苯基噌啉铱配合物的合成及电致发光性质
童碧海 董超振 张 曼 韩 召 张千峰
(安徽工业大学冶金与资源学院,分子工程与应用化学研究所,马鞍山 243002)
0 Introduction
Organic light-emitting diods(OLEDs)based on the phosphors have attracted much attention because of the great potential for low cost full-color flat-panel displays and light sources[1].Due to heavy atom induced spinorbit coupling,singlet as well as triplet excitons for the emission,phosphorescent complexes can theoretically achieve quantum efficiency up to 100%[2].Among all kinds of phosphorescent complexes studied,Irバcomplexes are very promising candidates due to rather short phosphorescence lifetimes,very high luminescence efficiencies and excellent color tunability[3].The Irバcomplexes get great development on account of the broad diversity of possible structures.For example,the most important cyclometalating ligands are available in a wide range,aromatic nitrogen heterocycle derivatives,such as pyridine[4],pyrimidine[5],pyrazine[6],pyridazine[7],oxadiazole[8],thiazole[9],imidazole[10]and pyrazole[11]etc.,had been used as the cyclometalating ligands.In adition,the ancillary ligands give additional possibilities to define structure and to tune the properties[12].
Several groups have demonstrated that the Irバcomplexes based on the C^N=N structure cyclometalating ligands, such as phenylpyridazine and phenylphthalazine,are strong electrophosphorescent materials[13-17].Devices based on these materials have a significantly improved performance.In the C^N=N structure cyclometalating ligands,a sp2-hybrid N is adjacent to the chelating N atom.According to Mi et al.[17],ligands bearing a C^N=N structure in Irバcomplexes could bond to the Ir atom more strongly,and could also create a “proximity effect”[18]compared to their counterpart ligands with chelating N atoms bearing adjacent sp2C atoms (represented as C^N=CH),such as Ir(ppy)3(tris(2-phenylpyridine)iridium).In our previous study,a series of tris-cyclometalated Irバcomplexes were directly formed by the reaction of phthalazine derivatives and IrCl3·3H2O[13-16].We believe this special reaction is relative to the strong bonding strength and the small steric hindrance between the ligand and the centric Ir-atom.These complexes have internal quantum efficiency of nearly 100%and good thermally stability.Phosphorescent OLED deviceswith thesecomplexesasdopant emitters exhibit red emission with high efficiency as wellas good operationalstability.By quantum chemistry calculations,Zhang et al.[19]even revealed that in our complexes the bulky phenolic group played a major role in the more promising photoluminescence(PL)and electroluminescence (EL)properties of 1.The large distortion between the phenolic and N=N moieties leads to negligible distribution on the Ph(B)moiety,which only acts as a pendant at the periphery of the chromophore core and protects the chromophore core from the hazardous intermolecular interaction of emitters and reduces luminescence quenching.Furthermore,the electron-withdrawing phenol increases the intramolecular charge separation and increases the dipole moment and therefore results in a smaller potential energy surface value,which is important for OLEDs.These results show that for Irバcomplexes the ligand with a C^N=N structure is superior to the one with a C^N=CH structure,and that this kind of Irバ complexes is promising for high-efficiency optoelectronic applications.
As another type of isomers of benzopyridazine besides phthalazine,cinnoline derivatives had also be used to synthesise cyclometalated Irバcomplexes in high yield by our group[20].A “turn-on” phosphorescent chemosensor of a neutral Irバ)complex Ir(dpci)2(dtc) (dtcH=diethyl dithiocarbamic acid)containing the dithiocarbamate ancillary ligand for Hg2+had been developed[21].In order to explore new efficient electroluminescentmaterials,a bis-cyclometalated Irバcomplex with dpci as the cyclometalating ligands and piclinic acid as the ancillary ligand was synthesized in the present paper.The photophysical and thermal properties of the complex were investigated.The performance of devices using the complex as the dopant was evaluated.
1 Experimental
1.1 Materials and characterization
The solvents were purified by routine procedures and distilled under dry nitrogen before use.All reagents,unless otherwise specified,were purchased from Sigma-Aldrich and were used as received.3,4-Diphenylcinnoline was prepared according to procedures described in the literature[20].UV-Vis absorption spectra were recorded on a Shimadzu UV-2501 PC spectrophotometer.1H NMR spectra were recorded on a Bruker-400 NMR spectrometer using CDCl3as solvent.Mass spectra were obtained on a Bruker Daltonics matrix-assisted laser desorption/ionization time-of-flight mass spectrometer(MALDI-TOF-MASS)with trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidenne]malononitrile (DCTB)as matrices and tetrahydrofuran as solvent.PL spectra were measured with a Shimadzu RF-5301PC fluorescence spectrophotometer.Fluorescence quantum yield of the complex was calculated using[Ru(bpy)3]Cl2in aqueous solution(Φ=0.042)as a standard[22].Luminescence lifetime was determined on an Edinburgh FL920 time-correlated pulsed single-photon-counting instrument with excitation at 405 nm.
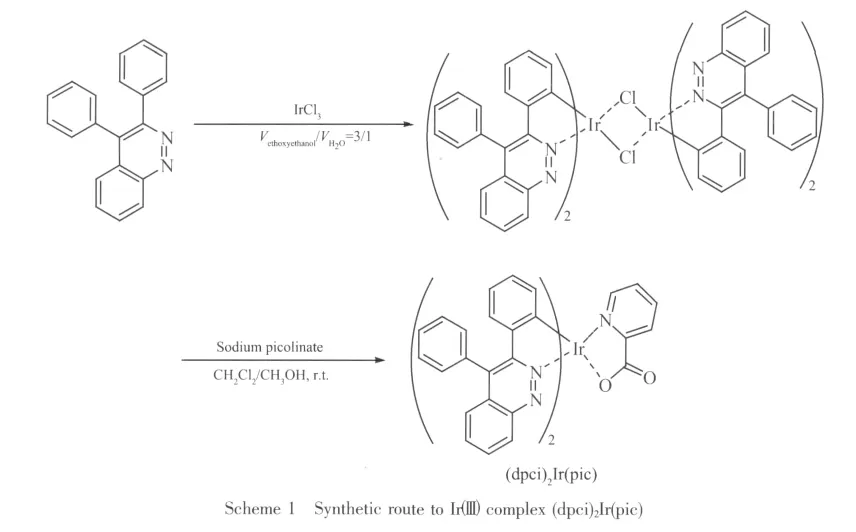
1.2 Synthesis of(dpci)2Ir(pic)
The synthetic route to Irバcomplex is given in Scheme 1.
To a round-bottomed flask (25 mL),2-ethoxyethanol(9 mL),3,4-diphenylcinnoline (0.5 g,2.0 mmol),IrCl3·3H2O (0.2 g,0.56 mmol)and water(3 mL)were added sequentially.The mixture was stirred under nitrogen at 120℃for 24 h and cooled to room temperature.The precipitate was collected and washed with ethanol and acetone,and then dried in vacuum to give a dark red cyclometallated IrⅢ-μchloro-bridged dimer(0.36 g,82%).
In a round-bottomed flask,0.08 g(0.05 mmol)of cyclometallated IrⅢ-μ-chloro-bridged dimer and 0.03 g(0.2 mmol)of sodium picolinate were mixed together in CH2Cl2/methanol(5 mL:5 mL).The mixture was stirred at room temperature for 1 h.The product was precipitated from water and dried.Dark red solid of(dpci)2Ir(pic)was obtained from column chromatography on a silica column using CH2Cl2and acetic ether(1∶1)as eluent,yield:0.07 g,79%.
1H NMR(CDCl3,400 MHz) δ(ppm)8.52(d,J=8.8Hz,1H),8.35(d,J=8.0Hz,1H),7.87(d,J=6.8 Hz,2H),7.57~7.74(m,7H),7.53(d,J=8.4 Hz,2H),7.48(d,J=8.8 Hz,2H),7.38~7.44(m,4H),7.31(t,J=6.8 Hz,3H),6.68 (t,J=7.4 Hz,1H),6.62 (t,J=7.6 Hz,1H),6.53(t,J=7.2 Hz,1H),6.43~6.48(m,3H),6.35(d,J=7.6 Hz,1H),6.17 (d,J=7.6 Hz,1H);13C NMR(CDCl3,100 MHz) δ (ppm)161.74,160.69,150.58,148.23,148.08,141.71,141.32,137.17,134.31,134.21,133.80,133.75,132.96,132.54,132.20,131.88,130.17,130.02,129.98,129.65,129.60.129.58,129.56,129.46,129.38,129.29,129.19,129.15,129.11,128.92,128.81,128.40,128.28,127.55,127.40,126.62,124.87,124.53,121.37,120.76;MADIL-TOF MS (m/z):Calcd.for[M+K+]C46H30N5O2IrK:916.078,Found:916.556.
1.3 OLED fabrication and measurements
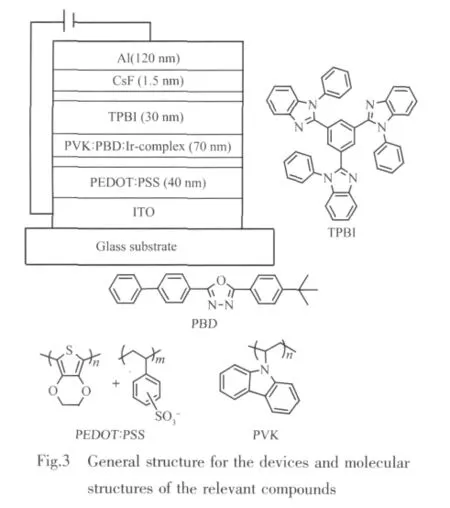
ITO (Tin-doped indium oxide)glass substrates were cleaned successively by ultrasonication in detergent,deionized water,acetone,and ethanol for 15 min and finally treated with ozone for about 20 min.PEDOT [poly(3,4-ethylenedioxythiophene)](Baytron P4083,Bayer AG)in water was spin-coated at a rate of 3 000 r·min-1on the ITO substrate and dried for 12 h at 80℃ under vacuum.Then the solution of PVK[poly(vinylcarbazole)](Mw=81 800)blended with PBD [2-tert-butylphenyl-5-biphenyl-1,3,4-oxadiazole]and(pqcm)2Ir(pic)(or(pqca)2Ir(pic))in chlorobenzene was spin-coated at a rate of 1 300 r·min-1on top of the PEDOT layer and annealed for 20 min at 100℃.The assembly was transferred into a deposition chamber with a base pressure of 4×10-3Pa,a film of TPBI[1,3,5-tris(N-phenylbenzimidazol-2-yl)-benzene]was evaporated onto the polymerlayer.Finally,the CsF and Al layers were evaporated.Current density-voltage-luminance data were collected using a Keithley 236 source measurement unit and a calibrated silicon photodiode.External quantum efficiency was obtained by measuring the total light output in all directions in an integrating sphere(IS080, Labsphere).Luminance and luminous efficiency were measured by a silicon photodiode and calibrated with a PR-705 photometer(Photo Research).
2 Results and discussion
2.1 Photophysical properties and electrochemical properties
UV-Visabsorption spectrum of(dpci)2Ir(pic)is shown in Fig.1.The band around 300 nm is assigned to a typical spin-allowed1π-π*transition of the ligands and band at 400 nm corresponds likely to spin-allowed singletmetal-to-ligand charge-transfer(1MLCT).On the other hand,the moderately intense absorptions at ca.450~600 nm can be assigned to a spin-forbidden triplet metal-to-ligand charge-transfer(3MLCT).
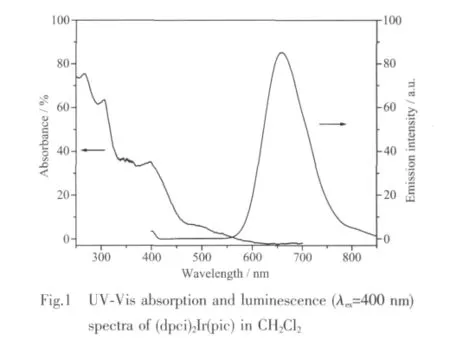
The room-temperature luminescence spectrum of(dpci)2Ir(pic)in CH2Cl2is also shown in Fig.1.Complex(dpci)2Ir(pic)emits intense luminnescence with emission wavelength at 657 nm,corresponding to pure red light emitting.The full width at half maximum (FWHM)of this transition is 100 nm.The phosphorescence quantum efficiency of(dpci)2Ir(pic)in degassed CH2Cl2solution with a excitation wavelength of 480 nm is ca.0.005.The excited state lifetime of(dpci)2Ir(pic)in CH2Cl2solution is determined to be 226 ns.The lifetime is remarkably shorter than those of(EO-CVz-PhQ)2Ir(pic)(1.3 μs)[23]and most of the other reported neutral Irバcomplexes[13-17].This shorter lifetime is beneficial to suppress the triplet-triplet annihilation,and this would be advantageous to the design of highly efficient OLED devices.
The electrochemical property of the complex was investigated by cyclic voltammetry in a CH2Cl2solution at room temperature using ferrocene as the internal standard.During the anodic scan,the cyclic voltammogram shows a reversible oxidation wave due to the IrⅣ/IrⅢ oxidation with the half-wave oxidation potential(E1/2ox)of 0.79 V vs Ag/AgNO3as shown in Fig.2.The highest occupied molecular orbital(HOMO)energy level is-5.16 eV relative to the value of-4.8 eV for ferrocene (Fc)with respect to zero vacuum level[24].The lowestunoccupied molecularorbital(LUMO)energy level is-3.16 eV from the HOMO energy level value and the energy gap derived from the UV-Vis absorption band.It is clear that the HOMO and LUMO levels of the complex are exactly between the HOMO energy level and LUMO energy level of PVK (HOMO=-5.8 eV and LUMO=-2.2 eV)and PBD (HOMO=-6.2 eV and LUMO=-2.4 eV),so the complex can function as a trap for both electrons and holes,thus resulting in high device efciency[25].
2.2 Electoluminescent OLEDs characterization
To illustrate the electroluminescent properties of Irバcomplex,the devices using the complex as dopants were fabricated with the following structures:ITO/PEDOT:PSS(40 nm)/Emitting layer(70 nm)/TPBI(30 nm)/CsF(1.5 nm)/Al(120 nm)(Fig.3).As a good hole-transporter,PVK was blended with PBD,which is an electrontransport material,to enable the host to transport both electrons and holes[16].TPBI acts as both a hole-blocker and an electron-transporter.
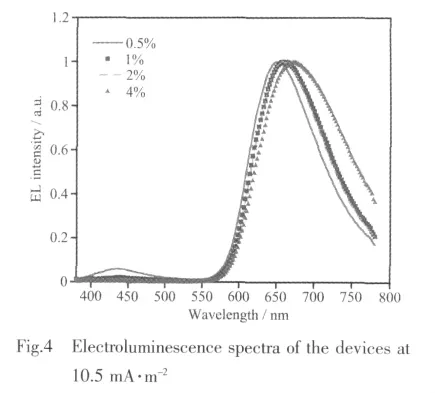
The EL spectra of OLEDs from 0.5 to 4wt%doping concentration are shown in Fig.4.At lower doping concentrations,two emission peaks are found in the EL spectra.The emission peak at 435 nm is corresponding to the residualemission ofhost materials,indicating incomplete energy transfer from host to(dpci)2Ir(pic).The other emission peak at 650 nm is the emission of(dpci)2Ir(pic).As the concentration of the dopant increases,the emission component at 435 decreases and completely disappears at higher concentrations(2wt%~4wt%).It can be speculated that the energy is completely transferred from the host to(dpci)2Ir(pic)in higher dopant concentration.The phenomenon with complete quenching of the host EL emission in higher dopant concentration indicates that energy and charge transfer from the host to the Irバ complex is efcient under electrical excitation.The EL spectra in higher dopant concentration have a slight red shift and this means that the dopant itself modifies substantially the polarity in the film[26].So the dopant concentration has a signicant effect on the mixing single and triplet emission of MLCT.All the optimization devices emit saturated red emissions and are promising candidates as red emitters in full-coloremitting OLEDs.
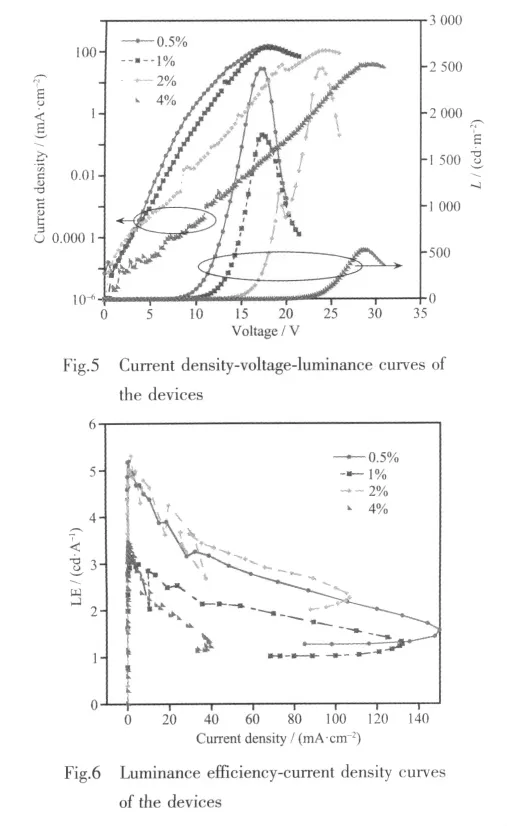
Plotsofcurrentdensity and luminance vs.voltage are shown in Fig.5,luminance efciencycurrent density curves are shown in Fig.6,and the detailed device properties are listed in Table 1.The maximum external quantum efficiency of two series devices increase from low to intermediate dopant concentration (0.5wt% ~2.0wt%)and then decrease with increasing concentration.For all the devices,the device at 2wt%doping concentration gives maximun external quantum efficiency.The maximum luminance is 2 484 cd·m-2at a current density of 100 mA·m-2,and the maximum external quantum efficiency is 9.11%photons/electron at 2.05 mA·m-2,corresponding to a luminous efficiency of 5.31 cd·A-1.The CIE chromaticity coordinate is (0.66,0.31)which is slightly deviated from the standard red values of(0.67,0.33)demanded by the National Television System Committee.This can be due to the long emission wavelength of the Irバ complex,so to increase the energy gap of this Irバcomplex will be beneficial.
The devices show a gradual increase in turn-on voltage with increased dopant concentration at a given current density from 0.5%to 4.0%,which is attributed to the signicant carrier-trap of Irバcomplex[27].Typical efficiency roll-off at higher current,attributable to a combination of triplet-triplet annihilation and fieldinduced quenching effect,is also observed here.
3 Conclusions
In conclusion,a novel saturated red-emitting cyclometalated Irバcomplex(dpci)2Ir(pic)based on phenylcinnoline ligand was synthesized and characterized.The device based on the complex at 2%dopant concentration has the best performance.A maximum electrophosphorescence peak at 656 nm with a maximum external quantum efficiency of 9.1%is obtained at this dopant concentration.
[1]Ulbricht C,Beyer B,Friebe C,et al.Adv.Mater.,2009,21:4418-4441
[2]Baldo M A,OBrien D F,You Y,et al.Nature,1998,395:151[3]Xiao L X,Chen Z J,Qu B.Adv.Mater.,2011,23:926-952
[4]Adachi C,Baldo M A,Thompson M E,et al.J.Appl.Phys.,2001,90(10):5048-5051
[5]Wang Z Q,Xu C,Dong X M.Inorg.Chem.Commun.,2011,14:316-319
[6]Ge G P,He J,Guo H Q.J.Organomet.Chem.,2009,694:3050-3057
[7]Gao Z Q,Mi B X,Tam H L.Adv.Mater.,2008,20(4):774-778
[8]Wu Z L,Zhu M X,Liu Y.Chinese Chem.Lett.,2005,16(2):241-244
[9]Xu M L,Zhou R,Wang G Y.Inorg.Chim.Acta,2009,362:515-518
[10]Huang W S,Lin J T,Chien C H.Chem.Mater.,2004,16(12):2480-2488
[11]Dedeian K,Shi J M,Shepherd N.Inorg.Chem.,2005,44:4445-4447
[12]You Y M,Park S Y.J.Am.Chem.Soc.,2005,127(36):12438-12439
[13]Tong B H,Mei Q B,Wang S J.J.Mater.Chem.,2008,18:1636-1639
[14]Fang Y,Tong B H,Hu S J.Organic Electronics,2009,10:618-622
[15]Fang Y,Hu S J,Meng Y Z.Inorg.Chim.Acta,2009,362:4985-4990
[16]Fang Y,Li Y H,Wang S J.Synthetic Met.,2010,160:2231-2238
[17]Mi B X,Wang P F,Gao Z Q,et al.Adv.Mater.,2009,21:339-343
[18]Siebrand W,Zgierski M Z.J.Chem.Phys.,1980,72:1641-1646
[19]Li X N,Wu Z J,Liu X J,et al.J.Phys.Chem.A,2010,114:9300-9308
[20]Tong B H,Wu F H,Mei Q B.Z.Naturforsch.B:Chem.Sci.,2010,65b:511-515
[21]Tong B H,Mei Q B,Lu M G.Inorg.Chim.Acta,2012,391:115-119
[22]Vanhouten J,Watts R J.J.Am.Chem.Soc.,1976,98:4853-4858
[23]Lee S J,Park J S,Song M.Adv.Funct.Mater.,2009,19:2205-2212
[24]Pommerehne J,Vestweber H,Guss W.Adv.Mater.,1995,7:551-554
[25]Whang D R,You Y,Kim S H.Appl.Phys.Lett.,2007,91:233501(1)-233501(3)
[26]Baranoff E,Suarez S,Bugnon P.ChemSusChem,2009,2:305-308
[27]Wong W,Zhou G,Yu X.Adv.Funct.Mater.,2006,16:838-846

