菊粉的酶法生物转化在食品中应用的研究进展
战荣荣,沐万孟,李赟高,张 涛,江 波*
(江南大学,食品科学与技术国家重点实验室,江苏 无锡 214122)
菊粉的酶法生物转化在食品中应用的研究进展
战荣荣,沐万孟,李赟高,张 涛,江 波*
(江南大学,食品科学与技术国家重点实验室,江苏 无锡 214122)
菊粉是我国分布广泛、含量丰富的重要农副产品,利用菊粉酶对其进行生物深加工可高效实现菊粉在食品领域的低成本、多方向、高附加值利用。本文综述国内外菊粉应用于食品的酶法研究开发现状,对不同类型菊粉酶生物转化产品的性质及食品应用、酶的微生物来源及菊粉酶的最新研究进展进行阐述。
内切型菊粉酶;外切型菊粉酶;菊糖果糖转移酶;低聚果糖;高果糖浆;双果糖酐
菊粉,又称菊糖,是D-呋喃果糖以β-2,1-糖苷键连结而成的多聚果糖,其还原端连接一个葡萄糖基,呈直链结构,聚合度一般在30左右。菊粉是我国种植广泛的重要农副产品,广泛存在于菊芋、菊苣、牛蒡、大丽花等多种植物的根茎中[1]。菊粉作为一种膳食纤维,由于具有类似脂肪的质构与口感,被长期作为简单的食品配料应用于食品加工[2]。然而,菊粉存在甜度低、水溶性较差等问题,进行更深层次、高附加值的生物产品开发成为充分利用我国丰富菊粉资源的新方向,也逐渐成为食品领域研究的热点。
利用微生物发酵或酶解等现代生物转化方法,菊粉有望实现多种形势的高附加值产品转化[3]。菊粉通过发酵策略可获得单细胞蛋白[4],柠檬酸[5],生物燃料如生物乙醇[6]、单细胞油[7],化学产品如丁二醇[8]、乳酸[9]及糖醇[10]等。菊粉高附加值酶法利用主要应用于食品领域,是借助生物菊粉酶生产食品行业重要的糖类或功能性糖类,其产物属于天然产品,具有适合现代人们对食品安全性、健康性及功能性等需求的特点,逐渐成为菊粉生物转化应用极其重要的方向。菊粉酶是菊粉酶法生物转化的媒介,为糖酐水解酶32家族(glycoside hydrolase family 32,GH32)酶类,按其水解菊粉的方式分为内切型菊粉酶、外切型菊粉酶及菊糖果糖转移酶3种类型[11],它们是一类作用于菊糖β-2,1-糖苷键的水解酶,可将菊糖水解为不同的单糖或低聚糖。现阶段发现的微生物菊粉酶多来源于霉菌、酵母菌和部分细菌[12],分布于胞外、胞内及胞壁中,各种微生物的产酶类型、酶活性及性质存在较大差异,且以产外切型菊粉酶微生物最多[13],更有微生物可以同时产多种类型菊粉酶。菊粉酶通常以I/S的大小来区分内切型菊粉酶和外切型菊粉酶,I、S分别是以菊粉、蔗糖作为底物时的酶活力,一般认为当I/S<10时为外切菊粉酶,I/S≥10时为内切菊粉酶[14],而菊糖果糖转移酶通常利用高效液相色谱对酶反应产物双果糖酐进行检测鉴定[11]。菊粉酶的纯化手段一般结合超滤、硫酸铵分段沉淀、离子交换和Sephadex凝胶过滤等,并利用HPLC进行产物分析。对于内、外切混合酶的分离一般采用NaCl线性梯度洗脱进行[15]。利用不同类型的菊粉作底物,菊糖内切型、外切型及转移酶的主要转化产物分别为低聚果糖、果糖和双果糖酐,并逐渐成为这些糖工业化生产的重要方式。需要指出的是,新型菊粉酶作为利用菊粉进行功能性糖——双果糖酐Ⅲ生产的重要方式,为菊粉在食品领域的应用提供最新研究方向,也为菊粉的酶学生物转化提供更广阔的开发空间,如下将以不同类型菊粉酶对菊粉在国内外食品领域的酶法研究及应用进展进行综述。
1 内切型菊粉酶——低聚果糖
内切型菊粉酶可随机断开菊粉链内部的β-1,2-糖苷键,主要水解产物为低聚果糖,是菊粉食品应用中已商业化的重要开发形式,成为低聚果糖工业化生产的重要工艺。从20世纪80年代初日本及欧美等发达国家开始利用微生物菊粉酶水解菊芋制取果糖,到目前对菊粉低聚糖生产的研究仍然十分火热。
1.1 低聚果糖的性质及食品应用
低聚果糖(inulooligosaccharides,IOS)为由2~10个果糖组成的低聚化合物,又称果寡糖。IOS具有抗肿瘤和刺激双歧杆菌生长的作用,也可作为膳食纤维用于防治糖尿病和减肥,还可用于改善肠胃功能降低血脂和预防高胆固醇等。在食品中具有广泛应用,常被用于糖果、水果制品、牛奶制品、酸奶、鲜奶酪、烘焙食品、冰激凌和调味料等[16]。
1.2 IOS生产现状及内切型菊粉酶生产IOS
利用微生物产内切型菊粉酶作用于菊粉可获得较高纯度的IOS,其副产物仅有少量果糖,逐渐取代以蔗糖为原料利用果糖基转移酶酶解制备低聚果糖(fructose oligosaccharides,FOS)的工艺,并成为现阶段IOS工业化生产的重要工艺。菊粉IOS的优良酶法生产工艺首先对微生物具有较高要求,是微生物产内切型菊粉酶在无外切酶及转移酶存在时对菊粉进行的转化方式,因此优良的内切型菊粉酶微生物资源对IOS的生产具有重要意义。能产生内切型菊粉酶的微生物有真菌如青霉属、曲霉属,酵母如克鲁威属、念珠菌属,细菌如杆菌属、假单胞菌属等[17],如表1所示。目前为止不同来源的菊粉资源,利用内切型菊粉酶固定化及现代分离纯化手段可实现70%~95%的IOS转化率。Nguyen等[18]通过壳聚糖固定化菊粉内切酶实现了菊芋汁固定床反应器的持续性发酵,其固定化酶的半衰期达48 d,IOS产率为66%。此外,菊粉底物的反应形式对IOS产量也有较为重要的影响。Jin Zhenyu等[28]发现,A. ficuum菌株所产菊粉内切酶利用菊芋汁的能力优于菊芋粉,可使IOS产量由50%增至80%。
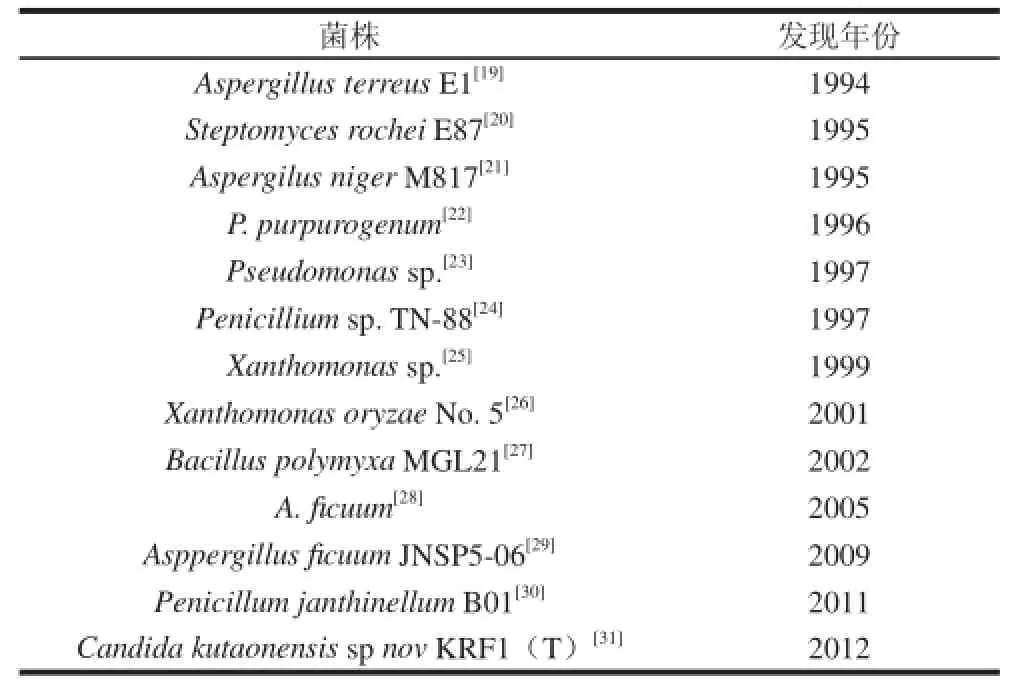
表1 生产IOS的内切菊粉酶微生物来源Table 1 Biochemical properties of endo-inulinase derived from various microorganniissmmss
1.3 内切型菊粉酶的分子水平研究现状
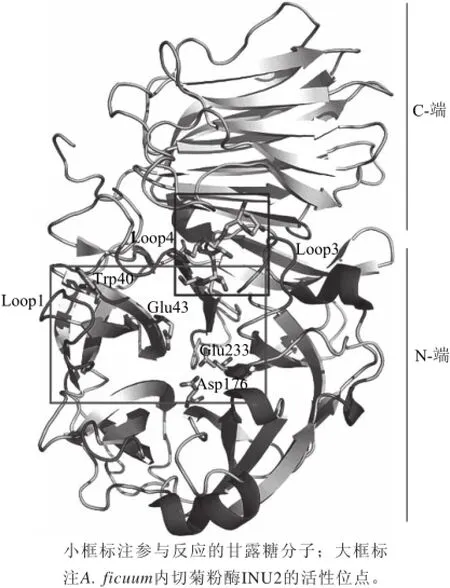
图1 A.ficuum菌株菊粉内切酶INU2的晶体结构[[3344Fig.1 The crystal structure of endo-inulinase INU2 from A. fi cuum[34]
野生型微生物菊粉内切酶产量及酶活力等都相对较低,单一产内切型菊粉酶的微生物较少,使得酶制剂的纯化工艺较难、生产成本较高,借助现代分子生物学技术构建因工程菌株、通过深入了解酶的结构及作用机制进行分子水平的基因改造等成为解决这一问题的有效途径,也成为现在内切型菊粉酶的研究重心和热点。Yun等[32]将Pseudomonas sp.内切菊粉酶基因进行原核表达使IOS的产率为78%,此后利用聚苯乙烯固定化及生物反应器等技术使菊粉内切酶50℃稳定工作17 d,IOS的产量达150 g/(L·h)。Kim等[33]先后探索了Arthrobacter sp.S37菌株菊粉内切酶基因EnIA的作用机制,E323、E519及D460是活性中心的关键氨基酸,E323及E519为亲核基团,而D460为酶催化的必需基团,并严重影响该酶的pH值特性。2012年Pouyez[34]首次将内切型菊粉酶INU2(Aspergillus ficuum)晶体结构成功解析,如图1所示,其晶体结构的催化中心比其他GH32水解酶类多一个催化反应口袋,这个口袋有两个loop环和一段W-M(I)-N-D(E)-P-N-G保守序列构成,这个额外的反应口袋可能是形成内切菊粉酶活性的关键,也可有效解释Trp40对其酶活具有重要作用及菊粉底物裂解机制。
2 外切型菊粉酶——高果糖浆
外切菊粉酶可作用于菊粉链非还原性末端的糖苷键,逐一水解释放出果糖,是工业化生产高果糖浆的重要生产方式;高果糖浆(high-fructose syrup,HFS)是果糖含量高达90%的第3代果葡糖浆产品,目前在美、日等国,果葡糖浆已成为最重要的甜味剂之一,并且其生产发展势头强劲,已越来越受到国内外的重视与欢迎[35]。
2.1 HFS的性质及食品应用
果糖是一种天然营养甜味剂,甜度为蔗糖的1.8倍,为山梨醇的1.5倍,其甜度高、热值低、具有类似蜂蜜的良好风味、结构组成等类似于蔗糖等特性使其成为优秀的果汁甜味剂和低热值食品原料。果葡糖浆是工业化生成果糖时的混合产物,因生产原料及工艺方法不同果糖含量在40%~90%,此外还含有葡萄糖及低聚糖等成分。HFS作为高浓度果糖类强化甜味剂,具有高渗透压、强保湿性、高发酵性、低冰点、甜味纯正和保健性等多种特性,被广泛应用于食品焙烤、饮料制品和食品配料等食品领域,成为蔗糖极具潜力的替代性糖产品之一。
2.2 HFS生产现状及外切型菊粉酶生产HFS
HFS的制备策略较多,主要包括以菊糖、玉米淀粉、葡萄糖和蔗糖为原料的酶法和化学法。美国、日本和我国HFS的传统工业化制备工艺是以玉米淀粉为原料利用异构酶酶法制备,然而该生产工艺复杂、生产成本也较高,成为HFS工业化生产及食品应用的瓶颈问题。20世纪70年代以后,各国开始关注以菊粉为原料,酸法和酶法水解制备果糖和高果糖浆的生产工艺。其中酸法制备高果糖浆果糖纯度较高,但副产物多、色素重、分离精制难,依然不能满足HFS的食品应用需求。而以菊粉为原料的酶法生产工艺为HFS的工业化生产及食品应用带来曙光。依靠外切型菊粉酶的HFS生产被称为一步法催化反应,它以其独特的工艺简单、成本低、得率高而备受青睐,逐渐成为国外HFS的主流生产工艺,外切型菊粉酶微生物来源如表2所示。Sirisansaneeyakul等[51]利用A. niger TISTR 3570(可产内切型及外切型菊粉酶)和Candida guilliermondii TISTR 5844菌株(仅产外切型菊粉酶)的菊粉酶混合物降解菊粉,25 h获得18.2 g/L的果糖产品。Singh等[36]利用K. marxianus YS-1分泌的菊粉内切酶固定化于杜奥莱A568后利用菊粉生产HFS,结果表明菊粉的纯度对HFS的产量影响不大,4 h产量达40 g/L左右。

表2 生产HPS的外切菊粉酶微生物来源Table 2 Biochemical properties of exo-inulinase derived from various microorganniissmmss
2.3 外切型菊粉酶的分子水平研究现状
随着菊粉外切酶的工业化发酵工艺日趋成熟,逐渐迎来了菊粉外切酶分子及原子水平的研究及机理研究的高潮。菊粉外切酶的基因INU1首次于1991年由Laloux等[55]从Kluyveromyces marxianus中克隆获得,此后Gao Wei[53]、Liu Bin等[56]又从Bacillus smithii T7中成功克隆INU1基因,并利用化学修饰研究证实其酶的活性中心必定包含2个Trp和一个His残基,组氨酸是酶与底物结合的关键位点,而色氨酸对其热稳定性有重要的作用。此后Cao Tianshu等[54]又克隆获得Cryptococcus aureus HYA的INU1基因,并利用pPICZαA载体在毕赤酵母X-33中成功表达。Emanuele等[52]利用动力学对其作用机制进行了研究,以工业化需求对进行动力学模型的温度及底物浓度进行定义(分别为40~60℃和3~60 g/L),获得产物果糖和菊糖底物的化学计量关系。目前,外切菊粉酶晶体结构已获得解析,如Aspergillus awamori[57]及Geobacillus srearothermophilus SK1289p[47]。Aspergillus awamori菊粉外切酶是一种可连接5个寡糖的糖蛋白酶,如图2所示,其催化区三级结构折叠为两个区域:独特的5片β-propeller折叠构成的N端催化区和折叠为β-sandwich结构的C端催化区,从催化中心侧链残基距离推测该酶遵循双位移机制,Asp41及Glu241分别作为亲核基团和催化基团,而Asp189通过氢键与底物作用,是重要的底物识别基团。
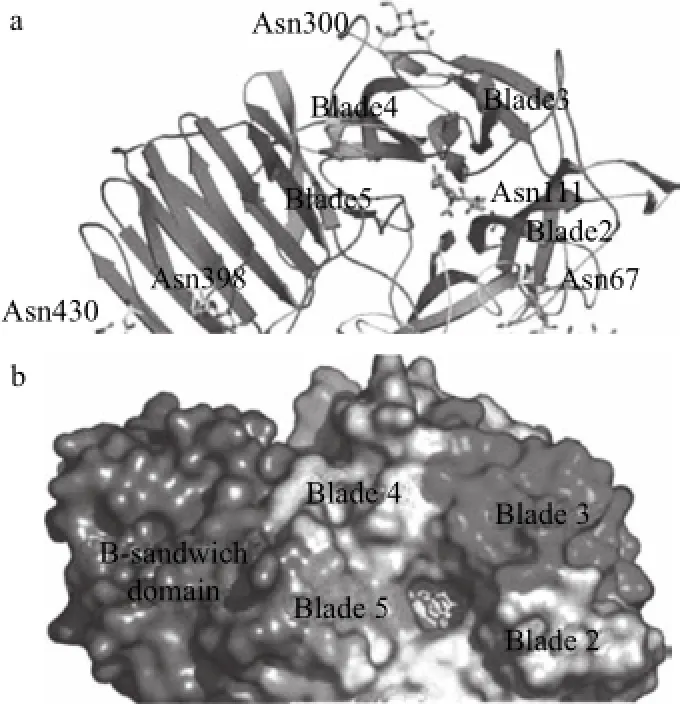
图2 Aspergillus awamori 菊粉外切酶二级晶体结构[[5577Fig.2 The secondary structure elements of exo-inulinase from Aspergillus awamori[57]
3 菊糖果糖转移酶——双果糖酐Ⅲ
菊糖果糖转移酶(inulin fructotransferase,IFTase)又称新型菊糖酶,是一种新型的菊粉水解酶类,可从菊糖非还原末端以相邻2个果糖基为单位水解菊糖糖苷键,同时伴随分子内转果糖基反应产生双果糖酐。利用IFTase生产双果糖酐的研究主要集中在日本和韩国,作为菊粉在食品应用的新型转化方式,现阶段主要应用于生物转化开发双果糖酐Ⅲ(difructose anhydride Ⅲ,DFAⅢ),目前已有日本甜菜制糖株式会社2009年采用北海道大学Arthrobacter sp. H65-7发酵产生IFTase[58],已实现工业化生产DFAⅢ。然而,目前国内的研究仅本课题组赵萌[11]筛选获得高产新菌株,并实现高效原核表达。随后,杭华[59-61]通过中试扩大化酶膜反应器利用IFTase进行DFAⅢ的应用性生产研究,实现400 g/L DFAⅢ的超高浓度生产,为利用菊粉工业化生产DFAⅢ提供了极为重要的探索性研究。
3.1 DFAⅢ性质、食品应用及IFTase酶法生产
双果糖酐又称二果糖二酐,是由两个果糖基组成的一类环状二糖,其中两个残基的还原性末端和相对残基的非还原性羟基连接,存在16种同分异构体[62]。通过微生物菊粉酶中的菊糖果糖转移酶转化菊糖可以合成双果糖酐Ⅰ[63]、Ⅲ,而其他同分异构体至今仍难以获得。到目前为止,发现产双果糖酐I的菌株仍然较少,国际产IFTase的菌株主要集中在日本和韩国。日本食品国家研究所Haraguchi先后发现4株产IFTase菌株可用于生产DFAⅢ[80,82,84],而现阶段最高酶活力是由江南大学Zhao Meng等[64]发现的A. aurescens SK8.001菌株经原核表达实现,该菌株可实现胞内胞外同时产酶,最高胞内酶活力达119 U/mL,胞外酶活力也高达81 U/mL,这一研究成果有望为国内外深化利用IFTase进行菊糖的DFAIII工业化生产提供可能。
DFAⅢ是近年来人们发现的一种新型天然功能性甜味剂,具有促进Ca、Fe、Mg、Zn、Cu等矿物质元素的吸收、增进骨骼生长[65]、利于排尿、改善便秘[66]、预防结肠直肠癌[67]及抑制蛀牙[68]等功能,同时其甜度较高、能量值低,具有良好的耐热、耐酸等理化特性,具有成为传统甜味剂蔗糖替代品的潜能。作为无糖或低糖食品的关键配料,适用于焙烤食品、饮料、糖果等食品领域,促使其逐渐成为菊粉资源食品开发的重点和热点方向。
3.2 IFTase生产DFAⅢ的微生物来源及分子水平研究现状
利用菊粉酶法合成DFAⅢ是利用微生物IFTase水解菊粉获得以DFAⅢ为主要产物的酶解策略,符合现代人们对食品领域天然产品的追求,也可有效实现菊粉的高附加值生物转化。此外,利用菊粉还可以通过化学合成实现DFAⅢ的制备(或利用果糖、低聚果糖、果糖多糖原料等[69-70]),然而化学合成过程会产生多种双果糖酐,且组分复杂,使DFAⅢ的分离纯化困难,产量较低。而酶法合成DFAⅢ的副产物只有少量低聚果糖,可全面有效的解决化学法合成DFAⅢ所存在的问题,成为利用资源丰富的菊粉资源进行DFAⅢ工业化生产的最佳途径,同时也对食品行业新糖源的开发具有重要意义。
酶解菊粉生产DFAⅢ的转化效率与IFTase的酶学性质有直接关系。不同菌株来源的IFTase的基因序列具有高度相似性,但酶活力、最适作用条件、分子质量等具有较大差异,表3给出了迄今为止发现的所有可产DFAⅢ的微生物IFTase性质。自1972年Tanaka[54]首次于产脲节杆菌中发酵生产新型菊粉酶可将菊糖降解为DFAⅢ和少量的低聚果糖以来,此后陆续有13种产DFAⅢ的野生型菌株被报道,至今只有Jung等[71]解析了IFTase三聚体晶体结构,如图3所示,并提出了分子内果糖基转移反应机制,如图4所示,IFTase三聚体结构形成仅可容纳一个二糖的底物结合口袋,为酶催化的前提条件,其活性位点分布在单体间的界面,并有6个直接的作用点,4个位于F1,2个位于F2,果糖基F1上的O-1’分别与Arg292、Ser133侧链结合,O-3’与Glu244羧基结合,O-’与Pro291结合;Asp233*位于另一个亚基上,Asp233*同时与果糖基F2上的O-3、O-4结合;Arg174*胍基与Asp233*羰基相互作用,可稳定Asp233*的定位;另外果糖基F1 O-4'及果糖基F2 O-6分别通过溶剂介导的氢键与Tyr197、Gln313结合。末端果糖基F1及相邻果糖基F2在催化过程中分别作为电子供体、电子受体;Glu244的羧基进攻F1上的3-OH使其去质子化,从而激活受体果糖基F2;Asp233通过氢键为F2定位,使得去质子化的F1 3-O更有效地亲核进攻F2 2-C,最终使得果二糖从菊糖上脱离。而其他可能存在的作用机理如活性位点残基的鉴定及作用机制仍不够清晰,这表明IFTase利用菊粉进行DFAⅢ的研究仍任重道远,许多基础性研究仍亟待开展。
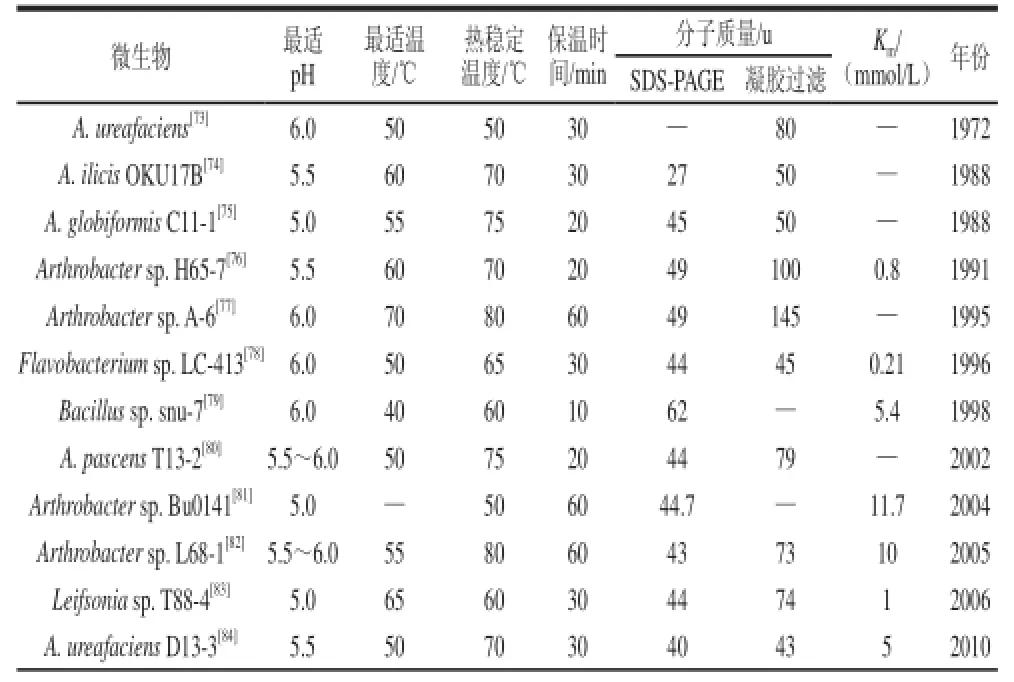
表3 利用菊粉生产DFAⅢ的IFTase的性质及微生物来源Table 3 Biochemical and enzymatic properties of inulin fructotransferases (producing DFAⅢ)from various microorganisms
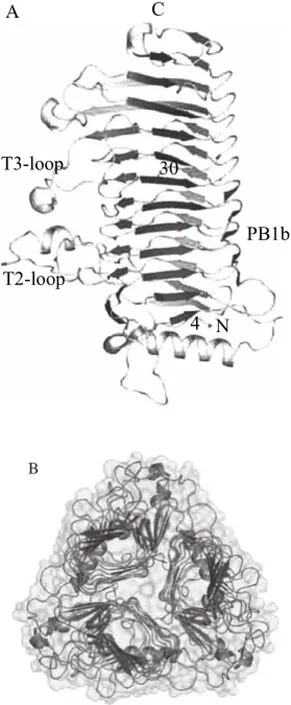
图3 Bacillus sp. 菊糖果糖转移酶的亚基结构(A)与晶体结构(B)Fig.3 Subunit structure (A) and crystal structure (B) of inulin fructotransferase from Bacillus sp
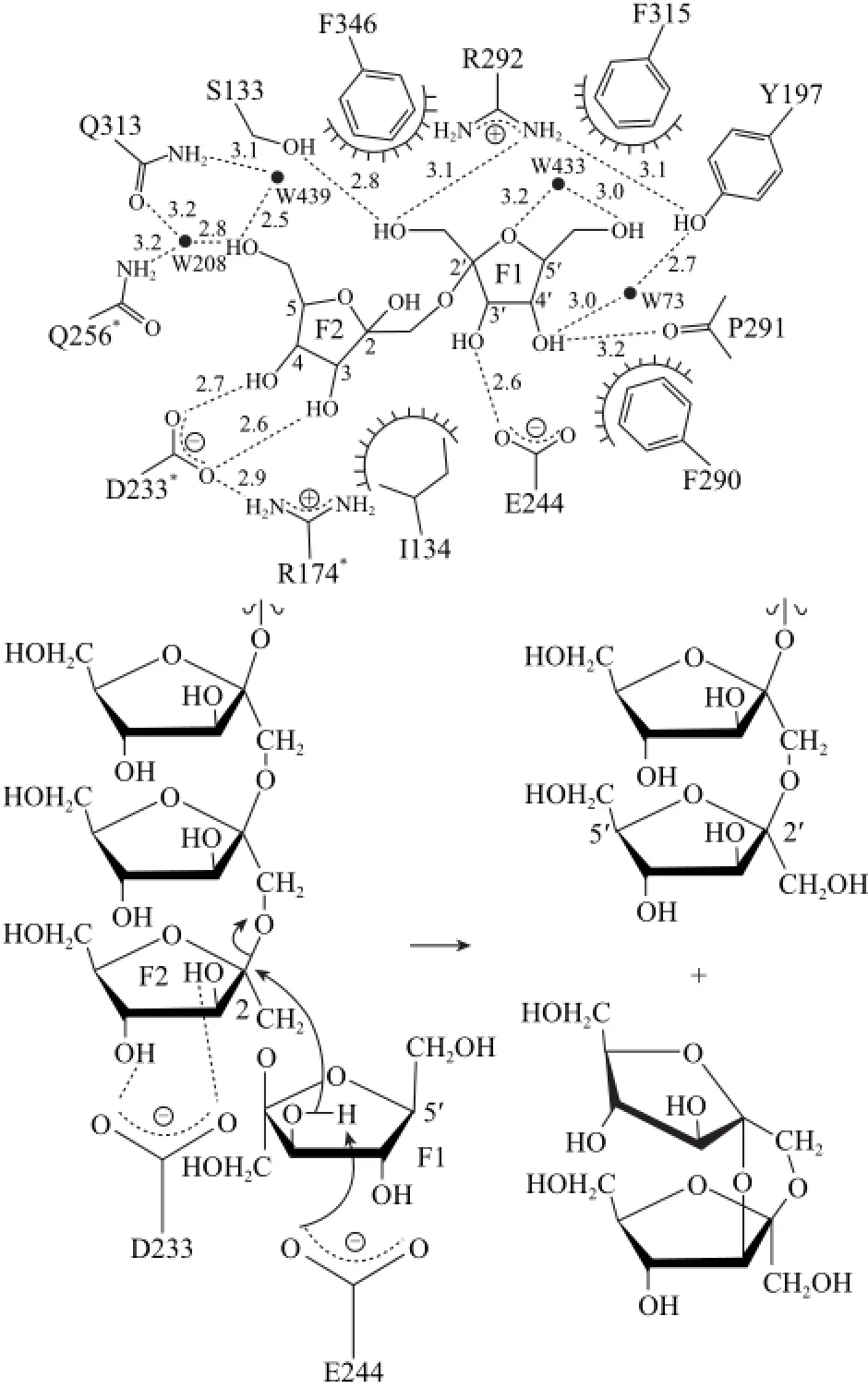
图4 4Bacillus sp. 菊糖果糖转移酶分子内果糖基转移反应示意Fig.4 Proposed catalytic mechanisms of inulin fructotransferase from Bacillus sp
此外,一些微生物IFTase具有降解菊糖为DFAI的活性,如Arthrobacter golobiformis S14-3所产IFTase(可产DFAIII)为胞外酶,且以单聚体形式存在,与产DFAⅢ的IFTase具有较高同源性[72]。DFAⅠ作为非还原性糖,甜度为蔗糖的一半,是一种低热量糖,现阶段研究较少,也可作为食品领域菊粉酶法转化的应用方向。
4 展 望
作为可再生资源的菊粉在食品应用中的高附加值开发应用研究仍处于初级阶段。随着不同微生物菊粉酶的开发,在食品中具有重要功能的IOS益生元和HFS甜味剂产品的研究已有较好基础,工业化应用也日趋成熟,新型菊粉酶应用于功能性甜味剂DFAⅢ的生产作为新的菊粉开发形式,进一步拓宽了菊粉在食品领域的应用,这也有望成为食品研究的新热点。此外,为实现菊粉在食品中的酶法高效转化,除却各种菊粉酶菌株的继续发掘外,以提高酶学性质及产量为目的的各种现有野生型菌株的菊粉酶结构、作用机理及分子水平的基因改造成为新的研究热点,这也必将为IOS、HFS及DFAⅢ等食品糖类的规模化生产提供更深入的理论支撑。而利用超滤或纳滤等现代酶膜反应器[85]进行新的酶底物反应模式研究,以及固定化技术、现代纯化手段等可以有效增进产物转化效率、降低能耗,也有望成为酶制剂的重要发展方向,这也必将对食品领域菊粉的酶法生物转化提供更为广阔的前景和发展空间。
[1] PANDEY A, SOCCOL C R, SELVAKUMAR P, et al. Recent developments in microbial inulinases, its production, properties and industrial applications[J]. Applied Biochemistry and Biotechnology, 1999, 81: 35-52.
[2] 王珊珊, 孙爱东, 何洪巨. 菊粉的功能性作用及开发利用[J]. 中国食物与营养, 2009, 15(11): 57-59.
[3] CHI Zhenming, ZHANG Tong, CAO Tianshu, et al. Biotechnological potential of inulin for bioprocesses[J]. Bioresource Technology, 2011, 102: 4295-4303.
[4] ZHAO Chunhai, CHI Zhe, ZHANG Fang, et al. Direct conversion of inulin and extract of tubers of jerusalem artichoke into single cell oil by co-cultures of Rhodotorula mucilaginosa TJY15a and immobilized inulinase-producing yeast cells[J]. Bioresource Technology, 2011, 102(10): 6128-6133.
[5] LIU Xiaoyan, CHI Zhe, LIU Guanglei, et al. Inulin hydrolysis and citric acid production from inulin using the surface-engineered Yarrowia lipolytica displaying inulinase[J]. Metabolic Engineering, 2010, 12(5): 469-476.
[6] HU Nan, YUAN Bo, SUN Juan, et al. Thermotolerant Kluyveromyces marxianus and Saccharomyces cerevisiae strains representing protentials for bioethanol production from Jerusalem artichoke by consolidated bioprocessing[J]. Applied Microbiology and Biotechnology, 2012, 95(5): 1359-1368.
[7] ZHAO Chunhai, CUI Wei, LIU Xiaoyan, et al. Expression of inulinase gene in the oleaginous yeast Yarrowia lipolytica and single cell oil production from inulin-containing materials[J]. Metabolic Engineering, 2010, 12(6): 510-517.
[8] GAO Jian, XU Hong, LI Qiujie, et al. Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R,R-2,3-butanediol[J]. Bioresource Technology, 2010, 101(18): 7076-7082.
[9] CHOI H Y, RYU H K, PARK K M, et al. Direct lactic acid fermentation of Jerusalem artichoke tuber extract using Lactobacillus paracasei without acidic or enzymatic inulin hydrolysis[J]. Bioresource Technology, 2012, 114: 745-747.
[10] SAHA B C. Production of mannitol from inulin by simultaneous enzymatic saccharification and fermentation with Lactobacillus intermedius NRRL B-3693[J]. Enzyme Microbial Technology, 2006, 39: 991-995.
[11] 赵萌. 菊糖果糖转移酶的菌种筛选、诱导合成、分离纯化及克隆表达[D]. 无锡: 江南大学, 2011.
[12] KANGO N, JAIN S C. Production and properties of microbial inulinases: recent advances[J]. Food Biotechnology, 2011, 25: 165-212.
[13] CABEZAS M J C, BRAVO R S, SHENE C. Inulin and sugar contents in Helianthus tuberosus and Cichorium intybus tubers: effect of postharvest storage temperature[J]. Journal of Food Science, 2002, 67: 2860-3865.
[14] 彭英云. Aspergillus fi cuum SK004产外切菊粉酶及其酶解菊粉制备高果糖浆的研究[D]. 无锡: 江南大学, 2005.
[15] 华成伟, 王建华, 滕达. 菊粉化学和微生物菊粉内切酶研究进展[J].中国食品学报, 2004, 4(4): 103-108.
[16] KAUR N, GUPTA A K. Applications of inulin and oligofructose in health and nutrition[J]. Journal of Biosciences, 2002, 27: 703-714.
[17] CHI Zhengming, CHI Zhe, ZHANG Tong, et al. Inulinase expressing microorganisms and applications of inulinases[J]. Applied Microbiology and Biotechnology, 2009, 82(2): 211-220.
[18] NGUYEN Q D, REZESSY-SZABO J M, CZUKOR B. Continuous production of oligofructose syrup from Jerusalem artichoke juice by immobilized endo-inulinase[J]. Process Biochemistry, 2011, 46(1): 298-303.
[19] 白春阳, 苏文金. 土曲霉金色变种AT8951菊粉酶的纯化和性质的研究[J]. 真菌学报, 1994, 13(4): 282-289.
[20] YOKOTA A, TAMAUCHI O. Production of inulotriose from inulin by inulin-degrading enzyme from Strepttomyyces rochei E87[J]. Letters in Applied Microbiology, 1995, 21: 330-333.
[21] NAKAMURA T, OGATA Y, SHITARA A, et al. Continuous production of fructose syrups from inulin by immol/Lobi-lized inulinase from Aspergillus nigermutant 817[J]. Journal of Fermentation and Bioengineering, 1995, 80: 164-169.
[22] ONODERA S, MURAKAMI T, ITO H, et al. Molecular cloningand nucleotide sequences of cDNA and gene encoding endo-inulinuase from Penicillium purpurogenum[J]. Bioscience Biotechnology and Biochemistry, 1996, 60: 1780-1785.
[23] YUN J W, KIM D H, KIM B W, et al. Production of inulooligosaccharides from inulin by immobilized endoinulinase from Pseudomonas sp.[J]. Journal of Fermentation and Bioengineering, 1997, 84: 369-371.
[24] NAKAMURA T, SHITARA A, MATSUDA S. Production, purification and properties of an endo-inulinase of Penicillium sp. TN-88 that liberates inulinase[J]. Journal of Fermentation and Bioengineering, 1997, 84(4): 313-318.
[25] PARK J P, BAE J T, YOU D J, et al. Production of inulooligosaccharides from inulin by a novel endoinulinase from Xanthomonas sp.[J]. Biotechnology Letter, 1999, 21: 1043-1046.
[26] CHO Y J, SINHA J, PARK J P, et al. Production of inulooligosaccharides from chicory extract by endoinulinase from Xanthomonas oryzae No. 5[J]. Enzyme and Microbial Technology, 2001, 28: 439-445.
[27] JEON S J, YOU D J, KWON H J. Cloning and characterization of cycloinulooligosaccharide fructanotransferase (CFTase) from Bacillus polymyxa MGL21[J]. Journal of Microbiology and Biotechnology, 2002, 12(6): 921-928.
[28] JIN Zhengyu, WANG Jing, JIANG Bo, et al. Production of inulooligosaccharides by endoinulinases from Aspergillus fi cuum[J]. Food Research International, 2005, 38(3): 301-308.
[29] CHEN Hanqing, CHEN Xiaoming, LI Yin. Purification and characterization of exo-and endo-inulinase from Aapergillus ficuum JNSP5-06[J]. Food Chemistry, 2009, 115(4): 1206-1212.
[30] WANG L, HUANG Y, LONG X. Cloning of exoinulinase gene from Penicillium janthinellum strain B01 and its high-level expression in Pichia pastoris[J]. Journal of Applied Microbiology, 2011, 111(6): 1371-1380.
[31] YUAN Bo, HU Nan, SUN Juan. Purification and characterization of a novel extracellular inulinase from a new yeast species Candida kutaonensis sp. nov KRF1(T)[J]. Applied Microbiology and Biotechnology, 2012, 96(6): 1517-1526.
[32] YUN J W, CHOI Y J, SONG C H, et al. Microbial production of inulooligosaccharides by an endoinulinase from Pseudomonas sp. expressed in Escherichia coli[J]. Journal of Bioscience and Bioengineering, 1999, 81: 291-295.
[33] KIM K Y, NASCIMENTO A S, GOLUBEV A. Catalytic mechanism of inulinase from Arthrobacter sp. S37[J]. Biochemical and Biophysical Research Communications, 2008, 371(4): 600-605.
[34] POUYEZ J, MAYARD A, VANDAMME A M, et al. First crystal structure of an endo-inulinase, INU2, from Aspergillus ficuum: discovery of an extra-pocket in the catalytic domain responsible for its endo-activity[J]. Biochimie, 2012, 94: 2423-2430.
[35] 王建华, 徐长警. 菊粉果糖的研究与开发[J]. 中国甜菜糖业, 2004(4): 10-14.
[36] SINGH R S, DHALIWAL R, PURI M. Production of high fructose syrup from Asparagus inulin using immobilized exoinulinase from Kluyveromyces marxianus YS-1[J]. Journal of Industrial Microbiology and Biotechnology, 2007, 34: 649-655.
[37] GUIRAUD J P. Inulin hydrolysis by an immol/Lobilized yeast-cell reactor[J]. Enzyme and Microbial Technology, 1983, 5: 185-190.
[38] MUKHERJEE K, SENGUPTA S. Purification and properties of a nonspecific belta-fructofuranosidase (inulinase) from the mushroom Panaeolus papillonaceus[J]. Canadian Journal of Microbiology, 1987, 33: 520-524.
[39] ROUWENHHORST R J, HENSING M, VERBAKEL J, et al. Structure and properties of the extracellular inulinase of Kluyveromyces marxianus CBS6556[J]. Applied Environmental Microbiology, 1990, 56: 3337-3345.
[40] AZHARI R, ALADA M S, EHUD I, et al. Purification and characterization of endo and exo-inulinase[J]. Biotechnology and Applied Biochemistry, 1989, 11: 105-117.
[41] KIM C H, RHEEh S K. Fructose production from Jerusalem artichoke by inulinase immobilized on chitin[J]. Biotechnology Letters, 1989, 11: 201-206.
[42] ETTALIBI M, BARATTI J C. Molecular and kinetic properties of Aspergillus ficuuminulinases[J]. Agricultural and Biological Chemistry, 1990, 54(1): 61-68
[43] ONODERA S, SHIOMI. Purification and subsite affinities of exoinulinase from Penicillium trebinskii[J]. Bioscience Biotechnology and Biochemistry, 1992, 56: 1443-1447.
[44] NAKAMURA T, OGATA Y, SHITARA A, et al. Continuous production of fructose syrups from inulin by immobilized inulinase from Aspergillus niger mutant 817[J]. Journal of Fermentation and Bioengineering, 1995, 80: 164-169.
[45] SCHORR G S, FONTANA A GUIRAUD JP. Fructose syrups and ethanol production by selective fermentation of inulin[J]. Current Microbiology, 1995, 30: 325-330.
[46] 魏文铃, 郑中辉, 郑志成, 等. 克鲁维酵母Y-85合成菊粉酶最适条件的研究[J]. 微生物学报, 1998, 38(3): 208-212.
[47] TSUJIMOTO Y, WATANABE A, NAKANO K. Gene cloning, expression, and crystallization of thermostable exo-inulinase from Geobacillus stearothermophilus KP1289[J]. Applied Microbiology and Biotechnology, 2003, 62(2/3): 180-185.
[48] KIM K Y, KOO B S, JO D. Cloning, expression, and purification of exoinulinase from Bacillus sp. sun-7[J]. Journal of Microbiology and Biotechnology, 2004, 14(2): 344-349.
[49] GILL P K, MANHAS R K, SINGH P. Comparative analysis of thermostability of extracellular inulinase activity from Aspergillus fumigatus with commercially available (Novozyme) inulinase[J]. Bioresource Technology, 2006, 97: 355-358.
[50] SINGH R S, DHALIWAL R, PURI M. Partial purification and characterization of exoinulinase from Kluyveromyces marxianus YS-1 for preparation of highfructose syrup[J]. Journal of Microbiology and Biotechnology, 2007, 17: 733-738.
[51] SIRISANSANEEYAKUL S, WORAWUTHIYANAN N, VANICHSRIRATANA W, et al. Production of fructose from inulin using mixed inulinases from Aspergillus niger and Candida guilliermondii[J]. World Journal of Microbiology and Biotechnology, 2007, 23: 543-552.
[52] EMANUELE R, VINCENZA C, STEFANO C. Fructose production by chicory inulin enzymatic hydrolysis: a kinetic study and reaction mechanism[J]. Process Biochemistry, 2009, 44: 466-470.
[53] GAO Wei, BAO Yongming, LIU Yang. Characterization of thermostable endoinulinase from a new strain Bacillus smthii T7[J]. Applied Biochemistry and Biotechnology, 2009, 157(3): 498-506.
[54] CAO Tianshu, WANG Guangyuan, CHI Zhen, et al. Cloning, characterization and heterelogous expression of the INU1 gene from Cryptococcus aureus HYA[J]. Gene, 2013, 516: 255-262.
[55] LALOUX O, CASSART J P, DELOUR J, et al. Cloning and sequencing of the inulinuase gene of Kluyveromyces marxianusvar. Marxianus ATCC 12424[J]. FEBS Letters, 1991, 289: 64-68.
[56] LIU Bin, WANG Jingyun, BAO Yongming. Characterization of key amino acid residues in active site of inulinase from Bacillus smithii T7 by chemical modification[J]. Chinese Journal of Catalysis, 2009, 30(7): 673-678.
[57] NAGEM R A P. ROJAS A L, GOLUBEV A M. Crystal structure of exo-inulinase from Aspergillus awamori: the enzyme fold and structural determinants of substrate recognition[J]. Journal of Molecular Biology, 2004, 344(2): 471-480.
[58] KIKUCHI H, INOUE M, SAITO, et al. Industrial production of difructose anhydride III (DFA III) from crude inulin extracted from chicory roots using Arthrobacter sp. H65-7 fructosyltransferase[J]. Journal of Bioscience and Bioengineering, 2009, 107(3): 262-265.
[59] 杭华. 菊糖果糖转移酶-模耦合反应器制备双果糖酐III的研究[D].无锡: 江南大学, 2012.
[60] HANG Hua, MU Wanmeng, JIANG Bo. Enzymatic hydrolysis of inulin in a bioreactor coupled with an ultrafiltration membrane[J]. Desalination, 2012, 284: 309-315.
[61] HANG Hua, MU Wanmeng, JIANG Bo. DFAIII production from inulin with inulin fructotransferase in ultrafiltration membrane bioreactor[J]. Journal of Bioscience and Bioengineering, 2012, 113(1): 55-57.
[62] MELLET C, FERNANDEZ J. Difructose dianhydrides (DFAs) and DFA-enriched products as functional foods[J]. Topics in Current Chemistry, 2010, 294: 49-77.
[63] UEDA M, SASHIDA R, MORIMOTO Y, et al. Purification of inulin fructotransferase(DFA I-producing) from Arthrobacter sp. MCI2493 and production of DFAI from inulin by the enzyme[J]. Bioscience Biotechnology and Biochemistry, 1994, 58(3): 574-575.
[64] ZHAO Meng, MU Wanmeng, JIANG Bo, et al. Purification and characterization of inulin fructotransferase (DFA III-forming) from Arthrobacter aurescens SK 8.001[J]. Bioresource Technology, 2011, 102: 1757-1764.
[65] TOMITA K, SHIOMI T, OKUHARA Y, et al. Ingestion of difructose anhydride III enhances absorption and retention of calcium in healthy men[J]. Bioscience, Biotechnology and Biochemistry, 2007, 71(3): 681-687.
[66] MINAMIDA K, SHIGA K, SUJAYA I N, et al. Effects of difructose anhydride III (DFA III) administration on rat intestinal microbiota[J]. Journal of Bioscience and Bioengineering, 2005, 99(3): 230-236.
[67] MINAMIDA K, OHASHI M, HARA H, et al. Effects of ingestion of difructose anhydride III (DFA III) and the DFA III-assimilating bacterium Ruminococcus productus on rat intestine[J]. Bioscience, Biotechnology and Biochemistry, 2006, 70(2): 332-339.
[68] KIKUCHHI H, NAGURA T, INOUE M, et al. Physical, chemical and physiological properties of difructose anhydride III produced from inulin by enzymatic reaction[J]. Journal of Applied Glycoscience, 2004, 51: 291-296.
[69] KIKUCHI H, NAGURA T, INOUE M, et al. Physical, chemical and physiological properties of difructose anhydride III produced from inulin by enzymatic reaction[J]. Journal of Applied Glycoscience, 2004, 51: 291-296.
[70] RUBIO E M, GARCIA MORENO M I, BALBUENA P, et al. Spacer-mediated synthesis of contra-thermodynamic spiroacetals: stereoselective synthesis of C 2-symmetric difructose dianhydrides[J]. Journal of Organic Chemistry, 2006, 71: 2257-2266.
[71] JUNG W S, HONG K, LEE S, et al. Structural and functional insights into intramolecular fructosyl transfer by inulin fructotransferase[J]. Journal of Biological Chemistry, 2007, 282: 8414-8423.
[72] KAZUTOMO H. Two types of inulin fructotransferases[J]. Materials, 2011, 4: 1543-1547.
[73] TANAKA K, UCHIYAMA T. Formation of difructofuranose 1,2’: 2,3’dianhydride from inulin by an extracellular inulinase of Arthrobacter ureafaciens[J]. Biochimimica et Biophysica Acta, 1972, 284: 248-256.
[74] KAWANMURA M, TAKAHASHI S, UCHIYAMA T. Purification and some properties of inulin fructotransferase (depolymerizing) from Arthrobacter ilicis[J]. Agricultural and Biological Chemistry, 1988, 52: 3209-3210.
[75] HARAGUCHI K, KISHHIMOTO M, SEKI K, et al. Purification and properties of inulin fructotransferase (depolymerizing) from Arthrobacter globiformis C11-1[J]. Agricultural and Biological Chemistry, 1988, 52: 291-292.
[76] YOKOTA A, HIRAYAMA S, ENMOTO K, et al. Production of inulin fructotransferase (depolymerizing) by Arthrobacter sp. H65-7 and preparation of DFA III from inulin by the enzyme[J]. Journal of Fermentation and Bioengineering, 1991, 72: 258-261.
[77] PARK J B, CHOI Y J. Purification and characterization of inulin fructotransferase (depolymerizing) from Arthrobacter sp. A-6[J]. Journal of Microbiology and Biotechnology, 1996, 6(6): 402-406.
[78] CHO C, LIM Y, KANG S, et al. Production of inulin fructotransferase (depolymerizing) Flavobacterium sp. LC-413[J]. Journal of Food Science and Nutrition, 1996, 1(1): 121-126.
[79] KANG S, KIM W, CHANG Y, et al. Purification and properties of inulin fructotransferase (DFA III-producing) from Bacillus sp. snu-7[J]. Bioscience, Biotechnology, and Biochemistry, 1998, 62(4): 628-631.
[80] HARAGUCHI K, YAMANAKA T, OHTSUBO K. Purification and properties of a heat stable inulin fructotransferase (DFA III-producing) from Arthrobacter pascens T13-2[J]. Carbohydrate Polymers, 2002, 50(2): 117-121.
[81] JAHNZ U. Screening-automation auf basis hohlkugelverkapselter zellen und enzymatische bildung von difructoseanhydrid III aus inulin unter thermophilen bedingungen (screening, charakterisierung, immobilisierung)[D]. Braunschweig: Technical University of Braunschweig, 2001.
[82] HARAGUCHI K, YOSHIDA M, OHTSUBO K. Thermostable inulin fructotransferase (DFA III-producing) from Arthrobacter sp. L68-1[J]. Carbohydrate Polymers, 2005, 59(4): 411-416.
[83] HARAGUCHI K, YOSHIHDA M, OHTSUBO K. Inulin fructotransferase (DFA-III producing) from Leifsonia sp. T88-4[J]. Carbohydrate Polymers, 2006, 66(1): 75-80.
[84] HARAGUCHI K. Inulin fructotransferase (DFAIII-Producing) from Arthrobacter ureafaciens D13-3[J]. Carbohydrate Polymers, 2010, 82: 742-746.
[85] HANG Hua, MU Wanmeng, JIANG Bo, et al. Enzymatic hydrolysis of inulin in a bioreactor coupled with an ultrafiltration membrane[J]. Desalination, 2012, 284: 309-315.
Enzymatic Biotransformation of Inulin for Application in Foods
ZHAN Rong-rong, MU Wan-meng, LI Yun-gao, ZHANG Tao, JIANG Bo*
(State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, China)
Inulin, which is widely distributed and abundant in China, is an important agricultural and sideline product. Biotechnological processing of inulin with inulinase can provide an efficient approach for low-cost, multi-directions, and high-value-added development and utilization of inulin in the field of food. This paper reviews the current situation of research on enzymatic biotransformation of inulin for application in foods. Additionally, the properties of inulinasecatalyzed bio-transformation products and their applications in the food industry as well as the microbial sources and the latest progress of different types of inullinase are elaborated in depth.
endo-inulinase; exo-inulinase; inulin fructotransferase; inulooligosaccharide; high-fructose syrup; difructose anhydride
Q539;TS210.1
A
1002-6630(2014)03-0226-08
10.7506/spkx1002-6630-201403046
2013-03-12
国家自然科学基金项目(21276001;31171705);江苏省科技支撑项目(BE2011622;BE2011766;BE2010678;BE2010626)
战荣荣(1984—),女,博士研究生,研究方向为食品生物技术。E-mail:newlife.zrr@163.com
*通信作者:江波(1962—),男,教授,博士,研究方向为酶在食品生物制造中的应用。E-mail:bjiang@jiangnan.edu.cn

