Involvement of anti-oxidative enzymes, photosynthetic pigments and flavonoid metabolism in the adaptation of Reaumuria soongorica to salt stress
YuBing Liu , Bo Cao, MeiLing Liu
Key Laboratory of Stress Physiology and Ecology in Cold and Arid Regions, Gansu Province; Shapotou Desert Research& Experiment Station, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
1 Introduction
Salinity can affect growth and yield of plants and can also generate secondary oxidative stress that occurs when there is a serious imbalance between the production of reactive oxygen species (ROS) and antioxidant defense, leading to oxidative damage of macromolecules and, eventually, of cellular structure (Meneguzzoet al.,1999). Plants have the ability to scavenge/detoxify ROS by producing different types of antioxidants. Antioxidants can be generally categorized into two different types,i.e., enzymatic and non-enzymatic. Enzymatic antioxidants include superoxide dismutase (SOD), catalase, ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase(DHAR) and glutathione reductase (GR). The commonly known non-enzymatic antioxidants are glutathione(GSH), ascorbate (AsA) (both water soluble), carotenoids, tocopherols (low molecular weight lipid soluble)and flavonoid (Ashraf, 2009).
Most plant antioxidant studies show that production of antioxidants is enhanced in response to salinity to counteract salt-induced elevated levels of ROS in plant cells. However, plants containing high levels of antioxi-dants can scavenge/detoxify ROS thereby contributing to increased salt tolerance (Wise and Naylor, 1987; Shalata and Tal, 1998; Ashraf, 2009). The over expression of GR,Peroxidase (POD) and APX activities inCalendula officinalisL. leads to an increase in the resistance to oxidative stress (Chaparzadehet al., 2004), whereas the change in levels of antioxidant molecules and enzyme activities inHelianthus annuusL. is among the first signals of plant tolerance and/or adaptation to stress conditions (Di Baccioet al., 2004).
It is evident that the over-expression of antioxidant enzymes or increase in the levels of antioxidative nonenzymatic metabolites is at least one component of the mechanism of salt tolerance in most plants. A significant part of antioxidants produced by plants in response to stress is secondary metabolites, including a vast array of simple and complex phenolic compounds derived primarily via the phenylpropanoid pathway (Dixon and Paiva, 1995). Phenolic compounds can also act as antioxidants by chelating metal ions, preventing radical formation and improving the antioxidant endogenous system (Al-Azzawie and Mohamed-Saiel, 2006). These compounds are known to act as antioxidants not only because of their ability to donate hydrogen or electrons but also because they are stable radical intermediates.Probably the most important natural phenolics are flavonoids because of their broad spectrum of chemical and biological activities, including antioxidant and free radical scavenging properties (Kahkonenet al., 1999). In fact, flavonoids have been reported as antioxidants,scavengers of a wide range of reactive oxygen species and inhibitors of lipid peroxidation (Williamset al.,2004).
The aim of this study is to investigate the role of enzymatic and non-enzymatic antioxidants on salt tolerance ofReaumuria soongorica(Pall.) Maxim. In particular, the flavonoid pathway of secondary metabolism was studied by determining the changes of total flavonoid content and the key enzyme activity.
2 Materials and methods
2.1 Plant materials and treatments
Reaumuria soongoricaseeds were planted in a field of the Botanical Garden of Lanzhou University and then after germination each seedling was transplanted into a 20-cm-diameter pot. The selected materials were three-year-old plants which were divided into four groups (15 pots per group), and then exposed for 8 and 14 days to salt stress by watering with different concentrations of NaCl solution (0, 100, 200, 400 mM) in each group, respectively. Plant treatment was carried out on Sep. 1, 2009. The treated plants were watered with saturated NaCl solution every three days at ambient air temperature and photoperiod.
2.2 Methods
Soil samples were selected from six pots per group and dried at 105 °C until constant weight, ground, and sieved through a 20-mesh screen. For each soil sample, a 1:5 soil-water extract was prepared and its electrical conductivity (EC) and salt content were measured by standard methods (Liuet al., 2006).
Total chlorophyll (Chl a+b), chlorophyll a(Chl a),chlorophyll b(Chl b), and carotenoids were determined spectrophotometrically using 80% acetone as a solvent(Lichtenthaler, 1987). Lipid peroxidation, antioxidant enzyme extraction and activity, flavonoid extraction and key enzyme activity of the flavonoid pathway were determined according to the methods of Liuet al.(2011).
All data were presented as means ± standard deviations of three determinations. Statistical analyses were performed using Students t-test and one-way analysis of variance. Multiple comparisons of means were done by LSD (least significant difference) test. Statistical assessments of differences between mean values were performed by Duncan’s multiple range test atP≤0.05.
3 Results
3.1 Soil salt content
Soil salt content increased significantly with increasing content of treated NaCl solution, and no variations between 8 and 14 days during treatment, except for treatment with 100 mM NaCl solution(Figure 1). After 14 days of 400 mM NaCl treatment, soil salt content was about eight times that of the control.
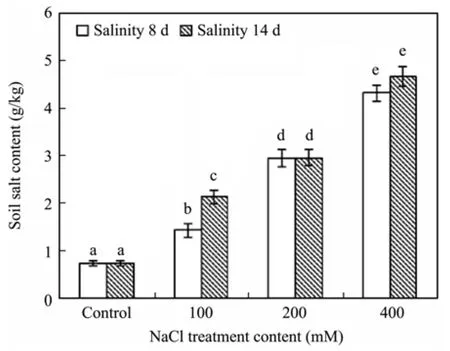
Figure 1 Variations of soil salt content of R. soongorica by watering with different solution contents of NaCl within 14 days
3.2 Lipid peroxidation and enzymatic antioxidants
To determine the extent of salt-induced oxidative damage, we monitored changes in lipid peroxidation by measuring the malondialdehyde (MDA) formation in leaves ofR. soongorica(Figure 2a). There was no significant effect on MDA content in eight days treatment below 200 mM NaCl. However, MDA content increased by about 30% treated with 400 mM NaCl in 14 days as compared to the control group.
SOD (Figure 2b) activity values show a significant increase under NaCl treatment in 8 and 14 days, and there was no variation among different content of NaCl treatment. POD activity (Figure 2c) increased with both increasing NaCl content and treatment time, arriving at a peak value in 14 days under 400 mM NaCl treatment.Compared with POD and SOD activity, APX activity data (Figure 2d) revealed the same trend with MDA content during treatment, exhibiting significant changes both in 8 and 14 days under 400 mM NaCl treatment.
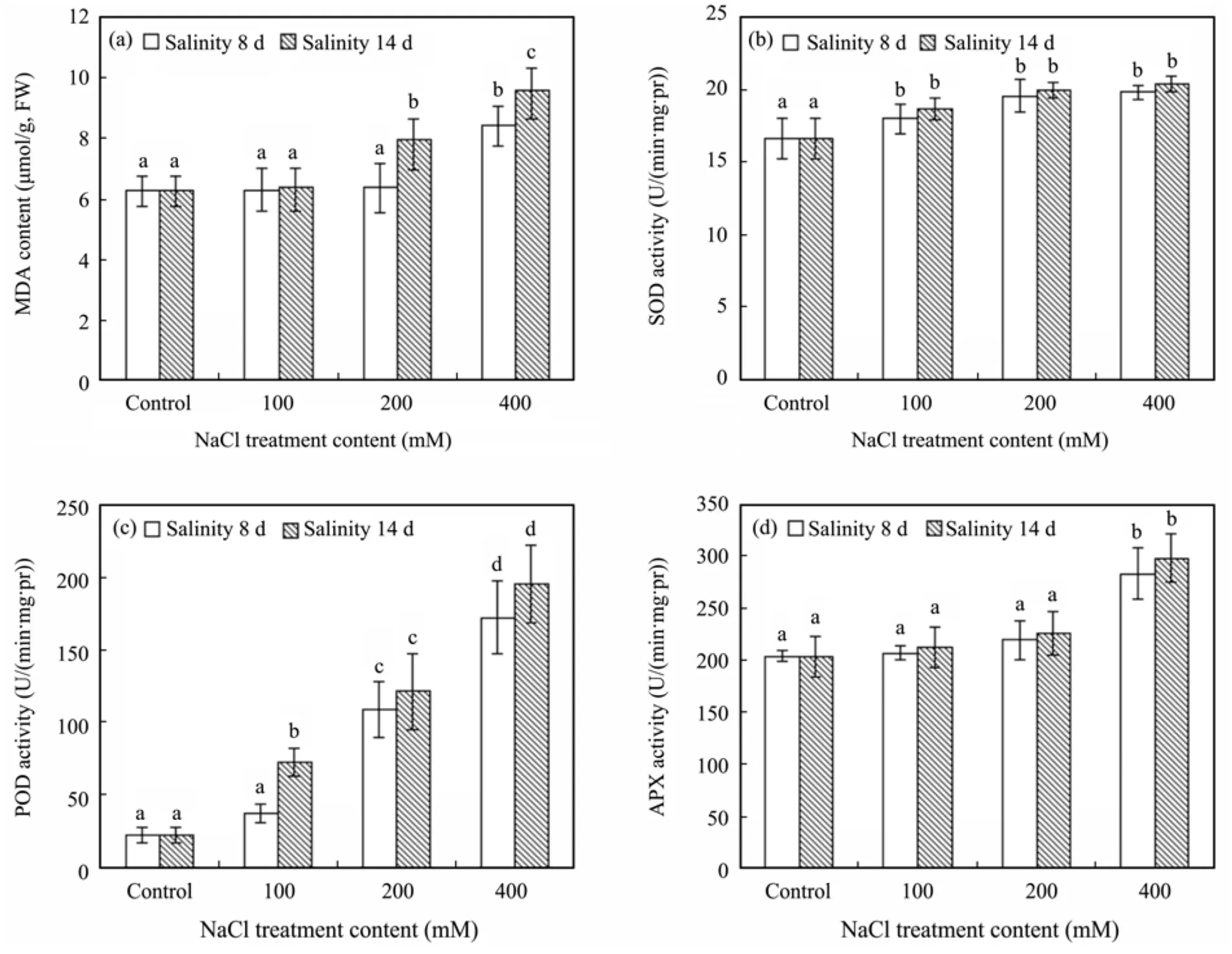
Figure 2 Effects of salt stress on the content of MDA (a) and the activities of SOD (b), POD (c) and APX (d) in leaves of R. soongorica, after 8 and 14 days of treatment by watering with different solution contents of NaCl
3.3 Photosynthetic pigments content
Photosynthetic pigments content are shown in Figure 3. All measured photosynthetic pigments including Chl a,Chl b, total chlorophyll and carotenoid contents (Figures 3a, 3b, 3c and 3d) decreased significantly above the 100 mM NaCl treatment, with few significant variations between 8 and 14 days treatments. The ratio of Chl a/Chl b(Figure 3e) displayed a slight increase above the 100 mM NaCl treatment in 8 and 14 days during the experiment. However, the ratio of carotenoid/Chl (Figure 3f)revealed a significant increase above the 200 mM NaCl treatment.
3.4 Flavonoid contents and key enzyme activities
Figure 4 shows the changes of total flavonoid and anthocyanin content, and the activities of PAL and CHI inR. soongoricaafter NaCl treatment. Total flavonoid,anthocyanin content and CHI activity decreased with increasing content of NaCl during the treatment (Figures 4a, 4b and 4d). PAL activity increased with the 100 mM NaCl treatment, and then decreased above the 100 mM NaCl treatment (Figure 4c). There were no significant differences in all enzymatic and non-enzymatic antioxidants between 8 and 14 days treatments.
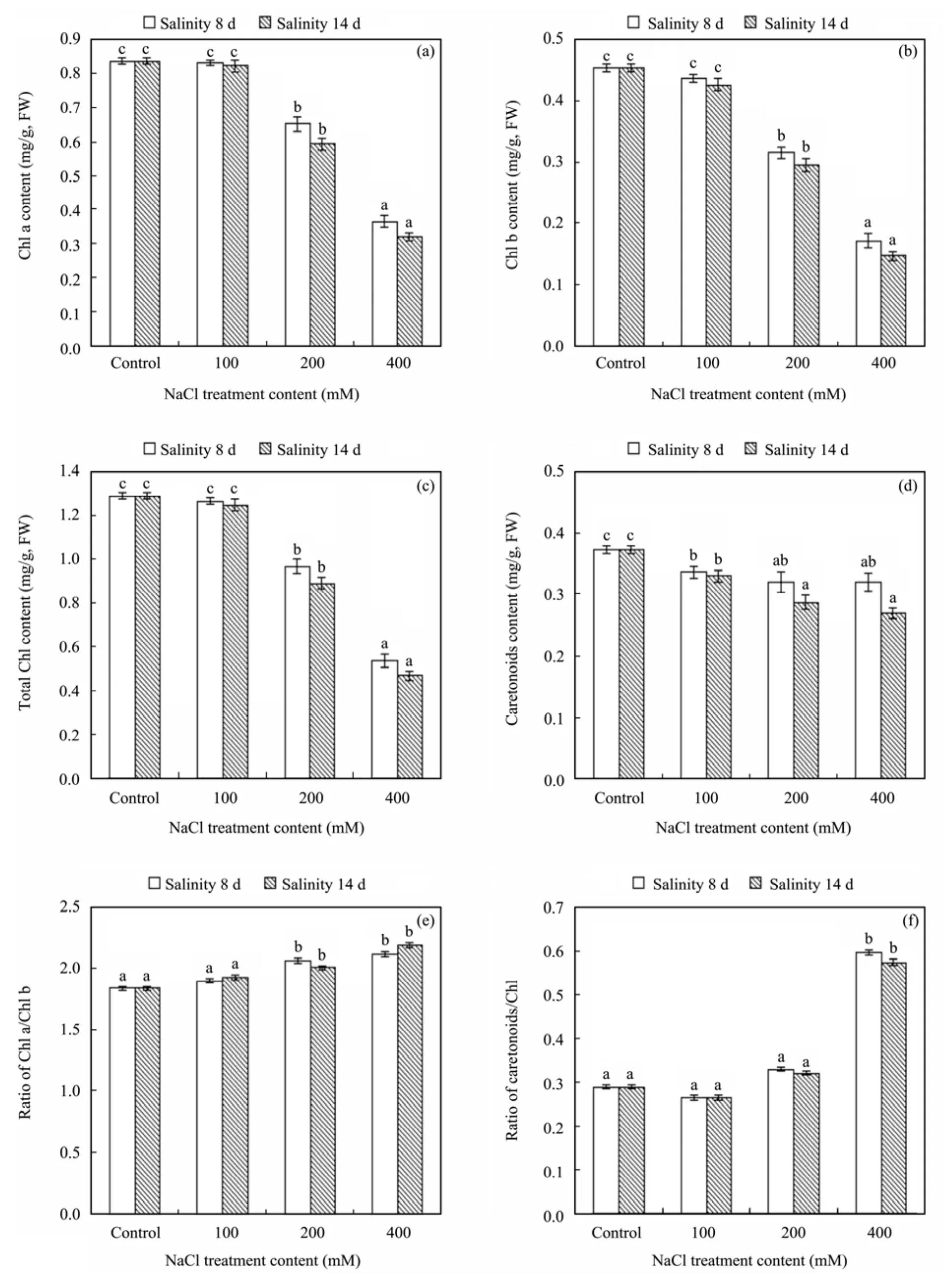
Figure 3 Effects of salt stress on contents of Chl a (a), Chl b (b), total chlorophyll (c) and caretonoids (d), and the ratio of Chl a/Chl b (e) and caretonoids/Chl (f) in leaves of R. soongorica, after 8 and 14 days treatment by watering with different solution contents of NaCl

Figure 4 Effects of salt stress on the contents of total flavonoid (a) and anthocyanin (b), and the activities of PAL (c) and CHI (d)in R. soongorica, after 8 and 14 days treatment by watering with different solution contents of NaCl
4 Discussion
Plants possess specific mechanisms to detoxify reactive oxygen species which include activation of antioxidant enzymes such as superoxide dismutase, catalase,peroxidase and those of the ascorbate–glutathione cycle(Noctor and Foyer, 1998; Smirnoff, 2005) as well as non-enzymatic antioxidants such as flavones, tocopherols, anthocyanins, carotenoids and ascorbic acid (Mittler,2002; Johnsonet al., 2003). Antioxidant enzymes such as SOD, CAT, APX, POD, GR and MDAR are known to substantially reduce the levels of superoxide and hydrogen peroxide in plants (Ali and Alqurainy, 2006). Superoxide dismutase catalyzes the dismutation of O2•-to molecular oxygen and H2O2(Giannopolitis and Ries,1977). SOD is one of the most important enzymes used against oxidative stress in the plant defense system, and it occurs ubiquitously in every cell of all types of plants.Like SOD, ascorbate peroxidases also play a vital role in plant defense against oxidative stress. Ascorbate peroxidases are the key enzymes for scavenging hydrogen peroxide in chloroplast and cytosol of plant cells (Asada,1992). In addition to enzymatic antioxidants, plants possess a variety of non-enzymatic molecules which play a substantial role in counteracting oxidative stress caused by stress. These non-enzymatic antioxidants include among others ascorbic acid, tocopherols, carotenoids,flavonoids, and glutathione (Noctor and Foyer, 1998;Tausz and Grill, 2000; Schaferet al., 2002).
In the present study, metabolism of MDA depends on various functionally interrelated antioxidant enzymes such as APX and POD. In particular, APX play a key role in the removal of H2O2in the chloroplast and cytosol (Noctor and Foyer, 1998; Moritaet al., 1999), and changes in the activity of this enzyme are strictly correlated with plant tolerance to oxidative stress (Ben Amoret al., 2006). NaCl stress changed the levels of MDA inR. soongorica, and induced the activation of antioxidant enzymes such as SOD, POD and APX, suggesting that enzymatic antioxidants play an important role under NaCl stress.
A research group in Japan (Usukiet al., 1984; Endoet al., 1985) first suggested a pro-oxidant activity of chlorophyll under light, which could be understood as an energy transfer of singlet-excited chlorophyll to oxygen that would form reactive oxygen species. However, the same authors also reported that chlorophyll and pheophytins provide protection by preventing autoxidation of vegetable edible oils stored in the dark and suggested a hydrogen donating mechanism breaking the radical chain reactions. Wanasundara and Shahidi (1998) reported that the presence of chlorophyll in tea extracts was responsible for a pro-oxidant effect on the oxidation of marine oils. Hoshinaet al.(1998) found that chlorophyll are better antioxidants than their metal free derivatives and confirmed the importance of the porphyrin ring on the inhibition of lipid autoxidation. Sakataet al.(1990) reported that a related compound to pheophorbide a, isolated from a species of clam, appeared to be the most important antioxidant in the organism. Reduction of pigments content, as a result of either slow synthesis or fast breakdown, has been considered as a typical symptom of oxidative stress (Smirnoff, 1993).
In the treatment of NaCl, chlorophyll content decreased significantly inR. soongoricaduring the experiment, which indicated that there was a photoprotection mechanism through reducing light absorbance by decreasing pigment content (Munné-Bosch and Alegre,2000; Galméset al., 2007; Elsheery and Cao, 2008).Higher ratio of Chl a/Chl b was also considered as a decreased emphasis on light collection in relation to the rates of PSII photochemistry (Demmig-Adams and Adams, 1996). Carotenoids are known to function as collectors of light energy for photosynthesis and as quenchers of triplet chlorophyll and O2. Moreover, they dissipate excess energy via the xanthophyll cycle and can act as powerful chloroplast membrane stabilizers that partition between light-harvesting complexes (LHCs) and the lipid phase of thylakoid membranes, reducing membrane fluidity and susceptibility to lipid peroxidation (Havaux,1998). Selected carotenoids are components of the light harvesting system in chloroplasts and play an important role in the protection of plants against photooxidative damage (Demmig-Adams and Adams, 2002). Caretonoid content decreased inR. soongoricaduring the experiment, which indicated that the protection by caretonoid was not one of the most important mechanisms under salt stress, while an increased ratio of caretonoid/Chl revealed a high tolerance inR. soongoricaat the 400 mM NaCl treatment. The increased ratio of caretonoid/Chl under stress conditions indicated a higher need of photoprotection (Elsheery and Cao, 2008).
Flavonoids also show antioxidant activity against a variety of oxidizable compounds. They belong to a large category of organic compounds,i.e., phenolics. Flavonoids occur widely in the plant kingdom, and are commonly found in leaves, floral parts, and pollen. Flavonoids usually accumulate in the plant vacuole as glycosides, but they also occur as exudates on the surface of leaves and other aerial plant parts (Vierstraet al., 1982).Several flavonoids act as potential inhibitors of the enzyme lipoxygenase, which converts polyunsaturated fatty acids to oxygen-containing derivatives (Nijveldtet al., 2001). In the present work, the contents of total flavonoid and anthocyanin decreased with increasing content of NaCl during the treatment. CHI activity also decreased under salt stress, but PAL activity increased in 100 mM NaCl and then decreased significantly above 200 mM NaCl treatment. This indicates that non-enzymatic antioxidant flavones, anthocyanins and carotenoids did not play a protection role inR. soongoricaunder salt stress, and only specific enzymes such as PAL was induced under lower content of NaCl treatment.
In conclusion, non-enzymatic antioxidant flavonoids,anthocyanin and carotenoids did not play an important role inR. soongoricaunder salt stress, but a steady level of caretonoid/Chl was exhibited under 400 mM NaCl treatment, suggesting that there was a high tolerance inR.soongoricaunder salt stress. These results may be due to antioxidant enzymes such as SOD, APX and POD scavenging ROS thereby contributing to increased salt tolerance.
This work was financially supported by the National Natural Science Foundation of China (31070358,91125029, 31160089 and 31000181).
Al-Azzawie HF, Mohamed-Saiel SA, 2006. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci.,78: 1371–1377.
Ali AA, Alqurainy F, 2006. Activities of antioxidants in plants under environmental stress. In: Motohashi N (ed.). The Lutein-prevention and Treatment for Diseases. Transworld Research Network, India,pp. 187–256.
Asada K, 1992. Ascorbate peroxidase—a hydrogen peroxide-scavenging enzyme in plants. Physiol. Plant, 85: 235–241.
Ashraf M, 2009. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv., 27:84–93.
Ben Amor N, Jiménez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C, 2006. Response of antioxidant systems to NaCl stress in the halophyteCakile maritime. Physiol. Plant, 126: 446–457.
Chaparzadeh N, D’Amico ML, Khavari-Nejad RA, Izzo R, Navari-Izzo F, 2004. Antioxidative responses ofCalendula officinalisunder salinity conditions. Plant Physiol. Biochem., 42: 695–701.
Demmig-Adams B, Adams III WW, 1996. Chlorophyll and carotenoid composition in leaves ofEuonymus kiautschovicusacclimated to different degrees of light stress in the field. Aust. J. Plant Physiol.,23: 649–659.
Demmig-Adams B, Adams III WW, 2002. Antioxidants in photosynthesis and human nutrition. Science, 298(5601): 2149–2153.
Di Baccio D, Navari-Izzo F, Izzo R, 2004. Seawater irrigation: antioxidant defense responses in leaves and roots of a sunflower (Helianthus annuusL.) ecotype. J. Plant Physiol., 161: 1359–1366.
Dixon RA, Paiva NL, 1995. Stress-induced phenylpropanoid metabolism. Plant Cell, 7(7): 1085–1097.
Elsheery NI, Cao KF, 2008. Gas exchange, chlorophyll fluorescence,and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant., 30: 769–777.
Endo Y, Usuki R, Kaneda T, 1985. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. I. Comparison of the inhibitory effects. J. Am. Oil Chem. Soc., 62(9):1375–1378.
Galmés J, Abadía A, Medrano H, Flexas J, 2007. Photosynthesis and photoprotection responses to water stress in the wild-extinct plantLysimachiaminoricensis. Environ. Exp. Bot., 60: 308–317.
Giannopolitis CN, Ries SK, 1977. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol., 59: 309–314.
Havaux M, 1998. Carotenoids as membrane stabilizers in chloroplasts.Trends Plant Sci., 3: 147–151.
Hoshina C, Tomita K, Shioi Y, 1998. Antioxidant activity of chlorophylls: its structure–activity relationship. Photosynthesis: Mechanisms Effects, 4: 3281–3284.
Johnson SM, Doherty SJ, Croy RRD, 2003. Biphasic superoxide generation in potato tubers. A self amplifying response to stress. Plant Physiol., 13: 1440–1449.
Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS,Heinonen M, 1999. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem., 47: 3954–3962.
Lichtenthaler HK, 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol., 148: 350–382.
Liu GM, Yang JS, Yao RJ, 2006. Electrical conductivity in soil extracts:chemical factors and their intensity. Pedosphere, 16(1): 100–107.
Liu YB, Liu ML, Cao B, 2011. Effects of drought stress on antioxidant enzyme, photosynthetic pigment and flavonoid pathway in two desert shrubs. Sciences in Cold and Arid Regions, 3(4): 332–338.
Meneguzzo S, Navari-Izzo F, Izzo R, 1999. Antioxidant responses of shoots and roots of wheat to increasing NaCl concentrations. J.Plant Physiol., 155: 274–280.
Mittler R, 2002. Oxidative stress, antioxidants and stress tolerance.Trends Plant Sci., 7: 405–410.
Morita S, Kaminaka H, Masumura T, Tanaka K, 1999. Induction of rice cytosolic ascorbate peroxidase mRNA by oxidative stress: the involvement of hydrogen peroxide in oxidative stress signaling.Plant Cell Physiol., 40: 417–422.
Munné-Bosch S, Alegre L, 2000. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss inRosmarinus officinalisplants.Planta, 207: 925–931.
Nijveldt RJ, van Nood E, van Hoorn DEC, Boelens PG, van Norren K,van Leeuwen PAM, 2001. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr., 74:418–425.
Noctor G, Foyer CH, 1998. Ascorbate and glutathione: keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol., 49:249–279.
Sakata K, Yamamoto K, Ishikawa H, Yagi A, Etoh H, Ina K, 1990.Chlorophyllone-A, a new pheophorbide a related compound isolated fromRuditapes philippinarumas an antioxidant compound.Tetrahedron Letters, 31(8): 1165–1168.
Schafer RQ, Wang HP, Kelley EE, Cueno KL, Martin SM, Buettner GR, 2002. Comparing carotene, vitamin E and nitric oxide as membrane antioxidants. Biol. Chem., 383(3–4): 671–681.
Shalata A, Tal M, 1998. The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relativeLycopersicon pennellii. Physiol. Plant., 104(2):169–174.
Smirnoff N, 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol., 125: 27–58.
Smirnoff N, 2005. Antioxidants and Reactive Oxygen Species in Plants.Blackwell Publishing Book.
Tausz M, Grill D, 2000. The role of glutathione in stress adaptation of plants. Phyton., 40: 111–118.
Usuki R, Endo Y, Kaneda T, 1984. Prooxidant activities of chlorophylls and pheophytins on the photooxidation of edible oils. J. Biol.Chem., 48(4): 991–994.
Vierstra RD, John TR, Poff KL, 1982. Kaempferol 3-O-galactoside 7-O-rhamnoside is the major green fluorescing compound in the epidermis ofVicia faba. Plant Physiol., 69(2): 522–525.
Wanasundara UN, Shahidi F, 1998. Antioxidant and prooxidant activity of green tea extracts in marine oils. Food Chem., 63(3): 335–342.
Williams RJ, Spencer JPE, Rice-Evans C, 2004. Flavonoids: Antioxidants or signaling molecules? Free Radical Bio. Med., 36(7):838–849.
Wise RR, Naylor AW, 1987. Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and endogenous antioxidants.Plant Physiol., 83(2): 278–282.
 Sciences in Cold and Arid Regions2013年6期
Sciences in Cold and Arid Regions2013年6期
- Sciences in Cold and Arid Regions的其它文章
- Identification of an AP2 gene related to open flowering in diploid wheat (Triticum monococcum)
- Spatial coupling relationships of gas hydrate formation in the Tibetan Plateau
- The morphological characteristics of glacial deposits during the Last Glaciation, taking the Parlung Zangbo River Basin as an example
- The change of Ningchan River Glacier No. 3 at Lenglongling, east Qilian Mountain, China
- The influence of human activity and precipitation change on mid-long term evolution of landslide and debris flow disasters
- Seasonal variation in soil microbial biomass carbon and nitrogen in an artificial sand-binding vegetation area in Shapotou, northern China
