Seasonal variation in soil microbial biomass carbon and nitrogen in an artificial sand-binding vegetation area in Shapotou, northern China
YuYan Zhou , XuanMing Zhang , XiaoHong Jia , JinQin Ma , YanHong Gao
1. Gansu Productivity Promotion Center, Lanzhou, Gansu 730000, China
2. Institute of Desertification Studies, Chinese Academy of Forestry, Beijing 100091, China.
3. Shapotou Desert Research and Experiment Station, Cold and Arid Regions Environment and Engineering Institute,Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
1 Introduction
Soil microbial biomass (MB) refers to the total mass of organisms with volume less than 5.0×103μm3,not including living plants, such as plant roots. Soil MB is the active portion of soil organic matter, and the most active factor in soil (Jenknson and Ladd, 1981;Dillyet al., 2003). A more generalized perspective of soil MB includes microbial carbon (MBC), microbial nitrogen (MBN), microbial phosphorus (MBP) and microbial sulfur (MBS), all of which may be measured using the chloroform fumigation extraction method.The role of soil MB is mainly shown in two aspects:first, soil MB is the driving force for the transformation and cycling of soil organic matter and nutrients,being involved in the decomposition of soil organic matter, formation of humus and transformation and cycling of soil nutrients; second, soil MB acts as the pool of soil nutrients, and is therefore an important nutrient source for plant growth.
Soil is a complex natural body possessing multiple media and components. Both the macro-and micro-structures of soil are quite complex, and specific characteristics remain unknown. In natural soil conditions,the composition and activities of soil microbes and their biochemical processes are very complex. Microbes, as the living organisms in soil, are relatively sensitive to environmental changes. Any changes in the biomass and composition of microbes will affect their nutrient cycle and its effectiveness (Davidson and Ackeman, 1993), thus can be used as an indicator in changes of ecosystem function and soil properties. In the context of climate change and human activities on ecosystems, soil MB plays an important role in the evaluation of soil fertility and health (Colemanet al.,1983; Insam, 1990; Smithet al., 1993; Wanderet al.,1994). Therefore, studies on the microbes will be beneficial to exploring the coupling mechanisms of ecological restoration and soil quality, and have important theoretical and practical significances in guiding the regional ecological environment construction and improvement of soil quality (Yaoet al., 1999; Wanget al.,2002).
In the arid and semi-arid areas of China’s Three-North region (North, Northeast, and Northwest China), the developments of artificial sand-binding vegetation, shrubs and grasslands have greatly improved the local ecological environment. Ecological restoration is a complex process in which vegetation and soil systems are developed with mutual drive and feedback, and the artificial vegetation causes changes in the soil properties during the process of natural succession of vegetation; in particular, the development of biological soil crust intensifies these changes, and has had a significant impact on soil organic carbon sequestration and the greenhouse effect. Soil MB and its influence on soil carbon pools and plant litter input not only affect the soil source of atmospheric carbon, but also indicate any small changes which may occur in the soil carbon pool. In order to maintain the safe operation of the Baotou-Lanzhou Railway, the Chinese Academy of Sciences and the Ministry of Railways established an artificial vegetation protection system in Shapotou (located on the southeast edge of the Tengger Desert),where the annual rainfall is less than 180 mm. The 1 km2artificial sand-binding observation field and 16 km long sand-binding vegetation succession observation field, both owned by the Chinese Academy of Sciences national testing station, provide study areas for the comparison of MBC and MBN. Using time instead of space method the variation characteristics of MBC and MBN in the restoration process of this artificial vegetation are investigated and the response mechanisms of MBC and MBN to the succession of artificial vegetation are discussed. The results may provide a theoretical basis for evaluating soil fertility modification and soil organic carbon pool during succession of sand-binding vegetation.
2 Materials and methods
2.1 Field sampling
The artificial vegetation area was first founded in 1956, and expanded in 1964, 1981, 1987 and 1990,forming a vegetation belt which is 16 km long and 500 m wide. Shrubs and sub-shrubs such asArtemisia ordosicaKrasch,Caragana korshinskiiKom. andHedysarum(sweat vetch) were used firstly in the area.Artemisia ordosicabecame the domain species for its better natural propagation. With the extension in sand-binding time, biological algae and moss crusts are formed, and their thickness increases year by year. The physical and chemical properties of soil, such as clay content, nutrients and organic matter have been greatly improved, and the number of plant species has increased dramatically,especially herbs (Li, 2005). The study area is located in the north of the railway crossing through the Chinese Academy of Sciences Shapotou Desert Research and Experiment Station. The experiment was carried out from March to December of 2011. Three points were randomly chosen in each age of artificial vegetation founded in 1956, 1964, 1981, 1987 and 1990, as well as in the natural vegetation and mobile dune area. Soil samples were collected at each point from three soil layers of 0–5, 5–10 and 10–20 cm, and the samples were sealed in plastic bags for laboratory. After being sifted through a 2 mm sieve and remove litter and roots,the soil samples were stored refrigerated at 4 °C for cultivation.
2.2 Experimental protocol
20 g of fresh soil through a 2 mm sieve was put into a 50 mL beaker. The beaker was placed in a vacuum drier filled with chloroform (with a small amount of zeolite added). The bottom of the vacuum drier was moistened with water and a suitable amount of NaOH solution. The drier was vacuumed by a vacuum pump until the chloroform reached boiling point, and was continuously boiled for 2 min. The drier valve was turned off and the samples were kept in the dark for 24 h at the temperature of 25 °C. The chloroform was then removed by vacuuming 3–4 times, for 2–3 min each time, until no chloroform odor was present. The soil was transferred to a 250 mL Erlenmeyer flask, 100 mL of 0.5 M K2SO4was added, and the solution was shaken for 30 min at 25 °C.The soil solution was filtered into a plastic bottle, and stored at -15 °C for future use. Soil samples without fumigation and reagent blanks were also prepared for contrast testing.
A 10.00 mL soil extract and 10.00 mL of 0.1 N K2Cr2O7-H2SO4were placed in a solid 150 mL test tube,along with a small amount of zeolite. The test tube was boiled in a high temperature oil-bath for 10 min at a temperature of 175±1 °C. After cooling down, all the solutions were relocated into a 150 mL flask. The solution was titrated to brick red with 0.05 N FeSO4.
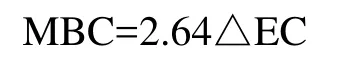
where △EC is the difference between the carbon extracted by 0.5 M K2SO4, with and without fumigation.

whereNis the concentration of FeSO4;V0is the volume of FeSO4for the reagent blank treatment (mL);Vis the volume of FeSO4for soil extraction (mL);Fis the dilution factor; andMis the weight of dry soil (g).
MBN was determined using the chloroform fumigation extraction method, and the experimental protocol was the same as that of microbial carbon.
The levels of nitrogen in the fumigated and non-fumigated soil solutions were determined using a Kjeldahl apparatus.
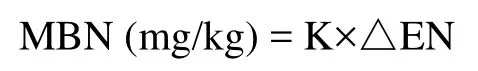
where △EN is the difference between nitrogen extracted by 0.5 M K2SO4with and without fumigation, andKis the water soil ratio.
3 Results
3.1 Seasonal variation of MBC and MBN
In the 0–5 cm soil layer, the contents of MBC and MBN in the artificial vegetation areas show a higher value during autumn than during spring and summer. In the soil depths of 5–10 and 10–20 cm, the seasonal variations of MBC and MBN were not significant (Figure 1).
3.2 Variation of MBC and MBN in the artificial vegetation succession process
In the 0–5 cm soil layer, the contents of MBC and MBN increased significantly with the age of the shrub plantation, particularly during autumn and summer (Figure 2). However, no significant variation was shown in spring, and the highest contents of MBC and MBN were found in the natural vegetation area.
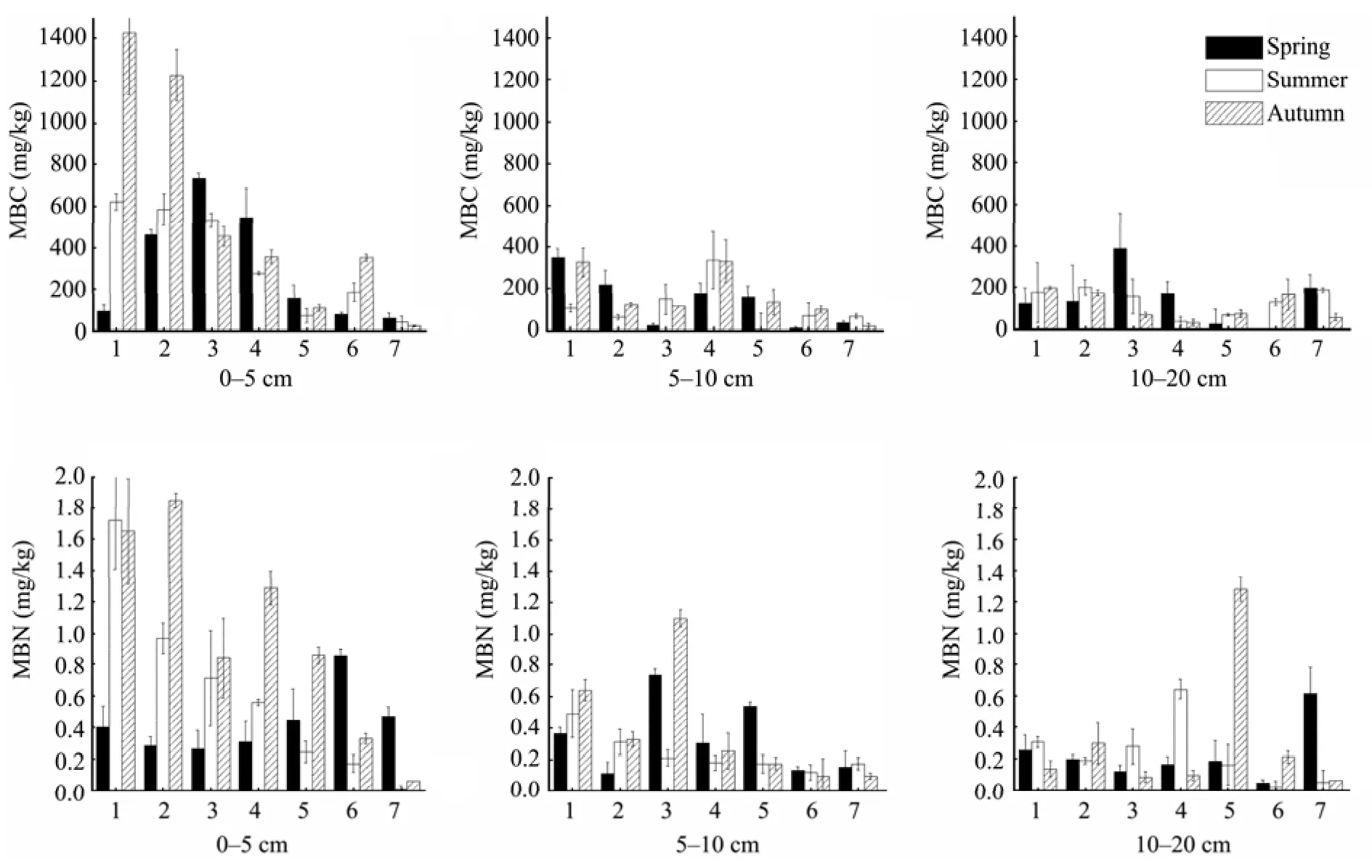
Figure 1 Changes of MBC and MBN in seasonal series. 1: A reference site with native vegetation;2, 3, 4, 5, 6: five different-aged revegetated sites (1956, 1964, 1981, 1987, and 1990); 7: mobile dunes
3.3 Variation of MBC and MBN in vertical sequence
Figure 3 shows the spatial variation characteristics of MBC and MBN during the artificial vegetation succession. During spring there was no significant variation of MBC and MBN with depth, while MBC and MBN decreased as the depth increased in summer and autumn.
In addition, as shown in Figure 3, in the mobile dune site, the MBC and MBN levels increased as the soil depth increased.

Figure 2 Changes of MBC and MBN in time series. 1: Topsoil (0–5 cm); 2: Soil depths of 5–10 cm; 3: Soil depths of 10–20 cm
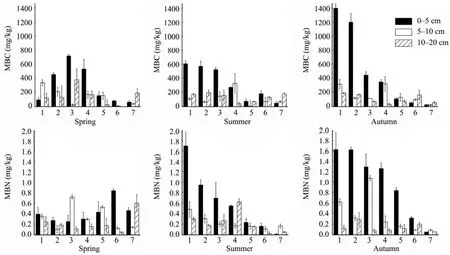
Figure 3 Changes of soil microbial biomass carbon and nitrogen in the vertical sequence. 1: A reference site with native vegetation; 2, 3, 4, 5, 6: five different-aged revegetated sites (1956, 1964, 1981, 1987, and 1990); 7: mobile dunes
4 Discussion
4.1 Seasonal variation characteristics of soil microbial biomass carbon and nitrogen
The experimental results showed that the contents of MBC and MBN in the 0–5 cm soil layer were higher during autumn than during spring and summer. For the deeper soil layers, the seasonal variation of MBC and MBN were not significant (Figure 1). The MBC and MBN contents were higher during autumn than in the other sample plots, which may have been the result of sampling after strong precipitation had occurred. Soil moisture significantly affects soil microbial biomass(Sparling and West, 1989); soil with higher moisture content may provide a favorable environment for microbial activity, and is conducive to the growth of soil microorganisms. The MBC and MBN contents are fairly sensitive to precipitation. The Shapotou region is located in arid and semi-arid zones, with an average annual rainfall of only 180 mm. Most of the precipitation consists of small amount of rainfall, which affected mainly the surface soil moisture. The relatively scarce precipitation in this region has a greater impact on the surface soil than on the deeper soil layers, which is likely to be the cause of the more apparent seasonal variations in the MBC and MBN levels of the 0–5 cm soil layer.
Temperature is another important indicator of seasonal sequence. A previous study (Wanderet al., 1994)analyzed the seasonal microbial biomass of theLeymus chinensis(Trin.) grassland in northeastern China; the results show that soil MB increased as temperature increased, and reached its highest point around mid-August (Chenet al., 1995). However, the results of this study did not show an increasing trend in the MBC and MBN levels as the temperature increased during the summer. This may be attributed to the fact that temperature affects soil microbial activity and plant root respiration enzyme activity. In the range of 0–35 °C, soil microbial activity and plant root respiration increased as temperature increased, and the optimum temperature is approximately 25–35 °C. When the temperature is higher than 45 °C, the activities of soil microbes and plant root respiration become inhibited (Chen and Hu, 1997;Chenet al., 1999; Caoet al., 2001), thus soil respiration is enhanced with an increase in temperature within a certain temperature range, and is depressed when the temperature exceeds a certain limit value.
Soil activity shows an increasing trend as temperature and moisture increases within a certain range, while soil activity is inhibited under extreme temperature and moisture conditions. In our study, surface soil temperature was often much higher than 45 °C in summer,reaching as high as 70 °C. In addition, a large number of observations showed that the seasonal variation of soil activity was significantly and positively correlated to seasonal variation of ground plant biomass, especially the seasonal variation of the weight of photosynthetic parts of the plants (Yanget al., 2004; Zhanget al., 2004).Therefore, the increase in soil MB during autumn may have resulted from the large number of leaves and dead roots, which caused transformation of the carbohydrates from the ground to the underground. The quantity and quality of the litter showed significant seasonal variation with the sequences of plant growth, and the variation showed a significant coincident relationship with the soil respiration rate. It was shown that, in general, low soil temperature during spring inhibits microbial and plant root activities, and strong solar radiation during summer inhibits soil activities. Soil temperature and moisture during autumn are suitable for soil microbial growth;therefore soil microbial biomass content is highest during autumn.
4.2 Variation characteristics of MBC and MBN during artificial vegetation succession process
After artificial vegetation was planted in mobile dunes, the mobile dunes changed from natural ecosystems into artificial ones, and then gradually reverted back to natural ecosystems. The succession process also altered soil MB. The experimental results indicated that MBC and MBN increased with the prolongation of vegetation restoration for the 0–5 cm soil layer during summer and autumn. No clear variation of MBC and MBN content during spring was shown. The highest MBC and MBN contents were found in the natural vegetation areas,mainly due to the fact that soils in native vegetation areas are more compacted, and with a greater area vegetation cover more water evaporation occurs, resulting in higher MBC and MBN contents. In the sand-binding areas, with the restoration of vegetation, the content of fine soil particles increases and water-holding capacity is enhanced, leading to higher MBC and MBN contents.These results are consistent with those of the study regarding the variation of MBC and MBN contents during the process of vegetation restoration in the Loess Hill Region (Huanget al., 2009). After the establishment of the artificial vegetation, with the continuation of vegetation succession, the community structure is changed from a single composition of shrubs and semi-shrubs to complex structures dominated by annual herbaceous plants (Liet al., 2005), which may also lead to a gradual increase in MBC and MBN.
4.3 Spatial variation of MBC and MBN
In spring there was no significant variation of MBC and MBN with depth, while MBC and MBN decreased as the depth increased in the summer and autumn (Figure 3). These results indicate that in the sand-binding process, nutrients in rainfall and dust are preserved, so that the soil nutrients accumulate in the surface layer (mainly concentrated in the 0–5 cm layer). In addition, plant litter containing fresh organic carbon and dense root secretions gradually accumulated, leading to enhanced soil biological activity in the surface soil. The microbial biomass in the deeper soils mainly originates from the decomposition of plant litter and scarce root exudates, resulting in soil activity variation in the vertical direction.The soil MB content in the sand-binding areas decreased with depth, and this change was similar to the process of accumulation of soil nutrients.
The average annual rainfall rate in the study area is less than 180 mm, and this rainfall is the region’s only source of soil water. In extreme conditions, particularly in arid areas, water distribution is the most limiting factor for plant growth, and may directly affect the restoration and reconstruction of degraded ecosystems in arid and semi-arid sand areas. As shown in Figure 3, the MBC and MBN levels increased as soil depth increased in mobile dunes, as a result of the loose soil matrix, soil infertility, and low water holding capacity (Jiaet al.,2006). Meanwhile, strong evaporation from the surface soil caused the occurrence of long-term low soil moisture content in the surface soil. The relatively high soil moisture conditions are the main cause of the MBC levels being higher in the deeper soil than in the surface soil.
5 Conclusions
(1) In the artificial vegetation, the MBC and MBN levels show the highest values in the 0–5 cm soil layer,and the highest values in the surface layer during autumn.The seasonal variations of the MBC and MBN levels were not apparent for the deeper soil layers.
(2) In the process of artificial vegetation succession,the MBC and MBN levels increased with the prolongation of vegetation restoration for the surface soil in summer and autumn. The highest MBC and MBN contents were found in the natural vegetation areas. No clear variations of MBC and MBN content were observed in spring. The MBC and MBN levels increased with the prolongation of vegetation restoration, indicating that the succession of sand-fixing vegetation will result in the accumulation of soil carbon and nitrogen, as well as the restoration of soil fertility.
(3) In the artificial vegetation areas, soil MB levels decreased with an increase in soil depth, while an opposite vertical variation trend was observed for mobile dunes.
This work was supported by the Chinese National Natural Scientific Foundation (41171077, 40801002,40971031). The authors would like to thank Dr. LiChao Liu who assisted with field work involved in the study.
Cao GM, Li YN, Zhang JX, Zhao XQ, 2001. Values of carbon dioxide emission from different land-use patterns of Alpine Meadow.Environmental Science, 22(6): 14–19.
Chen S, Zhang CZ, Liu DP, 1995. Seasonal variation in the biomass of soil decomposer microbes and its relaationship to the soil habitat in the leymus chinensis grasslands in northeast China. Acta Ecologica Sinica, 15(1): 91–94.
Chen SQ, Cui XY, Zhou GS, Li LH, 1999. Study on the CO2-release rate of soil respiration and litter decomposition instipa grandissteppe in Xilin River Basin, Inner Mongolia. Acta Botanica Sinica,41: 645–650.
Chen SY, Hu CS, 1997. Soil respiration rate of farmland ecosystem in Talhang Pledmont. Eco-agriculture Research, 5(2): 42–46.
Coleman DC, Reid CPP, Colo C, 1983. Biological strategies of nutrient cycling in soil systems. Advances in Ecological Research, (13):1–55.
Davidson EA, Ackeman IK, 1993. Changes in soil carbon inventories following cultivation of previously untilled soils. Biogeochemistry,20: 161–193.
Dilly O, Blume HP, Sehy U, Jimenez M, Munch JC, 2003. Variation of stabilized, microbial and biologically active carbon and nitrogen in soil under contrasting land use and agricultural management practices. Chemosphere, 52: 557–569.
Huang YM, An SS, Xue H, 2009. Responses of soil microbial biomass C and N and respiratory quotient (qCO2) to revegetation on the Loess Hilly-Gully region. Acta Ecologica Sinica, 29(6):2811–2818.
Insam H, 1990. Are the soil microbial biomass and basal respiration governed by the climatic regime? Soil Biology and Biochemistry,22(4): 525–532.
Jenknson DS, Ladd JN, 1981. Microbial biomass in soil: measurement and turnover. Soil Biochemistry Marcel Dekker, New York, (5):451–471.
Jia XH, Li XR, Chen YW, Li YS, 2006. The influence of vegetation recovery on organic carbon and nitrogen distribution in surface soil particle of dry area. China Environmental Science, 26(5): 560–564.
Li XR, 2005. Influence of variation of soil apatial heterogeneity on vegetation restoration. Science in China (Series D), 48: 2020–2031.
Li XR, Xiao HL, Liu LC, Zhang JG, Wang XP, 2005. Long-term effects of Sand-binding vegetation on the restoration of biodiversity in shapotou Region of Tengger Desert, Northern China. Journal of Desert Research, 25(2): 173–181.
Smith JL, Halvorson JJ, Papendick RI, 1993. Using multiple-variable indicator Kriging for evaluating soil quality. Soil Society of America Journal, 57: 743–749.
Sparling GP, West AW, 1989. Importance of soil water content when estimating soil microbial C, N and P by the fumigation-extraction methods. Soil Biology and Biochemistry, 21: 245–253.
Wander MM, Traina SJ, Stinner BR, Peters SE, 1994. The effects of organic and conventional management on biologically active soil organic matter fraction. Soil Society of America Journal, 58:1130–1139.
Wang GL, Liu GB, Hou XL, 2002. The research of species diversity after the vegetation restoration in loess hilly region. Journal of Mountain Research, 20(2): 182–187.
Yang Y, Hang JH, Zhan XM, Li X, Du LH, Li LH, 2004. The diurnal dynamic patterns of soil respiration for different plant communities in the agro-pastoral ecotone with reference to different measuring methods. Acta Phytoecologica Sinica, 28(3): 318–325.
Yao HY, He ZL, Huang CY, 1999. Turnover period of microbial biomass nitrogen in red soils and its significanse in soil fertility evaluation. Acta Pedologica Sinica, 36(3): 387–394.
Zhang XC, Yuan HL, Gao WS, 2004. Effect of crop-residue incorporation on soil CO2emission and soil microbial biomass. Chinese Journal of Applied Ecology, 15(3): 469–472.
 Sciences in Cold and Arid Regions2013年6期
Sciences in Cold and Arid Regions2013年6期
- Sciences in Cold and Arid Regions的其它文章
- Identification of an AP2 gene related to open flowering in diploid wheat (Triticum monococcum)
- Involvement of anti-oxidative enzymes, photosynthetic pigments and flavonoid metabolism in the adaptation of Reaumuria soongorica to salt stress
- Spatial coupling relationships of gas hydrate formation in the Tibetan Plateau
- The morphological characteristics of glacial deposits during the Last Glaciation, taking the Parlung Zangbo River Basin as an example
- The change of Ningchan River Glacier No. 3 at Lenglongling, east Qilian Mountain, China
- The influence of human activity and precipitation change on mid-long term evolution of landslide and debris flow disasters
