径向电场对纳米管中水分子通量的影响
葛振朋 石彦超 李晓毅
(中国科学院大学材料科学与光电技术学院,北京100049)
1 Introduction
Water transport in nanopores is crucial to biological activities1-3and important for designing novel fluidic devices.Hummer and coworkers4,5reported the fast water conduction ability of a short carbon nanotube by molecular dynamics(MD)simulations.In 2006,the enhanced water flow through carbon nanotubes(CNTs)was observed experimentally.6Holt et al.6found that the water flow rate through CNTs with radius of 1-2 nm was about 3 orders of magnitude faster than the conventional nonslip hydrodynamic flow.Zheng et al.3found that the relationship between the CNT wall-water interfacial friction stress and slip velocity follows a transition-state-theory-based inverse hyperbolic sine function.Bocquet et al.7studied the interfacial friction of water at graphitic interfaces with various topologies and found that the friction coefficient exhibits a strong curvature dependence,while friction is independent of confinement for the graphene slab.Thus controlling the transport of water across CNTs has attracted considerable attentions.8Osmotic or hydrostatic pressure gradient can drive water flow in a channel.1,2,9An imbalance of chemical,10thermal gradients11could change the movement of water in nanopores.
Joseph and Aluru12,13found that the water flow across the nanopores is strong coupled with the water dipole orientations and hydrogen bonds.It is easy to tune the dipole orientation of water by external electric field or charge when water molecules are confined.14,15Thus the external electric field or charge is one of the most promising method to control the gating and flowing behaviors of water molecules in a nanochannel.14,16-22Figueras and Faraudo23found that the application of an electric field perpendicular to the axis of the carbon nanotube would disrupt the hydrogen bond structure of the one dimensional(1D)water system and affect the transport properties of water inside the nanotube.Suk and Aluru18found a net flux of 3 water molecules per nanosecond by constantly maintaining water dipole vectors in the direction of the electric field.Su and Guo24have investigated the effect of axial electric field on the transport of single-file water molecules through a CNT.However,controlling of the water net flux in nanopores and the open/close behavior of nanopores for water molecules are far from satisfactory.
Molecular dynamics simulation has been proved to be a good method for the study of water molecules and nanomaterials.25-27In present work,we utilize the orthogonal electric field to control the net flux of water transport through a carbon nanotube and investigate the on-off gating behavior of a CNT.
2 Model design and simulation details
As shown in Fig.1,an uncapped(6,6)single-walled carbon nanotube of 2 nm in length and 0.81 nm in diameter was inserted along the z direction between two graphene layers.The distance between the graphene layers along z direction was 1.84 nm.822 water molecules were added in the up and down reservoirs thus the initial size of total system was 3 nm×3 nm×5 nm.
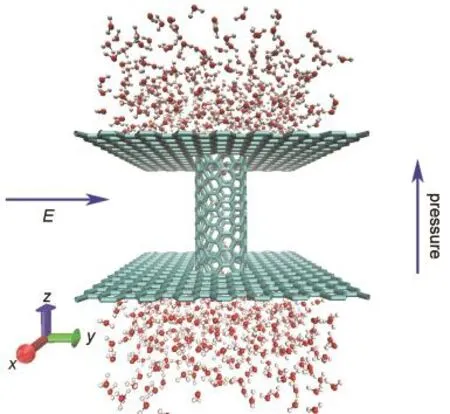
Fig.1 Snapshot of simulation system
All systems were run at an NPT ensemble(101 kPa and 300 K)using the software NAMD V2.828with periodic boundary conditions applied in all directions.CHARMM 27 force field29and TIP3P water model30were employed.The carbon atoms in CNT and graphene were assumed as the carbon atom in benzene which was named CA in CHARMM 27 force field.After 2 ns equilibrium molecular dynamics simulations without any external force,a uniform orthogonal electric field was added along+x direction during the rest 20 ns simulation time.The designed strengths of electric fields(E)are 0.5,1,1.5,2,2.5,2.7,2.8,2.9,3,3.5,4,4.5,and 5 V·nm-1,respectively.A system without any external electric field was also built for comparison.To accelerate the flux through CNT,a pressure difference of 200 MPa along z direction was utilized following the method proposed by Zhu et al.2Particle Mesh Ewald method31was used to calculate the full electrostatics interaction and the cutoff distance of van der Waals interaction was 1.2 nm.A time step of 2 fs was used.Structures were saved every 1 ps.20 ns MD simulation was run for each system and the data of last 15 ns were collected for statistics.To prevent the CNT from being swept away,the CNT and graphene layers were fixed during our simulations.
3 Results and discussion
The net flux of water molecules passing through a(6,6)tube for the different strengths of orthogonal electric field is shown in Fig.2a.According to the previous publications,4,9,16,24,32,33the upflux is defined as the total number of water molecules per nanosecond conducted through the CNT from top to bottom along with the direction of the pressure difference,so is the downflux but with the opposite direction.The net flux is the difference between upflux and downflux.We observed that the relationship between the net flux and the external orthogonal electric field strength E could be divided into three stages.At the beginning,when the orthogonal electric field strength E increases from 0 to 0.5 V·nm-1,the net flux of water maintains around 23 molecules·ns-1and is regardless of the E.The downflux is zero in this stage.With E increasing from 0.5 to 3 V·nm-1,the water net flux decreases linearly from 23 to 1 molecules·ns-1.The downflux is very small in this stage.When E increases to the stage of 3 to 5 V·nm-1,the water net flux is almost zero and regardless of E again.
Su and Guo24has studied the effect of an axial electric field strength in the range of 0-1 V·nm-1on the transport of water molecules through a carbon nanotube.They observed that the net flux increases with increasing E of axial electric field strength for E<0.07 V·nm-1and maintains 1.1-1.2 molecules·ns-1for E≥0.07 V·nm-1.The net flux is much sensitive to the axial electric field strength than the orthogonal electric field.However,it is easier to control the water net flux transport through CNT precisely by orthogonal electric field because the net flux is almost linear to the orthogonal electric field strength in a wide range of orthogonal electric field strength from 1 to 3 V·nm-1.The filling behavior of water molecules in CNTs can be described by the water occupancy <N>,as shown in Fig.2b.The water occupancy<N> also exhibits three stages of 7-6.5,6.5-1,and 1-0 molecules·ns-1corresponding to the 0-2,2-3,and 3-5 V·nm-1of orthogonal electric field strength,respectively.There is a steep slope of<N> from 6.5 to almost 0 molecules·ns-1when the orthogonal electric field increases from 2 to 3 V·nm-1,which indicates that the orthogonal electric field may be used to turn on/off the nanotube to water molecules.

Fig.2 (a)Averaged water flux through the CNT and(b)averaged number of water molecules inside the CNT with respect to the orthogonal electric field(E)
In order to understand the dependence of water net flux or occupancy on the dipole orientation of water molecules inside nanopores,we investigate the dipole orientations at different orthogonal electric field strengths.The probability distributions of the averaged angle <θ> are shown in Fig.3.The angle θ represents the angle between the dipole of a water molecule and the tube axis,and the average is taken over all water molecules inside the CNT.Apparently,the probability distributions with respect to the orthogonal electric strength E exhibit three stages too.In the first stage,when the orthogonal electric strength E increases from 0 to 2 V·nm-1,the peaks of the distribution of<θ> lie around 30°or 150°,in agreement with previous studies.16,24,32-35In the second stage,further increasing E from 2.5 to 3 V·nm-1,the first peak moves from 33°to 42°and the second peak vanishes gradually,as shown in Fig.3b.In the third stage,when E increases from 3.5 to 5 V·nm-1,the only peak moves from 49°to 71°,as shown in Fig.3c.This phenomenon is understandable according to the electrostatics theory.When the orthogonal electric field acts on the water molecules,water molecules rotate their dipole orientations to decrease the electrostatic energy of the system.However,the orthogonal electric field should be strong enough to make water molecules rotate.We have not observed the situation of having water molecules with both directions inside nanotube at the same time,for the nanotube is short in our simulations,which agrees with the results of Fang and co-workers.36
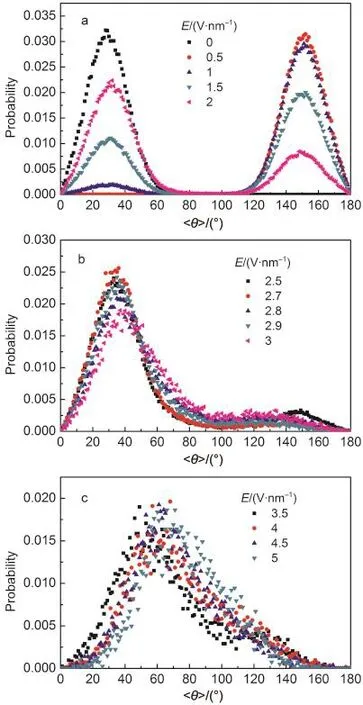
Fig.3 Probability distribution of the averaged angle<θ>between the water dipole and the tube axis at different orthogonal electric field strengths
According to Joseph and Aluru,12the water net flux should be in the direction of the aligned water dipoles when there is no other driving force.However,when the driving force is the 200 MPa pressure difference acting on the top of the first layer of water molecules as in our simulations,the averaged water dipole orientation might be opposite to the direction of net flux,which is exhibited in the case of E=0.5 V·nm-1.Comparing Fig.2 and Fig.3,we find that the probability distributions of<θ> is much stronger coupled with the water occupancy <N>rather than the net flux when 200 MPa pressure difference is being applied on water molecules.In the first stage when the peaks of the distribution of<θ> lie around 30°or 150°,the water occupancy <N> maintains a level of 6-7 molecules·ns-1.<N> decreases sharply to 1 molecules·ns-1in the second stage when the first peak moves from 33°to 42°and the second peak gradually vanishes.In the third stage when the only peak moves from 49°to 71°,<N> vanishes to zero.
The averaged angle<θ> with respect to the simulation time for different E values is also investigated to understand the flipping of water dipoles inside CNT and its sensitivity to the orthogonal electric field,as shown in Fig.4a.We observed that for E=0,0.5 V·nm-1,the water dipole orientations have not reversed their directions from the initial orientation within the 20 ns simulation time.When E is small(≤ 2 V·nm-1),the twostate(30°and 150°)orientation is very clear.With increasing E,the two-state orientation of water dipole is not so clear and there appear more and more middle-states,which means that angle<θ> moves away from 30°or 150°to the middle point of 90°.A flip is defined as <θ> passing through 90°.The average flipping frequency,fflip,is calculated and shown with respect to E in Fig.4b.At 1 V·nm-1of E,the flipping frequency is 0.15 ns-1corresponding to 6 ns of flipping period,which is within the range of 4-6 ns measured in Ref.20.The flipping frequency increases remarkably as the orthogonal electric field strength increases larger than 2 V·nm-1.This is due to the frequent back and forth flip around 90°,corresponding to the appearance of a lot of middle-states of the dipole orientation.It is found that the flipping frequency is also strong coupled with the water occupancy<N>rather than the net flux.
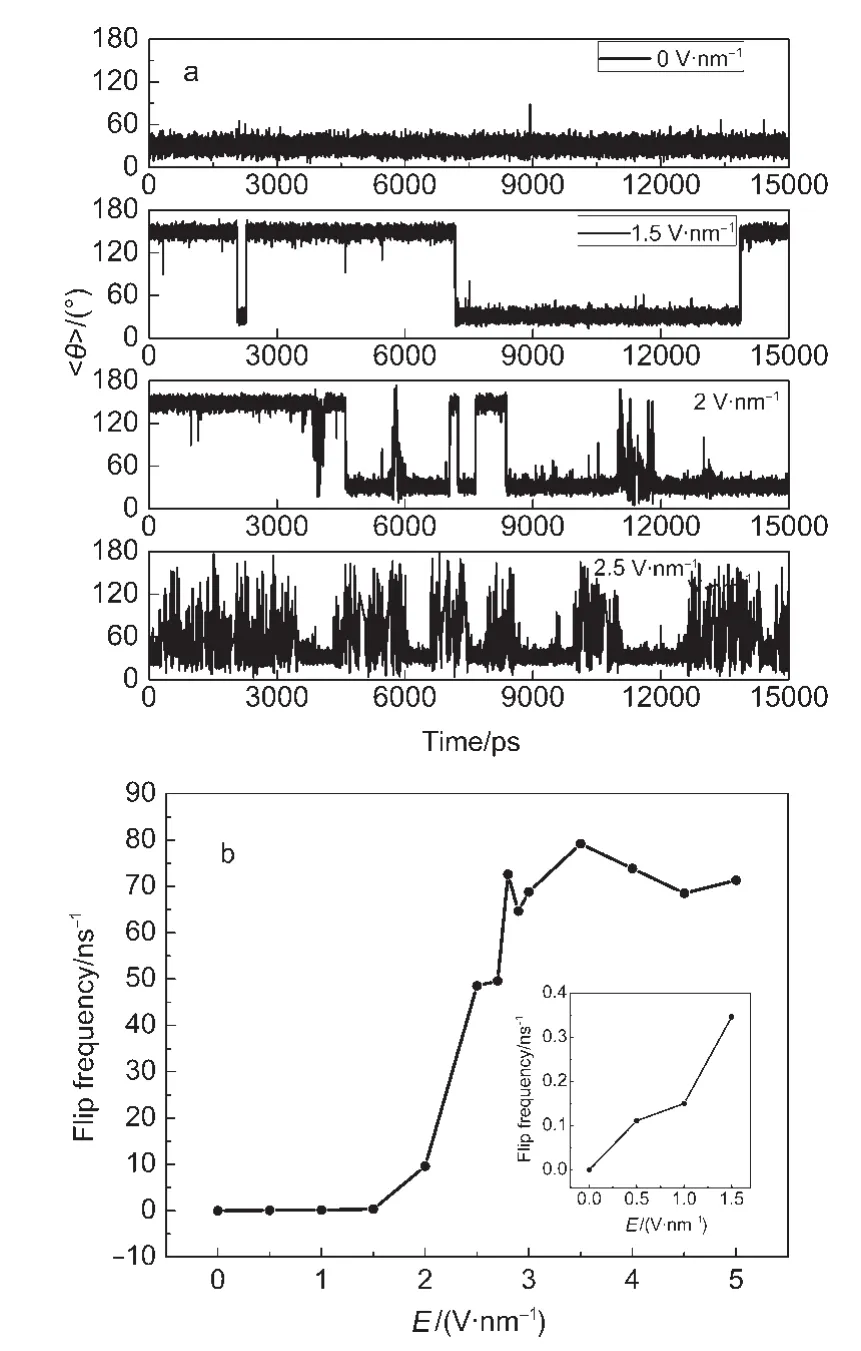
Fig.4 (a)<θ> vs time at different orthogonal electric field values,(b)flip frequency vs the orthogonal electric field
The water flux is related to the free energy profile along with the nanotube axis.As shown in Fig.5,the free energy barriers for water transport are less than 1kBT(kBT equals to 4.14×10-21J,which is twice the amount of a particleʹs one-dimensional freedom contributing to the internal energy in the ideal gas)when E is less than 2.5 V·nm-1.As E increases to 2.7 V·nm-1,the total tendency of free energy begins to increase along with z direction,which could act as the opposite force to the driving force of the pressure difference on water molecules.
The free energy barrier increases above 3.70kBT after E increases to 3 V·nm-1,and the nanotube is turned off.There is almost no net flux in this case,as shown in Fig.2a,although the pressure difference is still acting on water molecules.Apparently,the free energy barrier acts as the opposite force overcoming the driving force of the pressure difference in this case.
Hydrogen bond plays an important role in the transportion of water molecules.Water molecules are connected by hydrogen bonds in a special orientation and formed an ordered 1D structure water chain inside nanotube.They move concertedly passing through the nanotube.4,37Orthogonal electric field ruptures the hydrogen-bond network by making an influence on the orientations of water molecules.We oberved that the hydrogen-bond network inside the nanotube become shorter as the electrical field strength increased and we have even observed isolated water moleculars inside the nanotube.The average number of hydrogen bonds inside the nanotube of different orthogonal electric field strength was calculated and we found that the number of hydrogen bonds decreascd as the strength of electrical field increased,as can be seen in Fig.6.Therefore or-thogonal electric field could control the number of water molecules passing through the nanotube by influencing on the orientation of water molecules and the number of hydrogen bonds among water molecules inside nanotube.

Fig.5 Free energy profiles for water through the channels z is the position coordinate along the tube and F(z)=-kBTln[ρ(z)/ρ0],where ρ(z)is the water density along the channel pore.
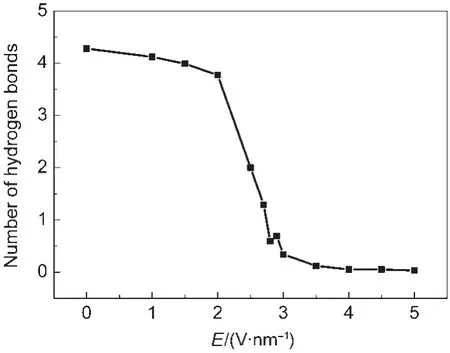
Fig.6 Average number of hydrogen bonds inside the nanotube with different orthogonal electric field strengths
4 Conclusions
In this study,we found that not only the net flux of water transported through a CNT could be controlled by an orthogonal electric field,but also the on-off gating behavior of the CNT.With a 200 MPa pressure difference acting on the top of the first layer of water molecules,as E increases from 1 to 3 V·nm-1,the net flux of water decreases linearly.When E increases over 3 V·nm-1,the flow of water molecules through the CNT is turned off and the net flux of water is almost zero.<N>decreases sharply from 6.5 to 1 molecules·ns-1when the E increases from 2 to 3 V·nm-1,which indicates that an orthogonal electric field may be used to turn on/off the flow of water molecules through a CNT.
When E was in the range of 0-2 V·nm-1,the angle<θ> between the water dipole and CNT axis clearly exhibited two states of 30°and 150°,resulting in <N> of 6.5-7 molecules·ns-1.Further increasing E from 2.5 to 3 V·nm-1caused the first peak of the probability distribution of<θ> to move from 33°to 42°and the second peak to gradually disappear,so <N> decreased rapidly to 1 molecules·ns-1.When E increased from 3.5 to 5 V·nm-1,the only probability distribution peak moved from 49°to 71°,and <N> decreased to zero.Both the orientation of water dipoles and the flipping frequency were strongly correlated with<N>in CNTs.
After the E was increased to 3 V·nm-1,the free energy barrier increased above 3.70 kBT and acted as an opposing force to overcome the driving force of the 200 MPa pressure difference on the water molecules,causing the net flux of water to decrease almost to zero.
We also found that the number of hydrogen bonds decreased as E increased.Therefore,an orthogonal electric field could be used to control the number of water molecules passing through a nanotube by influencing the orientation of water molecules and the number of hydrogen bonds between water molecules inside a nanotube.
Acknowledgment: The results described in this paper are obtained on the Deepcomp7000 of Supercomputing Center,Computer Network Information Center of Chinese Academy of Sciences.
(1) Zhu,F.Q.;Tajkhorshid,E.;Schulten,K.Biophys.J.2004,86,50.doi:10.1016/S0006-3495(04)74082-5
(2) Zhu,F.Q.;Tajkhorshid,E.;Schulten,K.Biophys.J.2002,83,154.doi:10.1016/S0006-3495(02)75157-6
(3) Ma,M.D.;Shen,L.;Sheridan,J.;Liu,J.Z.;Chen,C.;Zheng,Q.Phys.Rev.E 2011,83,036316.doi:10.1103/PhysRevE.83.036316
(4)Hummer,G.;Rasaiah,J.C.;Noworyta,J.P.Nature 2001,414,188.doi:10.1038/35102535
(5)Waghe,A.;Rasaiah,J.C.;Hummer,G.J.Chem.Phys.2002,117,10789.doi:10.1063/1.1519861
(6)Holt,J.K.;Park,H.G.;Wang,Y.M.;Stadermann,M.;Artyukhin,A.B.;Grigoropoulos,C.P.;Noy,A.;Bakajin,O.Science 2006,312,1034.doi:10.1126/science.1126298
(7) Falk,K.;Sedlmeier,F.;Joly,L.;Netz,R.R.;Bocquet,L.R.Nano Lett.2010,10,4067.doi:10.1021/nl1021046
(8) Zhang,Z.Q.;Ye,H.F.;Liu,Z.;Ding,J.N.;Cheng,G.G.;Ling,Z.Y.;Zheng,Y.G.;Wang,L.;Wang,J.B.J.Appl.Phys.2012,111,114304.doi:10.1063/1.4724344
(9)Zuo,G.C.;Shen,R.;Ma,S.J.;Guo,W.L.ACS Nano 2010,4,205.doi:10.1021/nn901334w
(10) Chaudhury,M.K.;Whitesides,G.M.Science 1992,256,1539.doi:10.1126/science.256.5063.1539
(11) Linke,H.;Aleman,B.J.;Melling,L.D.;Taormina,M.J.;Francis,M.J.;Dow-Hygelund,C.C.;Narayanan,V.;Taylor,R.P.;Stout,A.Phys.Rev.Lett.2006,96,154502.doi:10.1103/PhysRevLett.96.154502
(12) Joseph,S.;Aluru,N.R.Phys.Rev.Lett.2008,101,064502.doi:10.1103/PhysRevLett.101.064502
(13) Joseph,S.;Aluru,N.R.Nano Lett.2008,8,452.doi:10.1021/nl072385q
(14) Vaitheeswaran,S.;Yin,H.;Rasaiah,J.C.J.Phys.Chem.B 2005,109,6629.doi:10.1021/jp045591k
(15)Bratko,D.;Daub,C.D.;Leung,K.;Luzar,A.J.Am.Chem.Soc.2007,129,2504.doi:10.1021/ja0659370
(16) Li,J.Y.;Gong,X.J.;Lu,H.J.;Li,D.;Fang,H.P.;Zhou,R.H.Proc.Natl.Acad.Sci.U.S.A.2007,104,3687.doi:10.1073/pnas.0604541104
(17) Raghunathan,A.V.;Aluru,N.R.Phys.Rev.Lett.2006,97.
(18)Suk,M.E.;Aluru,N.R.Phys.Chem.Chem.Phys.2009,11,8614.doi:10.1039/b903541a
(19) Gong,X.J.;Li,J.Y.;Lu,H.J.;Wan,R.Z.;Li,J.C.;Hu,J.;Fang,H.P.Nat.Nanotechnol.2007,2,709.doi:10.1038/nnano.2007.320
(20) Won,C.Y.;Joseph,S.;Aluru,N.R.J.Chem.Phys.2006,125,117701.doi:10.1063/1.2338305
(21) Garate,J.A.;English,N.J.;MacElroy,J.M.D.J.Chem.Phys.2009,131,8.
(22) Dzubiella,J.;Allen,R.J.;Hansen,J.P.J.Chem.Phys.2004,120,5001.doi:10.1063/1.1665656
(23) Figueras,L.;Faraudo,J.Mol.Simulat.2012,38,23.doi:10.1080/08927022.2011.599032
(24) Su,J.Y.;Guo,H.X.ACS Nano 2011,5,351.doi:10.1021/nn1014616
(25) Lü,Y.J.;Chen,M.Acta Phys.-Chim.Sin.2012,28,1070.[吕勇军,陈 民.物理化学学报,2012,28,1070.]doi:10.3866/PKU.WHXB201202213
(26) LI,H.L.;Jia,Y.X.;Hu,Y.D.Acta Phys.-Chim.Sin.2012,28,573.[李海兰,贾玉香,胡仰栋.物理化学学报,2012,28,573.]doi:10.3866/PKU.WHXB201112191
(27)Zhang,X.;Zhang,Q.;Zhao,D.X.Acta Phys.-Chim.Sin.2012,28,1037.[张 霞,张 强,赵东霞.物理化学学报,2012,28,1037.]doi:10.3866/PKU.WHXB201203072
(28) Phillips,J.C.;Braun,R.;Wang,W.;Gumbart,J.;Tajkhorshid,E.;Villa,E.;Chipot,C.;Skeel,R.D.;Kale,L.;Schulten,K.J.Comput.Chem.2005,26,1781.
(29) Vanommeslaeghe,K.;Hatcher,E.;Acharya,C.;Kundu,S.;Zhong,S.;Shim,J.;Darian,E.;Guvench,O.;Lopes,P.;Vorobyov,I.;MacKerell,A.D.J.Comput.Chem.2010,31,671.(30) Jorgensen,W.L.;Chandrasekhar,J.;Madura,J.D.;Impey,R.W.;Klein,M.L.J.Chem.Phys.1983,79,926.doi:10.1063/1.445869
(31) Essmann,U.;Perera,L.;Berkowitz,M.L.;Darden,T.;Lee,H.;Pedersen,L.G.J.Chem.Phys.1995,103,8577.doi:10.1063/1.470117
(32) Wan,R.;Lu,H.;Li,J.;Bao,J.;Hu,J.;Fang,H.Phys.Chem.Chem.Phys.2009,11,9898.doi:10.1039/b907926m
(33) Wan,R.Z.;Li,J.Y.;Lu,H.J.;Fang,H.P.J.Am.Chem.Soc.2005,127,7166.doi:10.1021/ja050044d
(34)Yang,Y.L.;Li,X.Y.;Jiang,J.L.;Du,H.L.;Zhao,L.N.;Zhao,Y.L.ACS Nano 2010,4,5755.doi:10.1021/nn1014825
(35) Liu,B.;Li,X.Y.;Li,B.L.;Xu,B.Q.;Zhao,Y.L.Nano Lett.2009,9,1386.doi:10.1021/nl8030339
(36)Wu,K.F.;Zhou,B.;Xiu,P.;Qi,W.P.;Wan,R.Z.;Fang,H.P.J.Chem.Phys.2010,133,204702.doi:10.1063/1.3509396
(37) Zhu,F.;Schulten,K.Biophys.J.2003,85,236.doi:10.1016/S0006-3495(03)74469-5