药物及个人护理品在水中光解的研究进展
赵 群
(山东省环境保护科学研究设计院, 山东 济南 250013)

1 PPCPs的直接光解
许多PPCPs具有萘环、杂环、硝基等结构,能吸收自然光发生化学键断裂、电子重排以及异构化等转变成另外一种物质.有些PPCPs虽能吸收自然光,但将能量传递出去不发生光化学转化或转化较慢.如卡马西平吸收的光最多,但降解常数最小.氯贝酸虽吸收的光最小,但能迅速降解生成4-氯酚、对羟基苯酚、苯酚和对位苯醌[9].一般来说,PPCPs的直接光解取决于三方面:PPCPs分子结构、溶液pH值和PPCPs自敏化.

表1 一些PPCPs的直接光降解速率常数、半衰期和量子产率
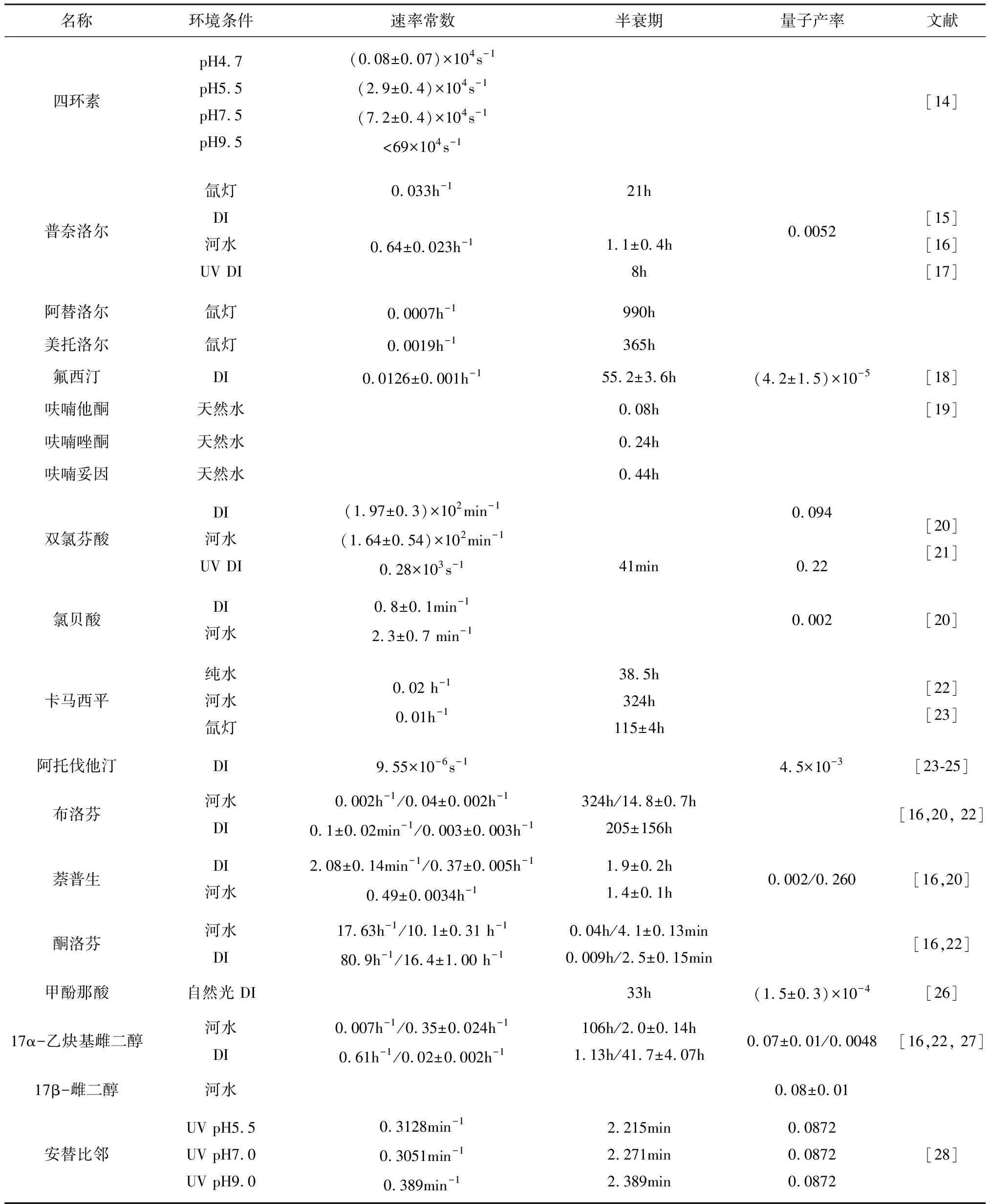
续表
注:表中DI为去离子水,UV为紫外光
1.1 分子结构
PPCPs对光的吸收与去定域化π电子体系有关,这类体系最可能发生π→π*跃迁.如果π电子体系存在带有杂原子,还可发生n→π*跃迁.通常一个分子的共轭效应越明显,其吸收峰向长波方向偏移越多,与太阳光谱重叠越多[29].普奈洛尔具有萘结构,吸收光谱与太阳光谱重叠最多,光解最快.而阿替洛尔和美托洛尔只有苯环结构,无论在低浓度(1mg/L)还是高浓度(10mg/L)二者光解速率均远远低于普奈洛尔[15].酮洛芬直接光解很快是由于其中羰基与两个苯环共轭,使n→π*跃迁能很低,易产生活性三重态.雌二醇和炔雌二醇的量子产率非常接近,分别为0.067和0.062,这与具有酚结构的物质很接近[27].
1.2 pH值
许多PPCPs阴离子形态倾向于红移,吸收光谱与自然光谱重叠的部分增加,利于光降解.在pH为5.9、8.0、9.1、11.0下,三氯生光解速率常数分别为(3.8±0.6)×10-4s-1、(6.9±0.5)×10-3s-1、(1.1±0.2)×10-2s-1、(1.2±0.3)×10-2s-1[13,30].雷尼替丁在pH为6时量子产率为(5.3±0.5)×10-3,pH为10时量子产率为(5.5±0.5)×10-3[10].pH升高使扑热息痛中酚羟基失去质子发生红移吸收更多自然光[31].
但有些PPCPs的光降解机理比较复杂,酸催化可使PPCPs在酸性下的降解速率大于碱性和中性.呋喃类抗生素(呋喃他酮、呋喃唑酮和呋喃妥因)的光解受pH的影响,光解速率在酸性条件下达到最大,pKa影响较小[19].也存在碱催化现象.自然光照下氟西汀在pH为9时的速率常数为pH为4和7时的二倍,在碱性条件下未观察到红移现象[18].
1.3 自敏化
PPCPs能自敏化产生活性氧(ROS)降解自身.四环素在水中光解并不满足一级动力学,这是因为降解产物发生自敏化产生ROS使其具有直接光解和ROS氧化两个途径,另外一个解释是四环素之间发生自敏化,这种自敏化在其带有两个负电荷时达到最大,甚至远大于直接光解,甚至加入金属离子后,这种自敏化仍能够发生[14].
一些磺胺类药物直接光解机理较为复杂,磺胺甲基异恶唑的光解速率随着吸收光量的增加而增加,在中性时光解速率常数最大,阴离子态光解速率则慢得多.磺胺异恶唑在中性时吸收光量最多,但阳离子态时光解速率最快.磺胺甲噻二唑和磺胺噻唑在中性时吸收光量最多,但是它们在阴离子态时光解速率最快,这些磺胺类物质结构相似,仅有杂环中杂原子及其取代基不同,但是光解特点却有很大差别[12].
2 PPCPs的间接光解
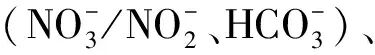
2.1 PPCPs与和体系的光反应

2.2 PPCPs与DOM体系的光反应
DOM与PPCPs的光反应机制较为复杂,一方面DOM通过能量转移、电子转移和氢转移或产生活性氧(HO·、1O2、H2O2等)促进PPCPs的光降解,另一方面DOM具有内滤作用,与PPCPs竞争吸收重要谱段的光抑制PPCPs的光降解,而且作为羟基自由基的淬灭剂DOM也会抑制PPCPs的光化学转化[36-37].DOM与三氯生竞争光减少三氯生的光降解[13].低浓度的DOM促进卡马西平的光解,浓度升高时,这种促进作用会减弱[9,22].阿托伐他汀与富里酸的光反应速率大于加入叠氮化钠的反应速率,阿托伐他汀与富里酸在D2O(重水(氧化氘)是由氘和氧组成的化合物)的反应速率也大于在水中光反应速率,单重态氧能被叠氮化钠淬灭,单重态氧在D2O中的存留时间比H2O中长,这说明阿托伐他汀与富里酸在光照下有单重态氧参与反应[24].DOM能够促进甲酚那酸的光降解,经过研究发现是DOM吸收光后向甲酚那酸传递能量或传递电子使其发生降解[26].扑热息痛与两种富里酸的光解反应速率均随着富里酸浓度的增加而加快,这是由于形成一系列活性中间体[38-39].
2.3 PPCPs与Fe3+的光反应
Fe3+主要通过两种途径参与PPCPs光降解:(1)Fe3+与有机物结合,使不吸收太阳光谱的有机物吸收太阳光谱发生光降解;(2)Fe3+通过光化学氧化还原循环产生羟基自由基参与PPCPs的氧化[40].卡马西平和Fe3+的光解速率随着pH的增加而减小,溶解态FeOH2+能在光照下产生羟基自由基降解卡马西平,随着pH增加,FeOH2+减少,自由基减少.但将Cl-加入卡马西平和Fe3+后,光解速率随pH的增加而增大.酸性条件下,Cl-是羟基自由基的淬灭剂,所以反应速率低,碱性条件下,Cl-对羟基自由基的淬灭减弱,Fe3+将Cl-氧化成Cl·/Cl2·参与卡马西平的氧化[41].雌酮水溶液几乎不发生光解,加入三价铁后,降解效率随光照时间延长而增加,但降解效率(55.1%)不高.pH为3~4时,雌酮与铁-草酸盐配合物有较高光解效率,pH降低或升高光解效率都有所下降[42].
2.4 PPCPs与生物体系的光反应
国外对PPCPs与生物体系的光反应研究较少,国内刘先利和葛利云研究了乙炔基雌二醇和雌二醇与藻类体系的光降解,发现鱼腥藻使乙炔基雌二醇光降解率增加.藻液中增多了活性氧H2O2、单重态氧和腐殖质,反应途径增加.在紫外光下,藻在107cells/L和109cells/L时具有催化作用,而在108cells/L则不理想,因为随着藻浓度增加,水体中光强受到部分削减,浓度继续增大,藻或腐殖质增大的综合催化补偿了光削减的影响[43].普通小球藻也能诱发雌二醇光降解,小球藻与17β-雌二醇结合吸收波长较长的光,藻液在光照下产生了活性氧以及藻类的死亡产生了具有催化作用的腐殖质[44].
3 展望
PPCPs种类繁多,不同种类PPCPs的化学结构不同,在水环境中的光解途径会有很大差别.同时,水环境介质是一个非常复杂的混合体系,即使是同种PPCPs,在不同水环境中光解途径也可能会有很大差别.目前已经有许多科研工作者对环境中出现的典型PPCPs进行了光化学降解研究,这些研究主要集中在:PPCPs在纯水中的直接光解和光解产物的鉴定以及根据光解产物推断直接光解机理;羟基自由基、单重态氧对PPCPs的光化学转化和产物鉴定以及根据产物推断机理;PPCPs在模拟水环境体系如硝酸根体系、碳酸根体系、DOM体系和金属络合物体系中的光解动力学及影响因素、产物鉴定及相关机理.也有人研究PPCPs在天然水包括海水环境中的光降解状况,并推断降解的机理.随着对PPCPs在水环境光降解研究的不断深入,人们发现有些PPCPs的光降解产物的毒性远大于母体化合物,如三氯生光解后能产生毒性极大的二噁英,所以很有必要对PPCPs的光降解产物进行毒性实验.一些PPCPs的光降解机理非常复杂,如磺胺类药物的降解无法用常规的直接光解、活性氧氧化进行解释,这就需要一些新的手段检测可能未被认识的物质如自由基或反应机理.随着QSAR技术的不断成熟,可对PPCPs的化学结构及空间构型通过合适的参数进行量化,建立光解速率模型来消减实验次数,减少人力、物力和财力的投入.
[1] EPA. Pharmaceuticals & personal care products in the environment: an emerging concern?[EB/OL]. (1999-12-24)[2005-3-20]. http://www.epa.gov/nerl/research/1999/html/g8-14.html.
[2] Carballa M, Omil F, Lema J M,etal. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant[J]. Water Research, 2004. 38(12): 2 918-2 926.
[3] Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data[J]. Toxicology Letters, 2002,131(1-2): 5-17.
[4] Daughton C G, Ternes T A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? [J]. Environmental Health Perspectives, 1999,107: 907-938.
[5] Jorgensen S E, Halling-Sorensen B. Drugs in the environment[J]. Chemosphere, 2000,40(7): 691-699.
[6] Kummerer K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources - a review[J]. Chemosphere, 2001,45(6-7): 957-969.
[7] Arnold W A, McNeill K. Transformation of pharmaceuticals in the environment: photolysis and other abiotic processes[J]. Comprehensive Analytical Chemistry, 2007,50: 361-385.
[8] Boreen A L, Arnold W A, McNeill K. Photodegradation of pharmaceuticals in the aquatic environment: a review[J]. Aquatic Sciences, 2003,65(4): 320-341.
[9] Doll T E, Frimmel F H. Fate of pharmaceuticals-photodegradation by simulated solar UV-light[J]. Chemosphere, 2003,52(10): 175 7-176 9.
[10] Latch D E, Stender B L, Packer J L,etal. Photochemical fate of pharmaceuticals in the environment: cimetidine and ranitidine[J]. Environmental Science & Technology, 2003,37(15): 3 342-3 350.
[11] Boreen A L, Arnold W A, McNeill K. Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: Identification of an SO2extrusion photoproduct[J]. Environmental Science & Technology, 2005, 39(10): 3 630-3 638.
[12] Boreen A L, Arnold W A, McNeill K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups[J]. Environmental Science & Technology, 2004,38(14): 3 933-3 940.
[13] Tixier C, Singer H P, Canonica S,etal. Phototransformation of triclosan in surface waters: a relevant elimination process for this widely used biocide - laboratory studies, field measurements, and modeling[J]. Environmental Science & Technology, 2002,36(16): 3 482-3 489.
[14] Werner J J, Arnold W A, McNeill K. Water hardness as a photochemical parameter: tetracycline photolysis as a function of calcium concentration, magnesium concentration, and pH[J]. Environmental Science & Technology, 2006,40(23): 7 236-7 241.
[15] Liu Q T, Williams H E. Kinetics and degradation products for direct photolysis of beta-blockers in water[J]. Environmental Science & Technology, 2007,41(3): 803-810.
[16] Lin A Y C, Reinhard M. Photodegradation of common environmental pharmaceuticals and estrogens in river water[J]. Environmental Toxicology and Chemistry, 2005,24(6): 1 303-1 309.
[17] Piram A, Salvador A, Verne C,etal. Photolysis of beta-blockers in environmental waters[J]. Chemosphere, 2008,73(8): 1 265-1 271.
[18] Lam M W, Young C J, Mabury S A. Aqueous photochemical reaction kinetics and transformations of fluoxetine[J]. Environmental Science & Technology, 2005,39(2): 513-522.
[19] Edhlund B L, Arnold W A, McNeill K. Aquatic photochemistry of nitrofuran antibiotics[J]. Environmental Science & Technology, 2006, 40(17): 5 422-5 427.
[20] Packer J L, Werner J J, Latch D E,etal. Photochemical fate of pharmaceuticals in the environment: aproxen, diclofenac, clofibric acid, and ibuprofen[J]. Aquatic Sciences, 2003,65(4): 342-351.
[21] Eriksson J J, Svanfelt L K. A photochemical study of diclofenac and its major transformation products[J]. Photochemistry and Photobiology, 2010,86(3): 528-532.
[22] Matamoros V, Duhec A, Albaiges J,etal. Photodegradation of carbamazepine, ibuprofen, ketoprofen and 17 alpha-ethinylestradiol in fresh and seawater[J]. Water Air and Soil Pollution, 2009,196(1-4): 161-168.
[23] Lam M W, Mabury S A. Photodegradation of the pharmaceuticals atorvastatin, carbamazepine, levofloxacin, and sulfamethoxazole in natural waters[J]. Aquatic Sciences, 2005,67(2): 177-188.
[24] Razavi B, Ben Abdelmelek S, Song W,etal. Photochemical fate of atorvastatin (lipitor) in simulated natural waters[J]. Water Research, 2011,45(2):625-631.
[25] Cermola F, DellaGreca M, Lesce M R,etal. Photochemical behavior of the drug atorvastatin in water[J]. Tetrahedron, 2006,62(31): 7 390-7 395.
[26] Werner J J, McNeill K, Arnold W A . Environmental photodegradation of mefenamic acid[J]. Chemosphere, 2005,58(10): 1 339-1 346.
[27] Mazellier P, Meite L, De Laat J. Photodegradation of the steroid hormones 17 beta-estradiol (E2) and 17 alpha-ethinylestradiol (EE2) in dilute aqueous solution[J]. Chemosphere, 2008,73(8): 1 216-1 223.
[28] 钟明洁, 陈勇, 胡春. 水中安替比林的紫外光降解研究[J]. 环境工程学报, 2009(6): 1 049-1 053.
[29] 瑞恩 P. 施瓦茨巴赫,菲利普 M. 施格文, 迪特尔 M. 英博登.环境有机化学[M]. 王连生,译. 北京:化学工业出版社,2003.
[30] Latch D E, Packer J L, Stender B L,etal. Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products[J]. Environmental Toxicology and Chemistry, 2005,24(3): 517-525.
[31] 刘钰, 杨曦, 高颖. 扑热息痛在硝酸根溶液中的光解研究[J]. 环境科学, 2007,28(6): 1 274-1 279.
[32] Zafiriou O C, True M B. Nitrite photolysis in seawater by sunlight[J]. Marine Chemistry, 1979,8(1): 9-32.
[33] Zepp R G, Hoigne J, Bader H. Nitrate-induced photooxidation of trace organic-chemicals in water[J]. Environmental Science & Technology, 1987,21(5): 443-450.
[34] Brezonik P L, Fulkerson-Brekken J. Nitrate-induced photolysis in natural waters: controls on concentrations of hydroxyl radical photo-intermediates by natural scavenging agents[J]. Environmental Science & Technology, 1998, 32(19): 3 004-3 010.
[35] Vione D, Khanra S, Man S C,etal. Inhibition vs. enhancement of the nitrate-induced phototransformation of organic substrates by the (OH)-O-center dot scavengers bicarbonate and carbonate[J]. Water Research, 2009, 43(18): 4 718-4 728.
[36] Canonica S, Jans U, Stemmler K,etal. Transformation kinetics of phenols in water - photosensitization by dissolved natural organic material and aromatic ketones[J]. Environmental Science & Technology, 1995, 29(7): 1 822-1 831.
[37] Richard C, Vialaton D, Aguer J P,etal. Transformation of monuron photosensitized by soil extracted humic substances: energy or hydrogen transfer mechanism? [J]. Journal of Photochemistry and Photobiology a-Chemistry, 1997, 111(1-3): 265-271.
[38] 高颖, 杨曦, 刘钰. 在Suwannee河富里酸溶液中扑热息痛的光解行为[J]. 环境化学, 2008, 27(4): 432-435.
[39] 高颖, 杨曦, 张金凤. 腐殖质NAFA溶液中的扑热息痛光解研究[J]. 环境保护科学, 2008, 34(3): 26-29.
[40] 邓南圣, 吴峰. 环境光化学[M]. 北京:化学工业出版社,2003.
[41] Chiron S, Minero C, Vione D. Photodegradation processes of the Antiepileptic drug carbamazepine, relevant to estuarine waters[J]. Environmental Science & Technology, 2006, 40(19): 5 977-5 983.
[42] 陈勇, 张长波, 吴峰,等. 雌酮在铁(Ⅲ)-草酸盐配合物体系中的光降解[J]. 环境污染治理技术与设备, 2006, 7(3): 45-47.
[43] 刘先利, 邓南圣, 徐栋,等. 含鱼腥藻水溶液中17α-乙炔雌二醇光降解[J]. 重庆环境科学, 2003, 25(8): 21-24.
[44] 葛利云, 邓欢欢, 吴峰, 等, 普通小球藻引发水中17β-雌二醇的降解[J]. 应用生态学报, 2004, 15(7): 1 257-1 260.

