sprague—Daw ley rats(2 months old)were random ly divided into five experimental groups:control group(C), 9-week exercise as model for EAMD(E),post-EAMD recovery group(R),oligosaccharide intervention group(O),and glucose intervention group(G).A ll rats were sacrificed at the end of 9 weeks.Serum samples were collected for measuring gonadotropin releasing hormone,follicle stimulating hormone,luteinizing hormone,17β-estradiol and progesterone levels.The ovaries were taken for investigation of exercise-and carbohydrate-induced follicular subcellular structure changes.
Results:Exercise induced irregular menstrual cycles and ovary subcellular structural damages,such as swollenness of m itochondria in rats from groups E and R.Both glucose and oligosaccharide supplements restored well-differentiated m itochondria in the ovarian follicular cells,and a significant improvementof endoplasmic reticulum and Golgi in swollenness in theca cells in groups O and G compared to groups C,E,and R. There was no difference in m itochondria subcellularstructuralchanges between groups O and G.Group E showed attenuation of serum levels of 17β-estradioland progesterone compared to C.There were no differences of 17β-estradiolserum levels among groups O,G,and R,while group G showed a lower level of progesterone than C.
Conclusion:Female adult rats w ith 9-week continuous exercise can cause menstrual dysregulation as a model for EAMD.Post-EAMD intervention w ith glucose and oligosaccharide intake can normalize the menstrual cycle,restore the follicular subcellular structure,and reverse the exercise-induced reduction of ovary sex hormones.Itsuggests a positive feedback of hypothalamus—pituitary—ovary axis mightbe involved in the molecular mechanisms of energy intake in treating EAMD.
CopyrightⒸ2014,Shanghai University of Sport.Production and hosting by Elsevier B.V.All rights reserved.
Carbohydrate supplement;Estradiol;Estrous cycle restrain;Follicular subcellular injury;Ovary;Progesterone;Rats
1.Introduction
The menstrual cycle occurs only in fertile female humans and other female rodentia,such as rats.Instead of 28 days as the average length in human,the length of rats menstrual cycle is about 4—5 days.1Based on vaginal smears,there are four phases of the menstrual cycle for rats:proestrus(12—14 h), estrus(25—27 h),metestrus(6—8 h),and anestrus(55—57 h)as described previously.2—5The menstrual cycle is mainly regulated by the endocrine system,including the intercoordination of the hypothalamus—pituitary—ovary(HPO) axis in the central nervous system.6
Female athlete triad(FAT)is a syndrome consisting of three necessary components:eating disorder,menstrualdysfunction, and loss of bone mass as osteoporosis.7A lthough its epidem iology remains unclear,studies demonstrated that FAT is closely linked to the imbalance between energy intake and exercise-associated energy requirement.One of major symptoms of FAT is exercise-associated menstrual disturbances (EAMD),which involves reduction of energy supply to the reproductive system due to energy redistribution throughout the body as to comprom ise movement related to energy consumption.8,9As a complementary mechanism,the exerciseinduced reduction of energy supply to reproduction system activates neurodocrinological pathways,such as the HPO axis, and rebalances the energy intake and energy expenditure to support the reproductive function.8,9Since low energy availability is the primary factor that causes EAMD,in this study, we examined whether carbohydrate supplements can reverse EAMD and protect against exercise-induced impairment in ovary as an important partof HPO axis regulation.
2.M aterials and m ethods
2.1.Animals
In the experiment,45 healthy mature 2-month-old female Sprague—Daw ley rats(Shanghai B&K Universal Group Ltd., Shanghai,China)were used.The average weight of rats was 200.0± 5.9 g.All rats were maintained on a 12 h:12 h reversed-light cycle(lights off at 08:00 pm)w ith continuous free access to food chew and water.The regular food chow (Q/TJCX1-2007;Shanghai Shi Lin Biological Technology Co., Ltd.,Shanghai,China),special formula w ith oligosaccharides and glucose10,11(QS130123010057;Hebei Hua Chen Starch Sugar Co.,Ltd,Gaoyi,China)were used as additional energy supplements.This study has been approved by Shanghai Institute of Nutrition(Ethical Certification 2013-002).Prior to the experiment,all rats were exam ined for vaginal cytology tw ice per day for two continuous estrous cycles to monitor menstrual cycles in all experimental rats.12At the end of experiments,all rats were anesthetized and the ovaries were removed for electron m icroscopy study.Blood was collected from inferior vena cava for detecting serum levels of 17βestradiol and progesterone,respectively.In order to compare the levels of ovarian hormone and m icrostructure of ovary at same stage of menstrual cycle,all rats were sacrificed during anestrous phase determined by vaginal smears.
2.2.Experimental design
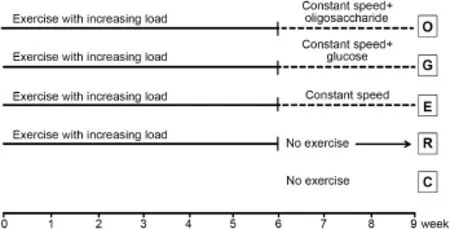
Fig.1.Treadmill running schedules show ing the specific timeline and various treatments of each groups.
As shown in Fig.1,rats were random ly divided into five groups,namely,normal controls(C)w ith no excise,EAMD model(E)having 6 weeks treadmill running w ith increasing work load followed by 3 weeks running w ith constant speed (Table 1),post-EAMD recovery model(R)having 3 weeks w ithout exercise after 6 weeks running,and 3 weeks carbohydrate supplements,oligosaccharide(O)or glucose(G),after 6 weeks running.Vaginal cytology examination was performed daily during the 9-week study to monitor the estrous cycle of each rat.All rats were fed w ith regular food chew throughout the study,except rats in groups O and G who received oligosaccharide and glucose for 3 weeks prior the end of the 9th week,respectively.We have previously identified that adult female rats developed EAMD after 6 weeks of exercise w ith incremental load with delayed the onset of estrus phase,much longer anestrous time,and irregular menstrual cycling(data notpublished).A ll rats were sacrificed at the end of the 9 weeks of exercise w ith 12 h fast.
2.3.Training program
A Duanshi PT98-type treadm ill(Xin Ruan Information Technology Ltd.,Shanghai,China)was used in this experiment.Animals were trained according to the Bedford animal training load standard13described in Table 1 and Fig.1.A fter 1 week of adaptation training,rats were exercising tw ice a day, one time in the morning and one time in the afternoon w ith m inimum 5 h restbetween the two exercise segments.Totalof 9 weeks exercise w ith each training cycle lasted for1 week(6 days exercise,1 day rest).Vaginal smear was performed daily to monitor menstrual cycle for each rat throughout the 9-week study.12Menstrual cycle dysfunction was diagnosed by the onset of estrus phase or length change of anestrus phase in menstrual cycle as described previously.14,15

Table 1 Daily exercise with treadm ill running schedule.
2.4.Determination of hormones
The serum levels of 17β-estradiol were detected by immunochemistry luminescence kit follow ing the instruction provided by manufactory(Beckman Coulter Inc.,Brea,CA,USA) and measured by Access 2 immunoassay system(Beckman Coulter).The follicle stimulating hormone(FSH),luteinizing hormone(LH),and progesterone serum levels were detected by double-antibody radioimmunoassay kit provided by Department of Neurobiology,Second M ilitary Medical University (Shanghai,China),and measured by an intellectγcounter (SN695B9;Chinese Academy ofSciences).The serum levelsof gonadotropin releasing hormone(GnRH)were detected by ELISA(R&D Systems,Inc.,M inneapolis,MN,USA)and measured by Thermo Scientific MK3 system(Thermo Fisher Scientific Inc.,Waltham,MA,USA).
2.5.Analysis of energy intakes
Energy intake was calculated by multiplying the amountof food ingested in gramsand the energy contentof the food(4 g of fat,19 g ofprotein,52 g ofcarbohydrate per100 g,totalenergy of 1377.6 kJ)and the carbohydrate supplementswhich energy were about30%of free intake food.Fig.2 showed the energy intakes by ratsfrom each experimentalgroup during the 9-week studies. Extraenergy intakesweregiven to ratsin groupsOand G in order to investigate whether carbohydrate supplements w ithout interfering in sports load and free food intake would preventEAMD. The targetof extra energy intake in groups O and G were about 30%of average energy intake of free regular diet.For example, started from 7th week,the target extra energy intakes were 72.38 kJand 66.59 kJfor rats in groups O and G,respectively.
2.6.Preparation for transmission electron microscopic examination

Fig.2.Changes in energy intakes in each group throughout 9 weeks study. Energy intakes were recorded and calculated weekly.The covariate in repeated measures general liner model to evaluate at the follow ing values: EI1=221.17 kJ.The levels of energy intake in group E remains at low levels during 9 weeks exercise study,while the energy intake of groups O,G,and R increased after 6 weeks,respectively.*p<0.05,compared w ith group C;#p<0.05,compared w ith group E.
The ovarian tissue wascutinto blocks(<1 mm3)and fixed by phosphatebufferand 2.5%glutaraldehyde for2 h.A 0.1 mmol/L phosphoric acid bleaching lotion wasused to rinse the blocks for 15 m in,repeat three times prior to be dehydrated by various concentrationsofethanoland acetone.Sampleswere then sliced into 50—60 nm by using an LKB-Iultra-thin m icrotomeand then negative-stained by uranyl acetate and lead citrate.A JEM-1200-ex transmission electron microscope(JEOL Ltd.,Tokyo, Japan)was used in this experiment.
2.7.Data statistics
Results were statistically analyzed by using SPSS17.0 software(IBM Corporation,New York,USA).Measured data in multiple groups were compared w ith random ized analysis of variance.Comparison among groups was performed by using SNK test.Weekly energy intake data during the 9-week study were analyzed by using repeated measures general linermodel. The difference was statistically significant whenp<0.05.
3.Results
3.1.Intensive exercise caused EAMD in adult female rats
To monitor the menstrualcycle,we performed daily virginal smears as previously described.14,15Each phase of menstrual cycle ischaracterized by superficialnucleated cellsforproestrus, superficialcytode cells forestrus,superficialnuclearted cellplus luekocyte for metestrus,and flourish leukocyte for anestrus, respectively.At the end of the 6-week intensive treadmill training,ovary epithelial cells changes were obvious as an outcome.Rats in group E showed longer duration of anestrus phase w ith a large numberofsmallunderlayercells and delayed onsetofestruswhile typicalm iddle layercellswere found in rats from groups R,G,and O,respectively(Fig.3).
3.2.Energy intake changes by EAMD
As shown in Table 2,a significant reduction of energy intake occurred in group E compared to the othergroups(p<0.05).In contrast,group C showed significantly higherenergy intakesthan the othergroups(p<0.05).The energy intake levelswere similar in groups R,O,and G.Asshown in Fig.2,energy intake ofgroup E remained at low levels throughoutthe 9 weeks exercise.At the end of6th week,there were no significantdifferences ofenergy intake among groups,exceptgroup C which showed significant greaterenergy intakesthan theothergroups(p<0.05).Thelevels of energy intake in groups O and G restored at7th week after taking carbohydrate supplements for 1 week,while a slow recovery ofenergy intake was also found in group R.
3.3.Carbohydrate supplements reversed EAMD-induced ovarian subcellular injury in adult female rats
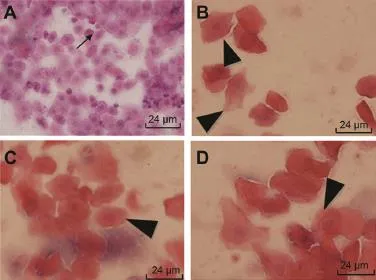
Fig.3.Ovary epithelial cells changes at the end of the 6-week intensive treadm ill running.Rats from group E(A)showed extension of anestrus phase as fl ourish underlayer cells(↑)while typical m iddle layer cells(▲)were found in rats from groups R,G,and O,respectively(B—D).
During anestrus phase ofmenstrual cycle,ovalshape nuclei were observed in the enlarged follicular cells from healthy adult female rats w ithout exercise(Fig.4A).In addition, abundant m itochondria,Golgi,and endoplasmic reticulum were also found in cytoplasm.However,significantsubcellular damages were observed in rats developed EAMD compared to control rats—follicular cells contained swollen mitochondria w ith broken cristae(Fig.4B).The exercise-induced m itochondrial damages were also observed in the EAMD rats w ith post-exercise rest,except a slight increase in number of m itochondria compared to group E(Fig.4C).A significant recovery of exercise-induced mitochondria impairment was found in rats treated w ith oligosaccharide and glucose, respectively(Fig.4D—F).Rats treated w ith carbohydrate supplements showed great reduction of swollen endoplasm ic reticulum and Golgi complex,and increase in abundant organelles.No significant difference was observed between groups O and G.
3.4.Carbohydrate supplements restored EAMD-induced reduction of HPO axis hormones in adult female rats
To exam ine whether energy intake would protect against EAMD through neuroendocrinological mechanisms,we exam ined the serum levels of GnRH,FSH,LH,17β-estradiol, and progesterone at the end of the 9 weeks study.As shown in Table 3,levels of serum GnRH,17β-estradiol and progesterone were significantly attenuated in group E compared to controls(p< 0.05).A significant reduction of serumprogesterone level was found in rats w ith glucose intake compared to control rats(p<0.05).However,there were no differences in 17β-estradiol levels among rats from groups C, R,O,and G.The levels of FSH and LH showed no change in rats w ith or without EAMD or carbohydrate supplements.
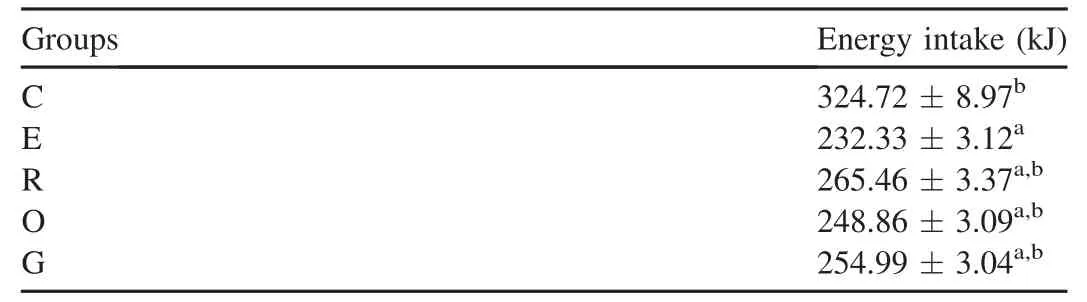
Table 2 Energy intakes in 9-week in experimental rats(mean±SD).
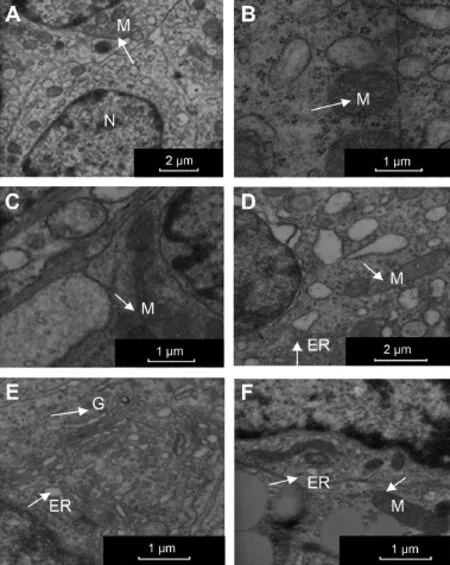
Fig.4.Ultrastructural changes in rat ovarian tissue by EAMD and carbohydrate supplements.Ovarian subcellular ultrastructures were examined and compared in rats from groups of control(A),EAMD(B),post-EAMD rest(C), carbohydrate supplements(D—F).N = nucleus;M = mitochondria; G=Golgi complex;ER=endoplasm ic reticulum.
4.Discussion
It is known that humans share sim ilar reproductive system w ith rats,including the regulatory HPO axis w ith GnRH,FSH, LH,estrogens,and progesterone.16While on exercise providing substantial health benefi ts,studies showed that women w ith excess physical activity could have negative consequences on the whole body,including reproductive system,such as FAT.Our data showed that adult female rats developed irregular menstrual cycle after 6 weeks of treadm ill running w ith increased speed followed by 3 weeks of exercise w ith constantspeed as shown in Table 1.As a consequence of intensive exercise,rats from group E had cycles w ith anestrus phases that were more than 4 days long.Endocrine system dysfunction is associated w ith strenuous exercise,and the resulting disturbance of sex hormones can cause disruption of menstrual cycling.17Our model showed significant disruption ofmenstrual cycle in consistentwith previous reported EAMD models.18—20
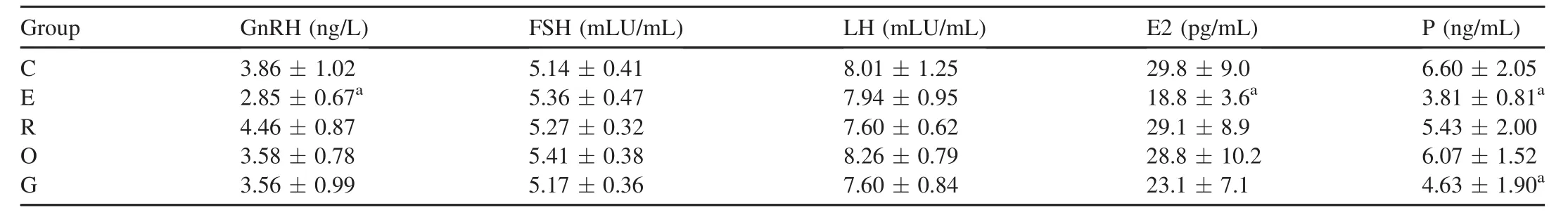
Table 3 Serum levels of hypothalamus—pituitary—ovary(HPO)axis hormones in experimental rats(mean±SD).
To examine whether EAMD is related to energy imbalance, we measured energy intake as showed in Table 2.Indeed, although our data showed that long post-exercise resting can restore the exercise-induced low level of energy intake,it is very difficult to practice in elite athletes training.Therefore, post-exercise carbohydrate supplements m ight be beneficial for preventing EAMD.Energy intake is part of energy availability,which is defined as dietary energy intake m inus exercise energy expenditure.The present study showed that adult female rats w ithoutexercise training had an increased energy intake along normalgrow th.If the energy availability is below 30 kcal/kg fat free mass per day,functions of reproductive system and other metabolic systems m ight be suppressed.21The reduction of energy intake in EAMD rats in our study is in consistent w ith human studies.For example,Tom ten and Høstmark22found calculated energy intake and total energy expenditure were in balance in athletes w ith regular menstruate,while a statistically significant negative energy balance was found in female athletes w ith irregular menstrual cycles.As previous studies had shown that disorder of the HPO axis in female athletes seemed to be rely on the recognition of an energy imbalance in human body,Stafford23considered this pathological phenomenon may be attribute to the lack of compensatory caloric intake confronting w ith significant energy expenditure.
To investigate whether EAMD induces pathological changes in HPO axis,we examined both ovarian follicular subcellular structures and circling ovarian hormones,such as 17β-estradiol and progesterone.We found rats w ith EAMD developed significant damages in follicular cells,such as swollen endoplasmic reticulum,Golgi complex,as well as m itochondria w ith broken cristae.Interesting enough,the exercised-induced follicular subcellular injuries were observed in the post-EAMD rats(Fig.4),suggesting a long lasting damages caused by EAMD in the adult female rats.The only difference between rats w ith EAMD and post-EAMD was a slight increase in number of microchondria in post-EAMD rats.Post-EAMD carbohydrate supplements adm inistration reversed the EAMD-induced impairment in ovarian follicular subcellular structure.Our data not only further supported the hypothesis of energy deficiency in EAMD,butalso provided a positive future translational approach to treat EAMD.
To understand whether excess exercise would alter hormones of HPO axis,we exam ined levels of HPO axis hormones of the female rats.We found that rats had excess treadm ill running in group E showed significantly reduction of GnRH,17β-estradiol,and progesterone levels compared to controls(p<0.05),while EAMD or carbohydrate supplements caused no changes in FSH and LH levels.Our finding is in consistentw ith other published reports from human studies, such as in female athletes w ith long intensive physical training,the level of progesterone was only 1/3 of that in normal control subjects,and the average estradiol level was also lower than controls.24In addition,comparing different sports,the long distance runners had the lowest level of estradiol and progesterone.7,14,25Although the mechanism of EAMD-induced damages of ovarian ultrastructure remains unclear,studies suggested that reduction of ovarian hormones by exercise may be associated w ith the hypothalam ic GnRH, which is known as an energy metabolism factor.26,27The reduction of GnRH release indirectly decreases the secretion of estradiol and progesterone.27
In 1984 Bonen and Keizer28fi rst reported that the primary locus of EAMD is the GnRH pulse generator athypothalamus. Metabolic challenges alter the GnRH,LH and FSH surge,and inhibitthe HPO system in partby increasing the sensitivity to the negative feedback of estradiol.Such an EAMD-induced deficiency in GnRH have been confi rmed by several independent research groups.29In addition,our previous studies demonstrated abnormal subcellular structural changes in GnRH neurons from hypothalamus of female rats w ith EAMD,which inevitably inhibit GnRH secretion.30However,no significant differences of serum GnRH level were found in rats among groups R,O,G,and C in this study,suggesting that good rest after intensive exercise or carbohydrate supplements intervention can effectively reverse the EAMD-induced inhibition of GnRH pulse generator.In addition,we found no significant difference on serum FSH and LH levels.Recent studies suggested that higher ghrelin and lower leptin secretion in female athletes may contribute to altered LH pulsatility and exerciseinduced amenorrhea.31Since levels of FSH and LH in anestrus were lower than in othermenstrual phases,the changes caused by EAMD and energy intake in this study m ight be limited. Therefore,further investigation of EAMD-induced changes in hormones of HPO axis in differentmenstrual cycle is needed.
To exam ine whether post-EAMD intervention w ith carbohydrate supplements could normalize the menstrual cycle and reverse the exercise-induced ovary dysfunction,we treated EAMD rats with glucose and oligosaccharide for3 weeks.Rats treated w ith carbohydrate supplements after EAMD showedsame menstrual cycles as rats from groups C and R,suggesting that extra energy intakes successfully restored the ovarian function in adult female rats.Furthermore,rats received glucose and oligosaccharide supplements reversed the EAMD-induced m itochondria morphology injuries.We found a slight increase in numberof mitochondria and a significant recovery of exercise-induced mitochondria impairment in rats treated w ith oligosaccharide and glucose,respectively.Our data also showed a significant reduction of serum progesterone level in rats with glucose intake compared to controls,while no differences in 17β-estradiol levels among rats from groups C,R, O,and G.While the reason of failing to restore EAMD-induced attenuation of progesterone in rats received post-EAMD glucose supplement needs further investigation,studies found an insulin sensitivity increases in exercise women,32which m ight counter the effectof glucose supplement in EAMD.
Consistentw ith previous findings,33—36our study shows the differences of the levels of 17β-estradiol and progesterone in each group are correlated w ith the ultrastructural changes of the ovarian cells observed under an electron microscope.It is reasonable to hypothesize that the reduction of estradiol and progesterone levels in serum is directly related to the impairmentof ovarian subcellular organelles,such as m itochondria, endoplasm ic reticulum,and Golgicomplex where endogenous estradiol and progesterone were synthesized.37,38Human studies indicates that athletes should follow diet and exercise regimens that provide energy of 30—45 kcal/kg/day fat free mass while training involving body weight control.21
5.Conclusion
Our study demonstrated that adult female rats developed EAMD after 6-week intensive treadm ill exercise training characterized by irregular menstrual cycles,significant ovary subcellular injuries,and reduction of ovarian hormone levels. The pathological changed caused by EAMD were reversed by post-EAMD resting,as well as post-EAMD carbohydrate supplements.A lthough the molecular mechanisms of energy intake in treating EAMD remain unclear,our data suggest a positive feedback of HPO axis m ightbe involved.Meanwhile, further research is needed to determ ine whether the suppression of HPO axis by exercise can be ameliorated by carbohydrate supplements in female athletes.
Acknow ledgment
This study is supported by Shanghai Key Laboratory of Human Sport Competence Development and Maintenance, Shanghai University of Sport(NO.11DZ2261100).
1.Ojeda SR,UrbanskiHF.Puberty in the rat.In:KnobilE,Neill JD,editors.The physiology of reproduction,vol.II.New York:Raven Press;1994. p.363—409.
2.Astwood EB.Changes in weight and water content of the uterus of the normal adult rat.Am J Physiol1939;126:162—70.
3.Hartman CG.Some new observations on the vaginalsmearof the rat.Yale J Biol Med1944;17:99—112.
4.Long JA,Evans HM.The oestrous cycle in the rat and its associated phenomena.Mem Univ Calif1922;6:1—148.
5.Mandl AM.The phases of the estrous cycle in the adult white rat.J Exp Biol1951;28:576—84.
6.Carr BR.Disorders of the ovaries and female reproductive tract.In: W ilson JD,Foster DW,Kronenberg HM,Larsen PR,editors.Williams textbookof endocrinology.9th ed.New York:Saunders;1998. p.751—817.
7.DiPietro L,StachenfelNS.The female athlete triad.Med Sci Sports Exerc1997;29:1669—71.
8.W illiams NI,Caston-Balderrama A,Helm reich DL.Longitudinal changes in reproductive hormones and menstrualcyclicity in cynomolgus monkeys during strenuous exercise training,abrupt transition to exercise induced amenorrhea.Endocrinology2001;142:2381—9.
9.Wang RW,Huang YJ.Advances in exercise-associated menstrual disorders.Chin J Sports Med2008;27:661—6[in Chinese].
10.Chen JL,Li KJ,Wu YZ.Effect of glucose polymer beverage on performance of young volunteers.Sports Sci1998;18:62—5[in Chinese].
11.Han XL,Ding SZ.The study procession of relationship between sports performance and carbohydrate supplementation.SportsSci2000;21:27—31[in Chinese].
12.Fu Y,Zhou MR,Xiong RH.Changes in histomorphology and ultrastructure of ovaries in exercise-induced estrus disorder rats.J Clin Rehab Tissue Engin Res2011;15:7643—6.
13.Bebford TG,Tipton CM,Wilson NC,Oppliger RA,Gisolfi CV.Maximum oxygen consumption of rats and its changes w ith various experimentalprocedures.J Appl PhysiolRespir Environ Exerc Physiol1979;47:1278—83.
14.Wang RW.The effect of incremental training on HPOU axis ultrastructure,gonadal hormones andβ-EP in female rats.Shanghai:Shanghai University of Sport;2000[Dissertation].
15.Cheng LZ,Zhong CP,Cai WQ.Modern histology.Shanghai:Shanghai Scientific and TechnologicalLiterature Publishing House;2003[in Chinese].
16.Staley K,Scharfman H.A woman’s pregative.Nat Neurosci2005;8:697—9.
17.Warren MP,Perlroth NE.The effects of intensive exercise on the female reporductive system.J Endocrinol2001;170:3—11.
18.Carlberg KA,Fregly M J.Disruption of estrous cycles in exercise-trained rats.Proc Soc Exp Biol Med1985;179:21—4.
19.Chatterton RT,Hrycyk L,Hickson RC.Effect of endurance exercise on ovulation in the rat.Med Sci Sports Exerc1995;27:1509—15.
20.W illiams NI,Helm reich DL,Parfi tt DB,Caston-Balderrama A, Cameron JL.Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training.J Clin Endocrinol Metab2001;86:5184—93.
21.Loucks AB,Thuma JR.Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women.J Clin Endocrinol Metab2003;88:297—311.
22.Tom ten SE,Høstmark AT.Energy balance in weight stable athletes w ith and w ithout menstrual disorders.ScandJMedSciSports2006;16:127—33.
23.Stafford DE.Altered hypothalamic-pituitary-ovarian axis function in young female athletes:implications and recommendations for management.Treat Endocrinol2005;4:147—54.
24.Loucks AB.Energy balance and body composition in sports and exercise.J Sports Sci2004;22:1—14.
25.Dimarco NM,Dart L,Sanborn CB.Modified activity-stress paradigm in an animal model of the female athlete triad.J Appl Physiol(1985)2007;103:1469—78.
26.Samuels MH,Sanborn CF,Hofeldt F,Robbins R.The role of endogenous opiates in athletic amenorrhea.Fertil Steril1991;55:507—12.
27.Pauli SA,Berga SL.Athletic amenorrhea:energy deficit or psychogenic challenge?Ann N Y Acad Sci2010;1205:33—8.
28.Bonen A,Keizer HA.Athletic menstrual cycle irregularity:endocrine response to exercise and training.Phys Sportsmed1984;12:78—94.
29.Warren MP,Goodman LR.Exercise-induced endocrine pathologies.J Endocrinol Invest2003;26:873—8.
30.Wang RW,Lu AY,Guo SD,Gao Y,Cai B,Duan ZC.The changes of ultrastructure of hypothalamus-pituitary axis for athletic menstrual cycle irregularities in rats.Chin J Sports Med2001;20:68—72[in Chinese].
31.Ackerman KE,Slusarz K,Guereca G,Pierce L,Slattery M,Mendes N, et al.Higher ghrelin and lower leptin secretion are associated w ith lower LH secretion in young amenorrheic athletes compared w ith eumenorrheic athletes and controls.Am J Physiol Endocrinol Metab2012;302:E800—6.
32.Krishnan S,Gustafson MB,Campbell C,Gaikwad NW,Keim NL.Association between circulating endogenous androgens and insulin sensitivity changes w ith exercise training in m idlife women.Menopause2014. http://dx.doi.org/10.1097/GME.0000000000000198[Epub ahead of print].
33.Zheng L,Xu HW.The features,mechanisms and significance of change in the function of HPO axis in incremental exercise process.Shanghai:East China Normal University;2004[Dissertation].
34.Vullie´moz NR,Xiao E,Xia-Zhang L,Wardlaw SL,Ferin M.Central Infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectom ized rhesus monkey.Endocrinology2005;146:784—9.
35.Tsigos C,Chrousos GP.Hypothalamic-pituitary-adrenalaxis,neuroendocrine factors and stress.J Psychosom Res2002;53:865—71.
36.Tena-Sempere M.Ghrelin:novel regulator of gonadal function.J Endocrinol Inves2005;28:26—9.
37.Yue J.Gynecotokology.Beijing:People’s Medical Publishing House; 2013.p.97—106[in Chinese].
38.Manabe N,Goto Y,Matsuda-M inehata F,Inoue N,Maeda A,SakamakiK, et al.Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia.J Reprod Dev2004;50:493—514.
Received 25 February 2014;revised 25 March 2014;accepted 10 April 2014
*Corresponding author.
E-mail address:renwwang@163.com(R.-W.Wang)
Peer review under responsibility of Shanghai University of Sport
2095-2546/$-see front matter CopyrightⒸ2014,Shanghai University of Sport.Production and hosting by Elsevier B.V.A ll rights reserved. http://dx.doi.org/10.1016/j.jshs.2014.04.006
 Journal of Sport and Health Science2014年3期
Journal of Sport and Health Science2014年3期
- Journal of Sport and Health Science的其它文章
- Women’s health in exercise and aging:What do we know?
- Why women see differently from the way men see?A review of sex differences in cognition and sports
- Sex differences in exercise and drug addiction:A m ini review of animal studies
- Women and exercise in aging
- Exercise training and antioxidant supplementation independently improve cognitive function in adult male and female GFAP-APOE m ice
- Surgical menopause enhances hippocampal amyloidogenesis follow ing global cerebral ischem ia
