2,6-双(1-苯基苯并咪唑-2-)吡啶铱バ配合物的合成、晶体结构和发光性质
刘生桂 陈自卢 苏文义 潘荣楷 石晓波
(1湛江师范学院化学科学与技术学院,广东高校新材料工程与技术研发中心,湛江 524048)
(2广西师范大学化学化工学院,桂林 541004)
0 Introduction
The efficiencies,brightness,and wavelength emissions of Irバcomplexes strongly depend on the molecular structure of cyclometalated ligand.The emission wavelength of Irバcomplexes normally can be altered by changing the electron density or position ofsubstituenton the main ligand[15].2,6-Bis -(benzimidazol-2-yl)pyridine is a luminescent tridentate ligand and can be further tailored[16-17].The substitution of the NH bond at the benzimidazole rings for the NR group will change their many properties (e.g.acidbase degree,photophysicalproperties)[18].In the present paper,We first adopted phenyl to substitute the NH hydrogen atoms of 2,6-bis(benzimidazol-2-yl)pyridine to obtain 2,6-bis(1,1′-phenylbenzimidazol-2-yl)pyridine,then 2,6-bis(1,1′-phenyl-benzimidazol-2-yl)pyridine coordinated to iridiumバion to produce a new cationic complex [Ir(bpbp)2](PF6)3.The complex thermal stability and photophsycal property were also investigated.
1 Experimental
1.1 General
o-Phenylenediamine, pyridine-2, 6-dicaboxyl acid,bromobenzene and KPF6were purchased from Shanghai Aladdin Reagent Company.IrCl3·nH2O was purchasedfrom ShanxiKaidaCompany.Allthe chemicals and solvents used were analytically pure and without further purification.The analyses (C,H and N)were made on a Perkin-Elmer 240C elemental analyzer.1HNMR spectroscopic measurements were carried out on a Bruker AM-300 NMR spectrometer,using TMS (SiMe4)as an internal reference.The solid infrared spectra (IR)were obtained from a Bruker IFS66V vacuum-typeFT-IR spectrophotometerby using KBr pellets.The UV absorption spectra were recorded on a modelUV-240 spectrophotometer(Shimadzu,Japan).Fluorescence measurements were performed on a ModelRF-5 spectrofluorimeter(Shimadzu,Japan).
1.2 Synthesis of 2,6-bis(1-phenylbenzimidazol-2-yl)pyridine(bpbp)
A mixture of bromobenzene (1.75 g,11 mmol),2,6-bis(benzimidazolyl)pyridine (1.65 g,5.0 mmol),CuI(0.40 g,2.1 mmol),1,10-phenanthroline (0.8 g,0.88 mmol),and Cs2CO3(14.5 g,45 mmol)was suspended in 50 mL of DMF.The mixture was refluxed for 24 h and then cooled to room temperature.The resulting sticky residue was purified by column chromatography and give colorless ligand(1.14 g,yield:45%).Its structure was confirmed by element analysis IR,and1H NMR.Element analysis for C31H21N5,Found (%):C,80.22;H,4.67;N,15.10,Calcd.(%):C,80.32,H,4.57,N,15.11.Selected IR data (KBr,cm-1):3 417,1 588,1 503,1 439,1 392,1 336,1250,1 191,1 147,1 072,1 002,933,874,830,744,685,615,502,421 and1H NMR:(CDCl3):6.99(d,4H),7.18 ~7.37(m,12H),7.88(t,3H),8.08(d,2H).
1.3 Synthesis of[Ir(bpbp)2](PF6)3·2DMF·2H2O
IrCl3·nH2O (406 mg,2.0 mmol)and bpbp(993 mg,2.0 mmol)were refluxed in ethylene glycol(35 mL)for 20 h at 180℃.After the solution was cooled to room temperature,a saturated aqueous solution of KPF6was added,and the resulting yellow precipitate was filtered off.After recrystallization from DMF,yellow crystals were obtained.Yield:1.193 mg 68.7%.Anal.Calcd.for C68H60N12O4P3F18Ir ([Ir(bpbp)2](PF6)3·2DMF·2H2O,%):C,47.04;H,3.48;N,9.68;Found(%):C,47.49;H,3.61;N,9.60.Selected IR data(KBr,cm-1):3428,3087,1605,1514,1455,1389,1338,1247,1156,1014,748,689,548.1H NMR:(CDCl3):6.29~6.31(d,4H)7.25~7.28(d,4H),7.40~7.45(t,4H),7.53~7.62 (q,8H).7.68~7.71 (d,8H),7.88~7.97(m,12H),8.66~8.72(t,2H).
1.4 X-ray crystallography
Single crystal structure determination of complex was performed on a Bruker SMART APEX CCD diffractometer equipped with a normal focus,3 kW sealed tube X-ray source and graphite monochromated Mo Kα radiation (λ =0.071073 nm)at 173 K,operating at 50 kV and 30 mA.The structures were solved by direct methods by using program SHELXTL.Absorption correction adopted Semi-empirical from equivalents.Fourier difference techniques,and refined by full-matrix least-squares.All non-hydrogen atoms in both structures were refined anisotropic displacement parameters.All hydrogen atoms were theoretically added.The crystal data are summarized in Table 1.
CCDC:913646.
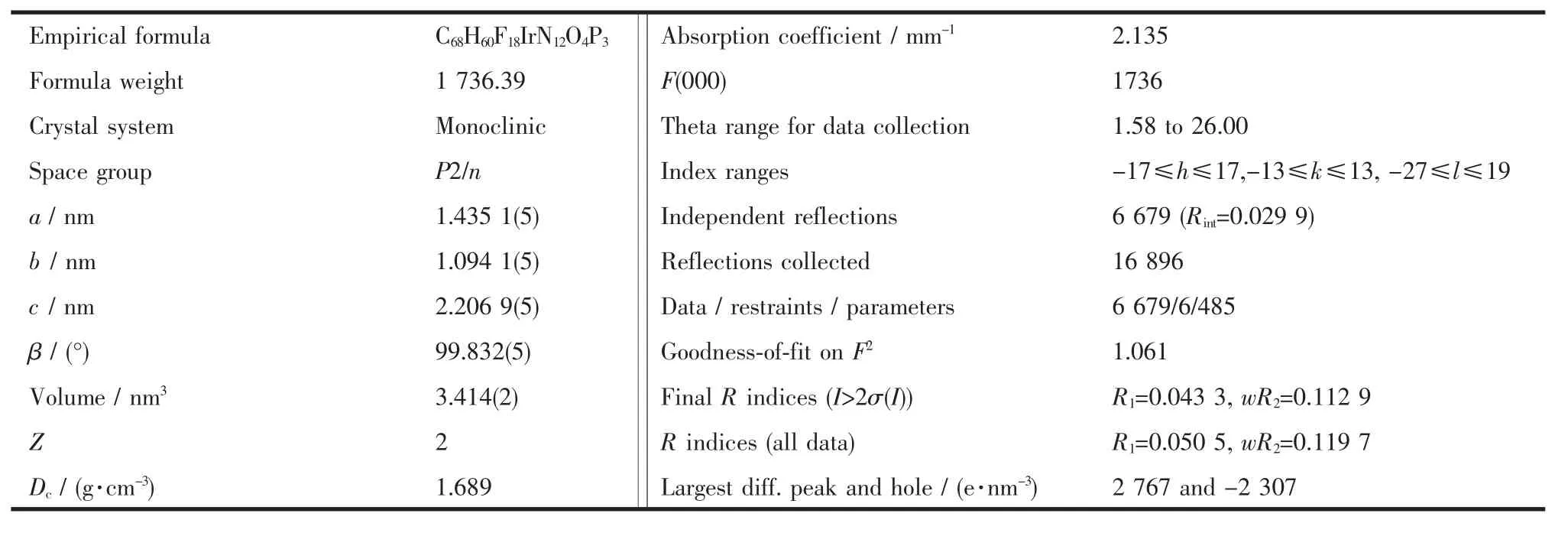
Table 1 Crystal data and structure refinement for complex
2 Results and discussion
2.1 Synthesis,IRandthermalstabilityforcomplex
The syntheses of Irバcomplexes with polypyridine ligands require rigorous condition because of the inertness of the Irバ core.Both high temperatures and a long reaction time were also necessary for the syntheses of Irバcomplexes with tridentate benzimidazole derivatives.Chromatographic purification for iridiumバcomplexes is commonly employed in order to separateadesired complexfrom undesired side products.Our synthetic reaction afforded a relatively pure crystalline product,which can be easily purified by recrystallization.Large negative hexafluorophosphate act as counterion to precipitate coordinated cation[Ir(bpbp)2]3+.The purity of the complex was carefully checked by elemental analysis,1H NMR.Single crystals suitable for X-ray crystallographic analysis were obtained by recrystallization from DMF.The IR spectra of the free ligand bpbp,complex show allabsorption bandsresulting from the skeletal vibration of the benzimidazole.In complex,there is a strong peak at 839 cm-1,which show PF6-in this complex.
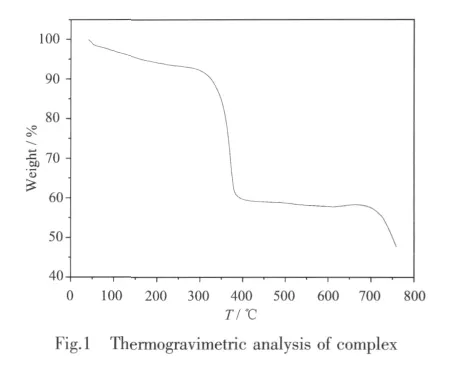
Thermogravimetric analysis(TGA)showed that the decomposition temperature of the complex is 330℃(Fig.1).The lost weight of 10.48%under 330 ℃ is due to the loss of coordinated H2O and the crystallized solvents,DMF,which is consistent with the theoretical weight-loss value of 10.77%.
2.2 Crystal Structure
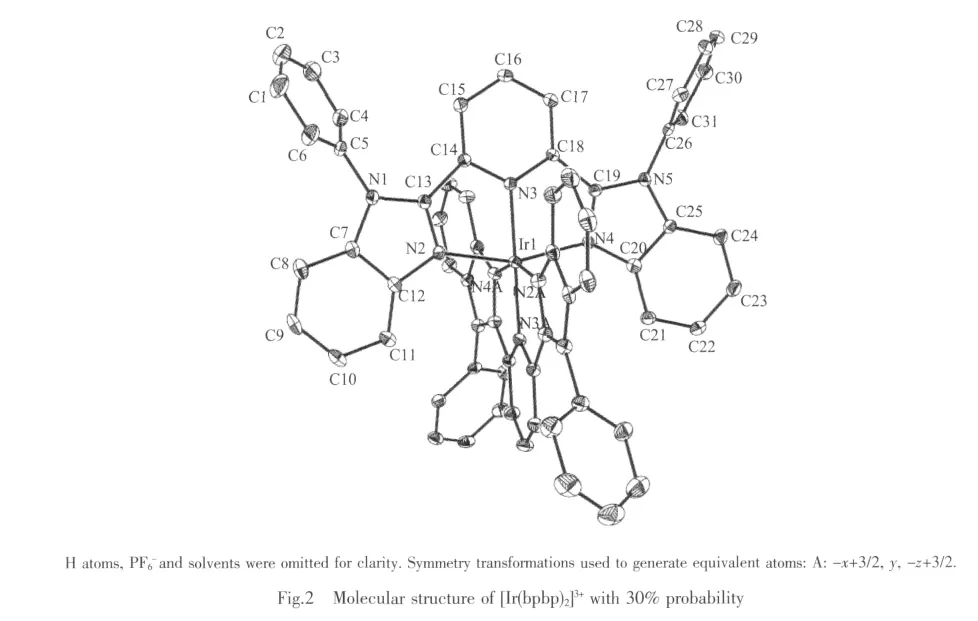
The ORTEP drawing for complex with atom numbering is shown in Fig.2.The center Irバ atom has N6coordination sphere,being bound by two bpbp ligand.In every ligand,the two benzimidazole rings and center pyridine ring and Ir1atom form a good planar (r.m.s.=0.006 2°),the dihedron between the planar(N2,N3,N4,Ir1)and the planar(N2A,N3A,N4A,Ir1)is 86.5°.The four substituted NH hydrogen phenyl rings are inclined with their attached benzimidazole rings plannar.
Selected bond lengths and angles for complex 1 are listed in table 2.The bond length of Ir-N fall into the range of the reported Ir-N distances[20].The average bond length of Ir1-N is 0.202 1 nm.The geometry of the IrN6 coordination is appreciably distorted octahedral.The significant deviation from 90°of the bond angles involving the chelation is observed(Table 2),which is presumably due to formation of fivemembered chelate ring.
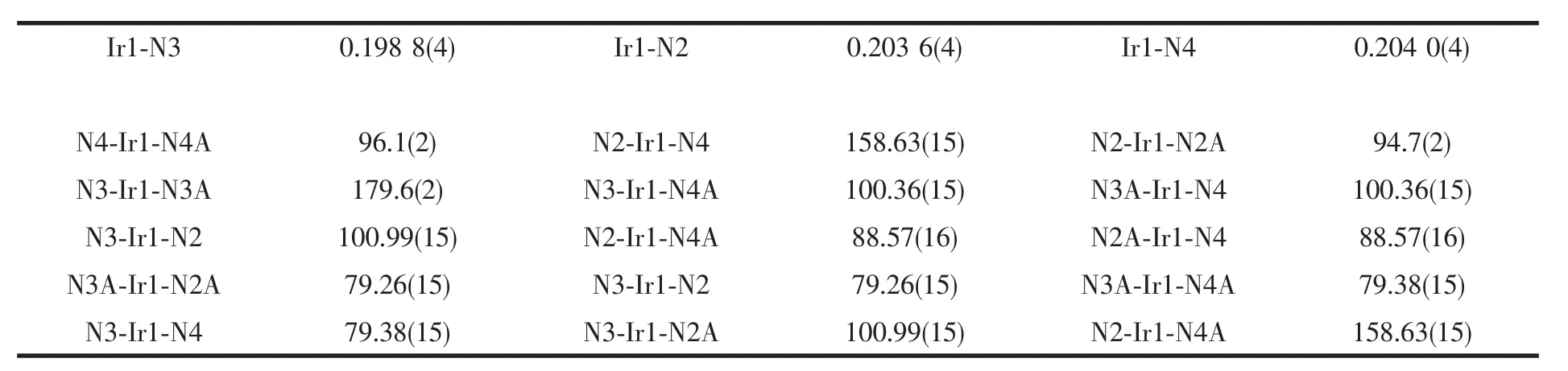
Table 2 Selected bong length(nm)and bond angles(°)
2.3 Photophysical properties
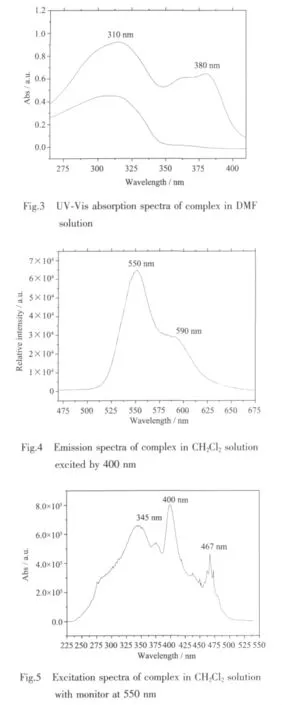
The UV-Vis absorption and liquid luminescence spectra were recorded at a concentration of 1.0×10-5mol·L-1in CH2Cl2at room temperature.The UV-Vis absorption spectra of free ligand and complex are shown in Fig.3.The ligand has absorption in the range of 270~350 nm and complex has absorption in the range of 270~400 nm.The ligand maximal absorption is located at about 310 nm,which can be assigned to intraligand π-π*transitions.There is one more peak observed at 380 nm for complex,which probably arise from metal-ligand charge transfer.As shown in Fig.4,excited by 400 nm wavelength light,the complex exhibits yellow green luminescence with the range of 520~650 nm in CH2Cl2solution at room temperature.In comparison with complex[Ir(bmbp)2](PF6)3(bmbp=2,6-bis(1-methylbenzimidazol-2-yl)pyridine),they all emission yellow green light with the peaks at 550 and 590 nm[20],but for complex [Ir(bpbp)2](PF6)3,the intensity of 550 nm is over 590 nm.It was suggested by reference that the emission was ascribed mainly to3LC and some contribution of3MLCT[21],which can be supported by the UV-Visabsorption spectra of complex.The complex excitation spectra is shown in Fig.5,monitor at 550 nm emission wavelength,the excitation band is within 230~500 nm with peaks at 345,400 and 467 nm.
3 Conclusions
In the complex,Irバion octahedral geometry is coordinated by six nitrogen atoms from two 2,6-bis(1-phenylbenzimidazol-2-yl)pyridine molecule. In complex absorption spectra,there is not only the free ligand absorption peak at 310 nm,but also shows strong metal-ligand charge transfer band within 348~400 nm.The complex exhibits yellow green luminescence in CH2Cl2solution.
[1]Nazeeruddin M K,Humphry-Baker R,Berner D,et al.J.Am.Chem.Soc.,2003,125:8790-8797
[2]Plummer E A,Hofstraat J W,De Cola L.J.Chem.Soc.Dalton Trans.,2003:2080-2084
[3]Tsuboyama A,Iwasaki H,Furugori M,et al.J.Am.Chem.Soc.,2003,125:12971-12979
[4]Lo K K M,Chung C K,Lee T K M,et al.Inorg.Chem.,2003,42:6886-6897
[5]SHEN Xuan(沈旋),HU Xiu-Hua(胡秀华),WANG Feng-Ling(王凤玲),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(4):651-656
[6]LI Chun-Xiang(李春香),BU Qing-Ming(卜清明),SUN Pei-Pei(孙培培),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22(8):1479-1483
[7]MEI Qun-Bo(梅群波),ZHANG Qian-Feng(张千峰),TONG Bi-Hai(童 碧 海 ).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(2):264-270
[8]LIU Jian(刘坚),WEI Chun(韦春),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(2):398-404
[9]LI Xiang-Hong(李襄宏),ZHAo Xin-Di(赵鑫帝),LÜ Kang-Le(吕 康 乐),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(2):303-307
[10]Pei Q,Yu G,Zhang C,et al.Science,1995,269:1086-1088[11]Slinker J,Bernards D,Houston P L,et al.Chem.Commun.,2003:2392-2399
[12]Rudmann H,Shimida S,Rubner M F.J.Am.Chem.Soc.,2002,124:4918-4921
[13]Wegh R T,Meijer E J,Plummer E A,et al.Proc.SPIE,2004,5519:48-58
[14]Nazeeruddin M K,Wegh R T,Zhou Z,et al.Inorg.Chem.,2006,45:9245-9249
[15]Wu H,Zhou G,Zou J,et al.Adv.Mater.,2009,21:4181-4184
[16]Liu S G,Zuo J L,Wang Y,et al.J.Phys.Chem.Solids,2005,66:735-740
[17]Liu S G,Zuo J L,Li Y Z,et al.J.Mol.Struct.,2004,705:153-157
[18]Bǒcaa M,Jamesonc R F,Linertb W.Coord.Chem.Rev.,2011,255:290-317
[19]Liu S G,Zhang L P,Liu J,et al.Spectrochimica Acta Part A,2012,97:464469
[20]Chen J L,Wu Y H,He L H,et al.Organometallics,2010,29:2882-2891
[21]Yutaka T,Obara S,Ogawa S,et al.Inorg.Chem.,2005,44:4737-4746

