Electrochemical Performances of Si-graphite/carbon Composites as Anode Materials for Lithium-Ion Batteries
Zhao Shuo, Chen Minglu, Xian Xiaochao, Zhang Yong, Xiang Jun, Sun Leiming
(School of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400030, China)
Si electrodes have not yet reached a reliable state for commercialization.One of the reasons is the deterioration of capacity due to the large volume expansion and contraction upon Li insertion and extraction[1-3].The other is the pulverization of active materials during cycling leading to the loss of electric conduction pathway.In addition,the low electronic conductivity of pristine Si makes it difficult to achieve good electrochemical performance at high current density[4-5].
Therefore,several strategies have been investigated to overcome the disadvantages of Si electrodes[6-9],such as reducing the particle sizes to nanoscale and introducing a second component to form nanocomposites.Recently,many researchers are paying attention to preparing Si/carbon composite anodes,in which the carbon acts as both a structural buffer and an electrochemically active material.Various carbonaceous materials have been employed to prepare Si/carbon composite anodes.Graphite, graphene[10-11], mesophase microbeads[12], pyrolyzed polyethylene glycol and other carbonaceous materials have been used as matrices for Si[13-15].
Starch after carbonization was honeycomb structure,which is expected to have well buffering effect.Therefore,potato starch was chosen as carbon source to prepare Si-graphite/carbon composite material in this work.The effect of preparation conditions(i.e.,m(Si)/m(graphite)in composite material,the ratio of composite/graphite/carboxylmethyl cellulose(CMC)/styrene butadiene rubber(SBR)and milling time)on the electrochemical performance was investigated.Meanwhile,the key factors affecting the electrochemical performance of the composite were investigated.
1 Material and methods
1.1 Preparation of the composite materials
Nano-Si(>99.9%,1 g,Shuitian Materials Technology Co.,Ltd)were dispersed in 200 m L deionized water by ultrasonication for 0.5 h.Then,potato starch(9 g, Shandong Jincheng Co., Ltd.)and graphite(Changsha Xingcheng Microlite Graphite Co.,Ltd)were added in the former solution.The mixed solution was stirred at 70℃for 1 h.The m(Si)/m(graphite)ratio was designed as 1∶0, 1∶2 and 1∶4, respectively.
Subsequently,the obtained emulsion was spread on the glass plate and dried at80℃for 2 h.The dried precursor was carbonized at 850℃for 1 h in a tube furnace with a flowing argon atmosphere.The final Sigraphite/carbon composite was obtained.
Mechanicalmilling of samples was carried out using YXQM-0.4 planetary ball-miller(Changsha MITR Instrument and Equipment Corporation).The rotation speed was 230 r/min.
1.2 Characterization techniques
The morphologies of samples were observed by FEINova 400 Nano scanning electron microscopy(SEM)with electron backscatter diffraction(EBSD)system.The crystalline structure of samples was characterized by X-ray diffraction(XRD)on Shimadzu XRD6000X diffractometer with Cu_Kαradiation.
1.3 Electrochemical measurements
The anode working electrodes were prepared by coating mixed slurry of Si-graphite/carbon composite,graphite,CMC and SBR on Cu foil current-collector.
CR2430 coin-type cells were assembled in an argon-filled glove box using lithium foils(ShanghaiOujin Lithium Industrial Co.,Ltd.)as the count electrode.A solution of 1 mol/L LiPF6in ethylene carbonate:dimethyl carbonate:methylethyl carbonate(1:1:1 in mass ratio)was employed as the electrolyte.The galvanostatic charge/discharge tests were performed on a NEWARE TC53 battery test systemin the voltage range of 0.001_2.5 V(versus Li/Li+)at the current density of 20 mA/g.
2 Results and discussion
The influence of the m(Si)/m(graphite)ratio in Si-graphite/carbon composite on the electrochemical performance has been studied,and the results are shown in Fig.1 .The m(composite)/m(graphite)/m(CMC)/m(SBR)ratio in working electrode is 93∶2∶2.5∶2.5.All samples have not been treated by mechanicalmilling.It can be seen that when m(Si)/m(graphite)ratio is changed from 1∶0 to 1∶4, the initial charge capacity decreases from 999 to 638 mA·h/g,while the first discharge capacity increase from 139 to 340 mA·h/g.Themain reason for the rapid capaci-ty loss may be the pulverization of Si anodes,which leads to loss of electrical contact and eventual capacity fading.A fter 20 cycles,the composite with m(Si)/m(graphite)ratio of 1∶4 has the highest reversible capacity.It indicates that graphite in the composite can alleviate the volume change and increase the conductivity of Si anode.Therefore, 1∶4 is chosen as the m(Si)/m(graphite)ratio in composite during the following study.
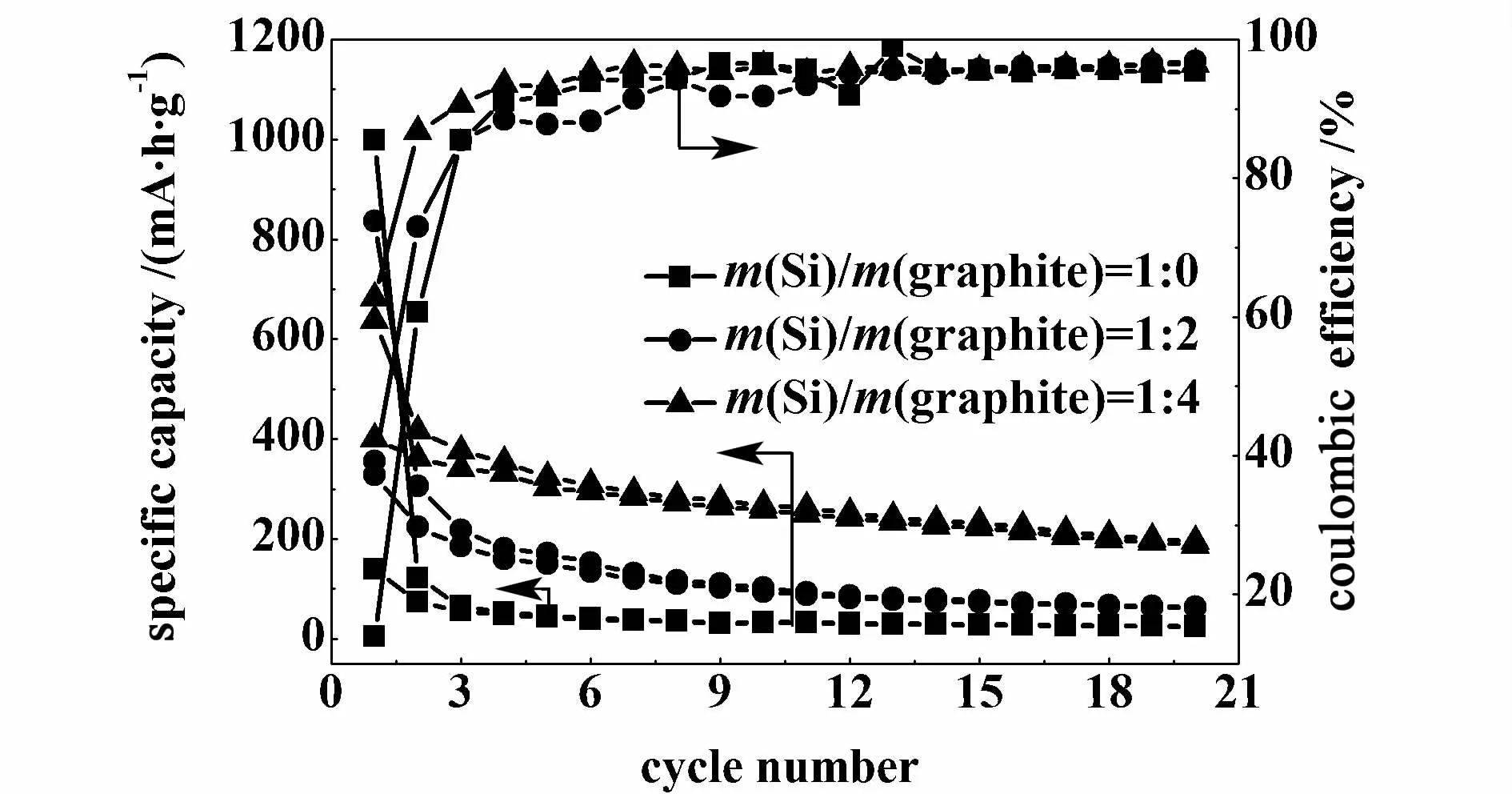
Fig.1 Effects of m(Si)/m(graphite)ratio in Si-graphite/carbon composite on charge-discharge capacities and cou lom bic efficiencies of composites
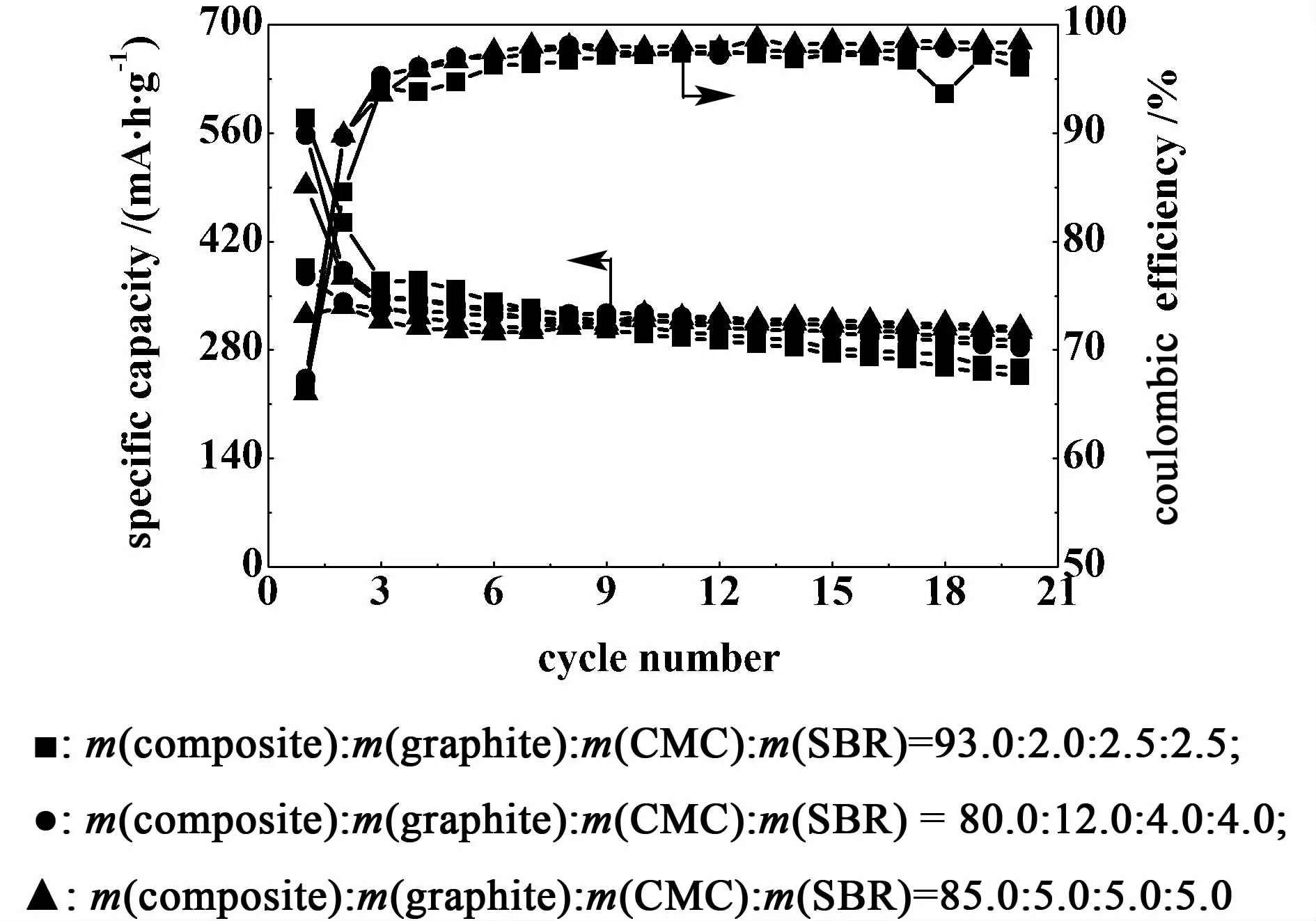
Fig.2 E ffects of the composite/graphite/CM C/SBR ratio on the charge-discharge capacities and the cou lom bic efficiencies of the working electrodes
The ratio of the composite,graphite and the binder in a working electrode is also an important parameter affecting the electrochemical performances of a working electrode.Their influences on the charge-discharge capacity and coulombic efficiency of the working electrodes were studied and the results are shown in Fig.2 .The working electrode with m(composite)/m(graphite)/m(CMC)/m(SBR)ratio of 85∶5∶5∶5 has the lowest initial reversible capacity of 325 mA·h/g with a coulombic efficiency of 86%.A fter 20 cycles,its reversible capacity remains the highest value of304 mA·h/g with a coulombic efficiency of 98%,which suggests that the electrode shows themore excellent capacity retention than the other two electrodes.Therefore,85∶5∶5∶5 is chosen as the m(composite)/m(graphite)/m(CMC)/m(SBR)ratio of working electrode during the following study.
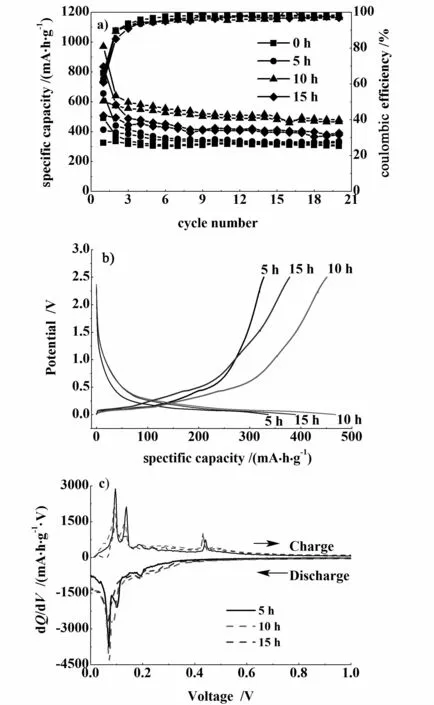
Fig.3 a)Effects of milling time on the charge-d ischarge capacities,b)charge-discharge cu rves and c)d ifferen tial capacity curves during the 20th cycles of samples milled for different times
In this section,the Si-graphite/carbon composite and conductive agent(graphite)was mechanically milled for 0,5,10 and 15 h, respectively.Then the obtained sampleswere furthermixed with binder(CMC and SBR).The effects ofmilling times on the electro-chemical performances of the working electrode are shown in Fig.3 a.The sample milled for 10 h has the largest reversible capacity and its cycle stability is comparable with the other three electrodes.In order to clarify the plateau region in Fig.3 b,differential voltage curves were plotted as shown in Fig.3 c.Itwas evident that the peaks at_0.07 V and_0.1 V during discharge and the peaks at_0.09 V and_0.13 V during charge were caused by intercalation and de-intercalation of lithium with the graphite[16-17].The peak at_0.44 V during charge can be attributed to the de-alloying process of the Li ions with the crystalline silicon[18-19].Due to an overlapping by an electrochemical respond of the graphite,a Si alloying peak around 0.04 V is absent during the discharge.
From the results of Fig.3 c,it indicates that Si and graphite in composites both contribute to the total capacity.For samples in Fig.3 ,all preparation conditions except milling times are the same.By milling method used in this study,themorphology and the particle size for nano-Si could not be changed.Therefore,it can be concluded that the difference of total capacity for the samp lesmay arise from two reasons.One is that Si and graphite were mixed more uniform ly with long ball-milling time,and another is the different crystalline structure and size of graphite with differentmilling times.In order to confirm the conclusion,SEMand XRD analysis were carried out.
Fig.4 presents SEM images of the graphite and milled composite materials for 5,10 and 15 h.The shape of the graphite is flake with an average particle size of~10μm(Fig.4 a).The composite material keeps the shape of graphite,when the powder was milled for 5 h(Fig.4 b).Most of the composite particles become potato shaped particles with the milled time is prolonged to 10 h(Fig.4 c).The potato shape of the particle is destroyed and aggregation increases when the milled time reaches 15 h(Fig.4 d).The formation of potato shaped particle in Fig.4 c is note worthy,as it has been reported that spherical particle improves the electrode performance[20].Therefore, the high capacity of sample milled for 10 h should be attributed to the potato shaped particles.

Fig.4 a)SEM images of graphite,the composite materialsmilled for b)5 h,c)10 h and d)15 h
The X-ray diffraction patterns of composites with different milling times are shown in Fig.5 .For the XRD curves of composites,it can be seen that there exists only the characteristic peaks of Si and graphite,but absent of silicon oxide and Si-C alloy diffractions.For the 5 h and 10 h-milled samples,the XRD patterns of graphite showa significant decreasing and broadening of the[002]Bragg peak at 27°, indicating that graphite is transformed into amorphous state.Upon increasing the milling time to 15 h, the[002]Bragg peak at 27°becomes broader and its intensity also decreases further,indicating increased disorder of the graphite structure[21].
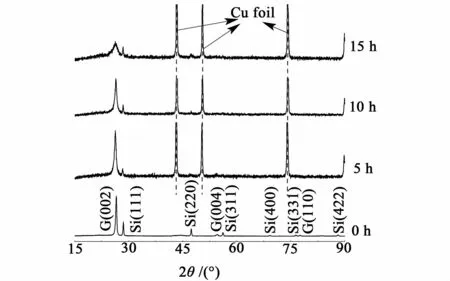
Fig.5 XRD patterns of Si-graphite/carbon composite with different milling times
For Si, the intensity of[111]peak does not change obviously as themilling time are prolonged from 5 h to 15 h,indicating that the longer milling time does not change further the crystallinity of Si[22].Therefore,results of SEMand XRD confirm further that the different crystalline structure and morphology of graphite with differentmilling times mainly contributes the difference of total capacity.
3 Conclusions
Si-graphite/carbon composites were prepared using nano-Si(~30 nm),graphite and potato starch as raw materials.The effects of preparation conditions,including m(Si)/m(graphite)ratio in composite,m(composite)/m(graphite)/m(CMC)/m(SBR)ratio and milling time during preparation of the working electrode,on the capacity and the cycling stabilities of the composites were investigated.It indicates that the composite prepared under the optimum conditions m(Si)/m(graphite)=1∶4, m(composite)/m(graphite)/m(CMC)/m(SBR)=85∶5∶5∶5, and 10 h of milling)has a reversible capacity of 466 mA·h/g with an initial coulombic efficiency of 62.3%
Under the same m(Si)/m(graphite)ratio and m(composite)/m(graphite)/m(CMC)/m(SBR)ratio,the effect of the milling time on the electrochemical performances of the composite was studied by differential capacity curves,SEMand XRD analysis.The results indicate that both Si and graphite in the composite contribute to the total capacity.The difference of the capacity comes from graphite.The total capacity of the composite can be improved further by a proper increase of Si content.
Acknow ledgment
The project supported by the National Natural Science Foundation of China(Grant No.21203258),and sharing fund of Chongqing University’s large-scale equipment(Grant No.2013121543);the Fundamental Research Funds for the Central Universities(Grant No.CQDXWL-2013-018).
[1]Zhao G,Zhang L,Meng Y,et al.Decoration of graphene with silicon nanoparticles by covalent immobilization for use as anodes in high stability lithiumion batteries[J].JPower Sources, 2013, 240:212_218
[2]Touahir L,Cheriet A,Dalla C D A,et al.Methylated silicon:A longer cycle-life material for Li-ion batteries[J].JPower Sources, 2013, 240:551_557
[3]Fan Y,Zhang Q,Xiao Q,et al.High performance lithiumion battery anodes based on carbon nanotube-silicon core-shell nanowires with controlled morphology[J].Carbon,2013,59:264_269
[4]Delpuech N, DupréN, Mazouzi D, et al.Correlation between irreversible capacity and electrolyte solvents degradation probed by NMR in Si-based negative electrode of Li-ion cell[J].Electrochem Commun, 2013,33:72_75
[5]Kim K W,Park H,Lee JG,et al.Capacity variation of carbon-coated silicon monoxide negative electrode for lithium-ion batteries[J].Electrochim Acta, 2013,103:226_230
[6]Xun S,Song X,Grass M,et al.Improved initial performance of Si nanoparticles by surface oxide reduction for lithium-ion battery app lication[J].Electrochem Solid-State Lett, 2011, 14(5):A61_A63
[7]McDowell M T,Lee SW,Ryu I,et al.Novel size and surface oxide effects in silicon nanowires as lithium battery anodes[J].Nano Lett, 2011, 11(9):4 018_4 025
[8]Song T,Xia J L,Lee JH,et al.Arrays of sealed silicon nanotubes as anodes for lithiumion batteries[J].Nano Lett, 2010, 10(5):1 710_1 716
[9]Park M H, Kim M G, Joo J, et al.Silicon nanotube battery anodes[J].Nano Lett, 2009, 9(11):3 844_3 847
[10]Wang D, Li F, Ping G, et al.Preparation and Enhanced electrochemical anode performance of Si/graphene nanocomposites[J].In JElectrochem Sci, 2013,8(7):9 618_9 628
[11]Guzman R C,Yang JH,Cheng M M,et al.A silicon nanoparticle/reduced graphene oxide composite anode with excellent nanoparticle dispersion to improve lithiumion battery performance[J].J Mater Sci, 2013, 48(14):4 823_4 833
[12]Zhang Z,Zhang M,Wang Y,et al.Amorphous siliconcarbon nanospheres synthesized by chemical vapor deposition using cheap methyltrichlorosilane as improved anode materials for Li-ion batteries[J].Nanoscale, 2013,5(12):5 384_5 389
[13]Lee E H,Jeong B O,Jeong SH,et al.Effect of carbon matrix on electrochemical performance of Si/C compos-ites for use in anodes of lithium secondary batteries[J].Bull Korean Chem Soc,2013,34(5):1 435_1 440
[14]Shin K, Park D J, Lim H S, et al.Synthesis of silicon/carbon,multi-core/shell microspheres using solution polymerization for a high performance Li ion battery[J].Electrochim Acta, 2011, 58:578_582
[15]Lai J, Guo H J, Wang Z X, et al.Preparation and characterization of flake graphite/silicon/carbon spherical composite as anodematerials for lithium-ion batteries[J].J Alloy Compd, 2012, 530:30_35
[16]Yoon Y S, Jee S H, Lee S H, et al.Nano Si-coated graphite composite anode synthesized by semi-mass production ballmilling for lithium secondary batteries[J].Surf Coat Technol, 2011, 206(2/3):553_558
[17]Jo Y N,Kim Y,Kim JS,et al.Si-Graphite composites as anode materials for lithium secondary batteries[J].J Power Sources, 2010, 195(18):6 031_6 036
[18]Chan C K, Ruffo R, Hong SS, et al.Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes[J].J Power Sources, 2009, 189(2):1 132_1 140
[19]Lee B S, Son S B, Park K M, et al.Fabrication of Si core/C shell nanofibers and their electrochemical performances as a lithium-ion battery anode[J].J Power Sources, 2012, 206:267_273
[20]Natarajan C, Fujimoto H, Mabuchi A, et al.Effect of mechanicalmilling of graphite powder on lithiumintercalation properties[J].J Power Sources, 2001, 92(1/2):187_192
[21]Gan L, Guo H J, Wang Z X, et al.A facile synthesis of graphite/silicon/graphene spherical composite anode for lithium-ion batteries[J].Electrochim Acta, 2013,104:117_123
[22]Si Q, Hanai K, Ichikawa T, et al.A high performance silicon/carbon composite anode with carbon nanofiber for lithium-ion batteries[J].J Power Sources, 2010, 195(6):1 720_1 725

