丙烯酸分子的激发态超快预解离动力学
张蓉蓉 秦朝朝 龙金友 杨明晖 张 冰
(中国科学院武汉物理与数学研究所,波谱与原子分子物理国家重点实验室,武汉430071;中国科学院研究生院,北京100049)
丙烯酸分子的激发态超快预解离动力学
张蓉蓉 秦朝朝 龙金友 杨明晖 张 冰*
(中国科学院武汉物理与数学研究所,波谱与原子分子物理国家重点实验室,武汉430071;中国科学院研究生院,北京100049)
利用飞秒泵浦-探测技术结合飞行时间质谱(TOF-MS),研究了丙烯酸分子被200 nm泵浦光激发到第二电子激发态(S2)后的超快预解离动力学.采集了母体离子和碎片离子的时间分辨质谱信号,并利用动力学方程对时间分辨离子质谱信号进行拟合和分析,揭示了预解离通道的存在.布居在S2激发态的分子通过快速的内转换弛豫到第一电子激发态(S1),时间常数为210 fs,随后再经内转换从S1态弛豫到基态(S0)的高振动态,时间常数为1.49 ps.分子最终在基态高振动态势能面上发生C-C键和C-O键的断裂,分别解离生成H2C=CH和HOCO、H2C=CHCO和OH中性碎片,对应的预解离时间常数分别约为4和3 ps.碎片离子的产生有两个途径,分别来自于母体离子的解离和基态高振动态势能面上中性碎片的电离.
时间分辨质谱;超快动力学;泵浦-探测;飞行时间质谱;内转换
1 Introduction
The real-time investigation of the ultrafast dynamics of excited molecules in the gas phase has attracted a great deal of attentions over the last two decades.1-4With the development of ultrashort-pulse laser technology,5femtosecond pump-probe technique has been proved to be a powerful tool to study the ultrafast phenomena and obtain new insights into the detailed mechanisms of excited molecules.6In a typical pump-probe experiment,a pump pulse excites the molecule to an excited state and a second delayed probe pulse monitors the change of the population in the excited state.By varying the time delay between the pump and probe pulses,it is possible to assemble measurements as a function of delay time.The time-dependent population can reveal the ultrafast relaxation processes7such as internal conversion(IC)and intersystem crossing(ISC).
The gas-phase photochemistry of unsaturated carboxylic acids has been the subject of numerous interests and investigations.8-16As the smallest α,β-unsaturated carboxylic acid,acrylic acid(H2C=CHCOOH)is an ideal system for investigating photochemistry of this kind of molecules and provides an important dynamic model for studying configuration interactions between C=C and C=O double bonds.The ultraviolet photodissociation of acrylic acid has been studied experimentally and theoretically in some detail.12-22Several processes have been proposed as possible dissociation pathways:

Reactions(1)and(2)involve the cleavage of single bonds CC and C-O,respectively,while reactions(3)and(4)are decarboxylation and decarbonylation processes.
Rosenfeld and Weiner12studied the photodissociation dynamics of acrylic acid at 248 and 193 nm using infrared fluorescence technique and concluded that the decarboxylation was the primary pathway at both wavelengths.On the basis of the mass fragmentation spectral data,Miyoshi et al.19have pointed out that,in addition to the C-C bond fission,reactions(2)and (4)occurred after excitation at 193 nm.Afterwards,Kitchen et al.17carried out an intensive study of acrylic acid upon 193 nm irradiation in a cross laser-molecular beam apparatus by measuring the photofragment velocity distributions.They only observed products which resulted from primary C-O and C-C bond fissions,while decarboxylation was the minor channel of dissociation.However,their experimental data were still not enough to precisely describe the mechanism for how the dissociation happened.Recently,in the photodissociation study of acrylic acid excitation with ArF(193 nm)laser carried out by Upadhyaya et al.,13the initially prepared S2state underwent nonradiative transitions to S1state,where the molecule subsequently dissociated to give OH fragments and a risetime of 2 μs was seen from the time-resolved fluorescence signal.However,the lifetime of S2and S1states was not determined due to the time resolution limitation of the excimer laser.The dissociation time constant and mechanism also need to be further confirmed.Apart from the experimental studies,Fang and Liu22determined the dissociation of acrylic acid to CH3CH+CO2, CH2CHOH+CO,CH2CH+COOH,and CH2CHCO+OH in the ground state surface as well as in the excited state surface using ab initio quantum chemical methods after excitation to S2state.They proposed the most probable dissociation mechanism,but no sufficient experimental evidence to support their calculated results.It is obvious that the previous studies of acrylic acid were mainly focused on the determination of the dissociation channels.However,the researchers are still not clear about how the dissociation happens,what intermediate states and ultrafast processes involved and what kind of dissociation is(direct dissociation or predissociation).Femtosecond time-resolved experiments can figure out these questions very well.
Therefore,the femtosecond time-resolved study of acrylic acid after UV excitation of 200 nm is reported in this paper. We measure the mass spectra signals of the parent ion and fragment ions as a function of the delay time.How the evaluation of the excited molecule over time after preparing the optically bright S2(1ππ*)state will be identified and the mechanism of the generation of the fragment ions will be discussed in detail. Moreover,we will try to determine the predissociation time constant for different dissociation pathways.The goal of this research is attempted to give a clear description of the ultrafast dynamics and dissociation mechanism of acrylic acid via S2excited state.
2 Experimental
The experimental setup consists of a regenerative amplified Ti:sapphire femtosecond laser system(Coherent Int.,America), a molecular beam apparatus,a time-of-flight mass spectrometer(TOF-MS),and a two-dimensional(2D)position sensitive detector.The femtosecond laser system has been described in detail elsewhere.23Briefly,it produces a pulse of 45 fs duration centered at 800 nm with maximum energy of 1 mJ·pulse-1at 1 kHz repetition rate.This fundamental light was frequency doubled in a 0.5 mm thick BBO crystal(type I,cut angle 29.2°)to generate the second harmonic 400 nm pulse.The 266 nm probe pulse(third harmonic)was generated in a 0.2 mm thick BBO crystal(type I,cut angle 44.3°)by sum frequency mixing of the second harmonic and the fundamental,while the 200 nm pump pulse(fourth harmonic)was generated in a 0.2 mm thick BBO crystal(type I,cut angle 64.7°)by sum frequency mixing of the third harmonic and the fundamental.The pump and probe beams with vertical polarization were merged by a dichroic mirror and directed into the molecular beam chamber. The time delay between the pump and probe pulses was accurately monitored by a computer-controlled linear translation stage(PI,M-126.CG1,Germany).
The experiment was performed in a homebuilt apparatus, which has been described elsewhere.24-26The liquid sample (acrylic acid,99.9%purity),typically 5%seeded in helium carrier gas at a background pressure of 2×105Pa,was expanded through a pulsed valve(Parker,General Valve,America)with the aperture 0.6 mm to generate a pulsed molecular beam.The molecular beam was skimmed and introduced into the ionization chamber where it intersected perpendicularly with the pump and probe laser beams in the extraction field region of the TOF-MS to produce the ions.The field-free region(360 mm)of the TOF-MS was shielded with a μ-metal tube to suppress outer magnetic fields.The generated ions were monitored by the detector assembly consists of a dual microchannel plate(MCP,Photek,Britain)and a fast P47 phosphor screen (3040FM,Galileo Electro-Optic Corp,America).A photomultiplier tube(PMT,931A,Hamamatsu,Japan)is mounted behind the screen to collect TOF mass spectra.The output mass spectra signal was displayed by a digital oscilloscope(Tektronix, TDS 2012,America)and then transferred to a computer that controlled the experiment.
3 Results and discussion
The TOF mass spectrum of acrylic acid are obtained at the temporal overlap of the 200 nm pump and the 266 nm probe light,as shown in Fig.1.The peaks of parent ion and fragment ions have been observed in the spectrum.The peaks yielded at m/e of 72,55,45,27,and 17 corresponding to C3H4O+2parent ion,H2C=CHCO+,HOCO+,H2C=CH+,and very weak OH+fragment ions,respectively.The TOF mass spectra show that the cleavage of C-C bond and C-O bond happened after excitation of acrylic acid.
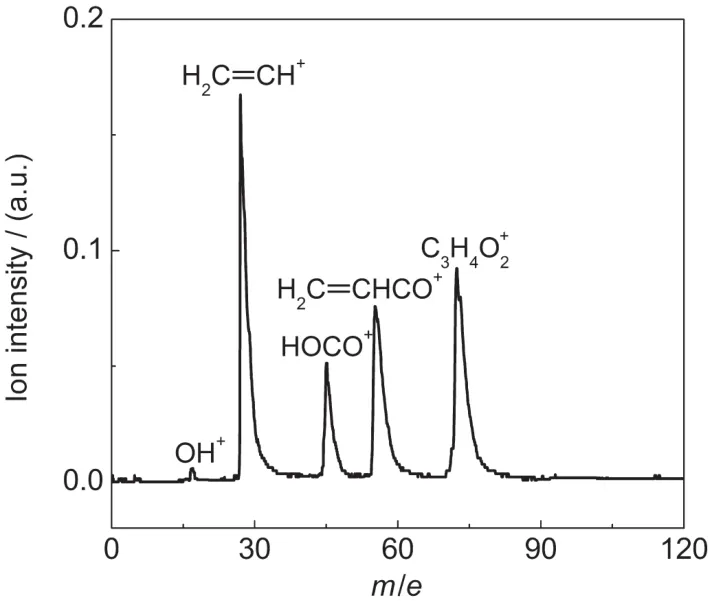
Fig.1 TOF mass spectrum of acrylic acid obtained at the temporal overlap of 200 nm pump and 266 nm probe light
Fig.2 shows the recorded time-resolved mass spectra ion signals of,HOCO+,and H2C=CH+as a function of the pump-probe delay time.The signal of OH+fragment ion is too weak to extract the time-resolved ion signal.It is obvious that the signal profile of fragment ions shows different decay behavior compared to parent ion after several picoseconds.The dynamics concealed in the decay of these ion signals will be discussed later by our kinetic equation fitting and analysis.
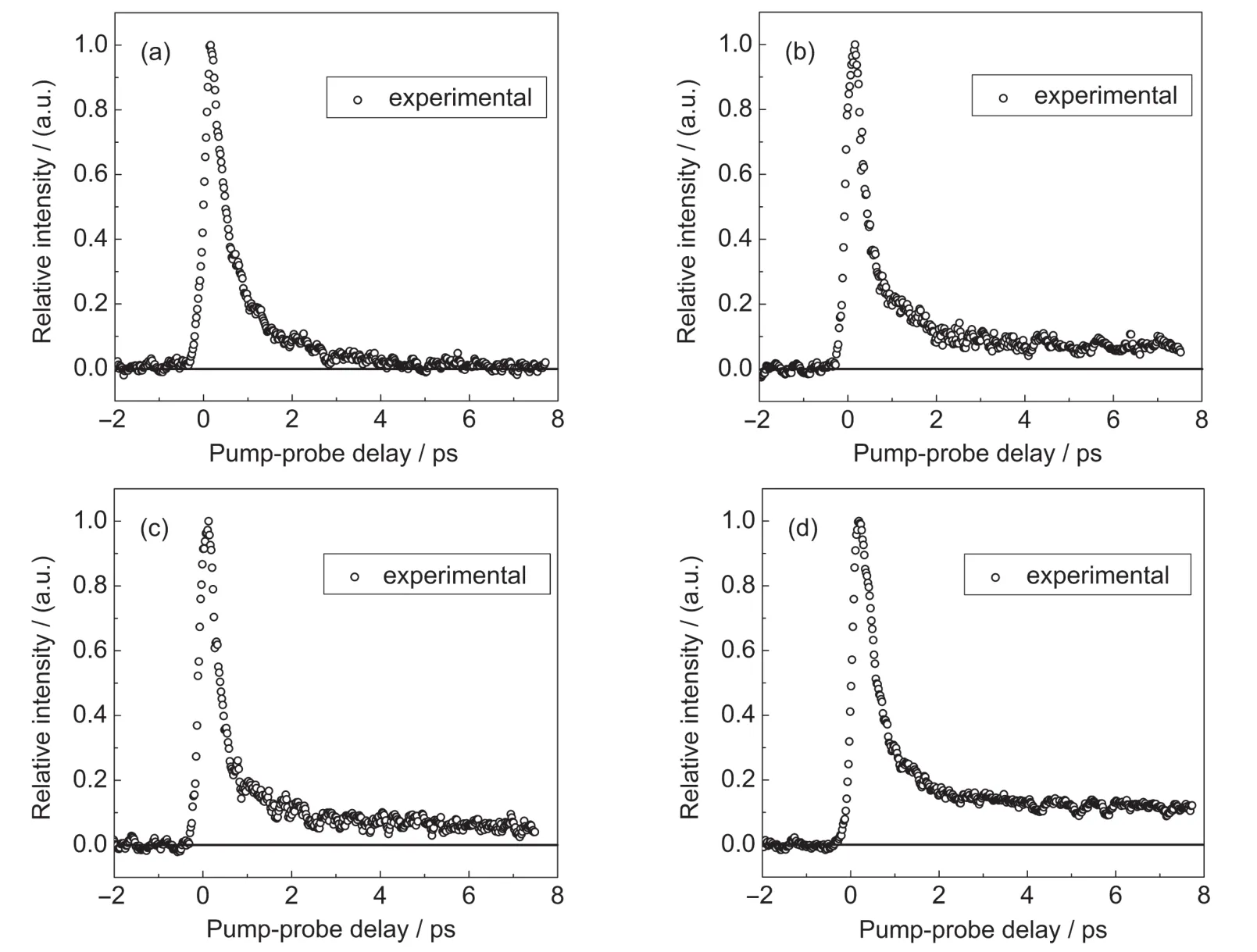
Fig.2 Time-resolved mass spectra ion signals recorded as a function of the pump-probe delay(a)C3H4O2+,(b)H2C=CHCO+,(c)HOCO+,(d)H2C=CH+;The black line is the zero baseline.
Since the ionization energy(IE)of acrylic acid is 10.77 eV,27the ionization of acrylic acid can be obtained by one 200 nm (6.32 eV)photon pump and one more 266 nm(4.65 eV)pho-ton probe.It is believed that the excitation of acrylic acid at 200 nm is populated on the S2(1ππ*)state centered at vinyl group with an admixture of π*(C=C)/π*(C=O)character, which is confirmed by the emission spectrum reported by Arendt et al.10The S1state of acrylic acid is of nπ*character localized on the carbonyl group,which is a dissociative state.13From the theoretical calculation of acrylic acid,Fang and Liu22concluded that after the molecule populated on the S2state,the system may decay through three radiationless routes,internal conversion(IC)to S0,to S1and intersystem crossing(ISC)to T2.Generally speaking,an intersystem crossing from excited singlet to triplet state occurs with very low efficiency in the carbonyl compounds.28Moreover,Upadhyaya et al.13reported that when(π,π*)transition of acrylic acid is excited by ArF(193 nm)laser pulses,the initially prepared S2state through nonradioactive transition to higher vibrational levels of the S1state is the dominant pathway because it seems unlikely that a spin-forbidden ISC to T2state can compete with spin-allowed IC to S1state.
In our experiment,the ground state acrylic acid absorbs one 200 nm photon to the S2excited state,then the excited molecule can be ionized by absorbing one 266 nm probe photon forming parent ion.According to the discussion above,the S2state most probably through IC decays to the S1state and S0state,the S1state then relaxes to the S0state,here S1and S0refer to the higher vibrational levels of the S1state and the vibrationally hot ground state,respectively.The excited molecule populated on the S1state can also be ionized by absorbing probe light,while in the S0state,the molecule could not be directly ionized since very high energy required for ionization according to Franc-Condon principle.Thus,we believe that the parent ion comes from the ionization of S2and S1excited states.In order to confirm the proposed complex kinetic schemes,we use coupled kinetic equations to fit the time profile of parent ion.It is known that the photoionization cross sections of S2and S1states depend on the electronic configuration of these states and cation state.Thus,the relative ratio of ionization cross section of the two states is an unknown quantity. We assume that the relative ratio of ionization cross section of the S1and S2states is P1and the possibility of simultaneous excitation of the S1state is neglected in our fitting processes. Then,the coupled kinetic equations are given as follows:
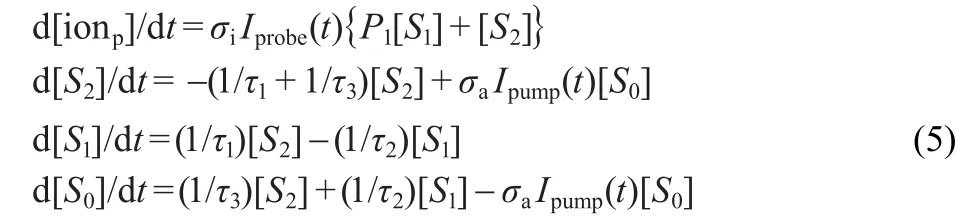
where[ionp],[S2],[S1],and[S0]represent the relative population of parent ion,second excited state,first excited state, and vibrationally hot ground state of acrylic acid,respectively. d[X]/dt(X=ionp,S2,S1,and S0)represents the relative population changing over time.σiis the photoionization cross section and σais the photoabsorption cross section.Ipump(t)and Iprobe(t) are the pump and probe laser intensities,respectively.1/τ1,1/τ2, and 1/τ3represent the S2-S1,S1-S0,and S2-S0IC rates,respectively.Fluorescence from S2and vibrationally excited S1states is ruled out due to their very low quantum yield.
The fitting results of the time-resolved parent ion signal using kinetic equations are shown in Fig.3.Open circles are the experimental results and solid lines are the fitting results.The kinetic fitting is consistent with our experimental data very well.It gives time constants of τ1=210 fs,τ2=1.49 ps,P1=0.21, and τ3is very long relative to τ1and τ2.The results indicate that IC of the S2state to the vibrationally hot S0state is negligible and the relative ratio of ionization cross section of S1and S2states is 0.21.The fast red component(210 fs)in the time profile corresponding to the lifetime of S2state is mainly decayed through S2
-S1IC,while the slow purple component(1.49 ps) corresponding to the lifetime of S1state is possibly decayed through S1
-S0IC. In Fig.2(b-d),the time profiles of the fragment ions have same kinetic behavior as parent ion at the beginning of about 2 ps,but after that the fragment ion signals show almost no decay even at long delay time,which suggests that the generation of the fragment ions should have two different originations, from the dissociation of parent ion and the ionization of neutral fragment.From kinetic analysis of the parent ion signal,the results have shown that the excited molecule was finally relaxed to the vibrationally hot ground state.Thus,we concluded that the dissociation of acrylic acid molecule should happen on the vibrationally hot ground state surface,which is the type of predissociation.This can be supported by the theoretical study of the photodissociation of acrylic acid carried by Fang and Liu.22They pointed out that on the ground-state surface is the most probable pathway for the cleavage of C-C and C-O bonds, producing H2C=CH and HOCO,H2C=CHCO and OH,respectively.Based on the above discussion,the signal change of fragment ion over time can be expressed as the signal change of parent ion over time superimpose the ion signal change on the vibrationally hot ground state surface over time.The kinetic equation is given as follows,
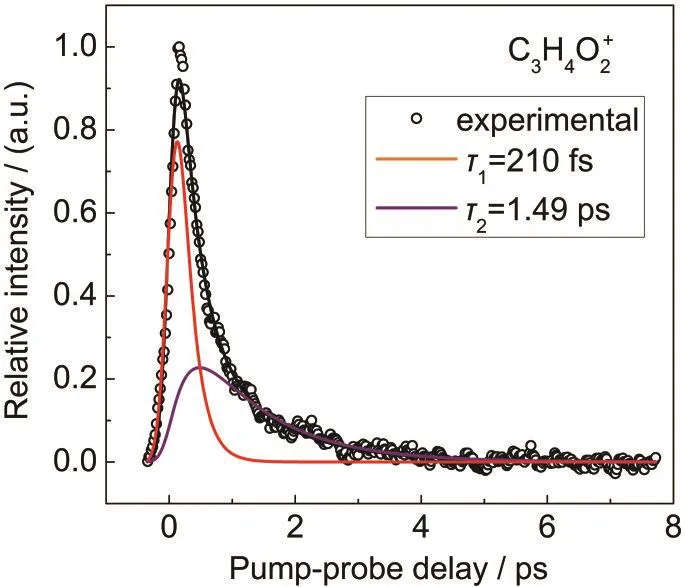
Fig.3 Fitting results of the time-resolved parent ion signal using kinetic equations
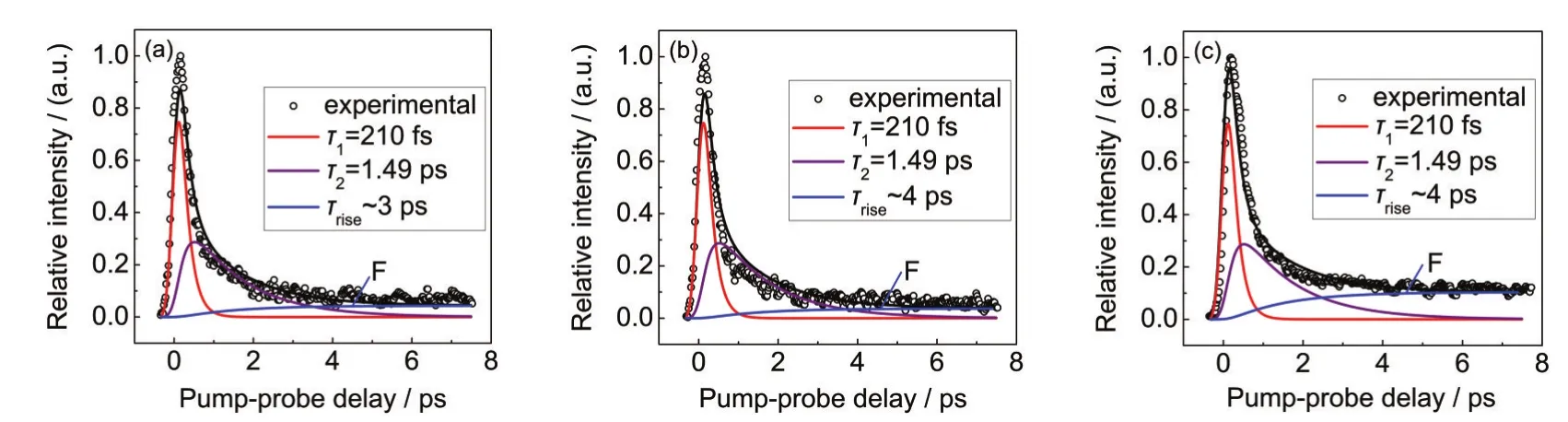
Fig.4 Kinetic fitting of the time-resolved signal of fragment ions (a)H2C=CHCO+,(b)HOCO+,(c)H2C=CH+
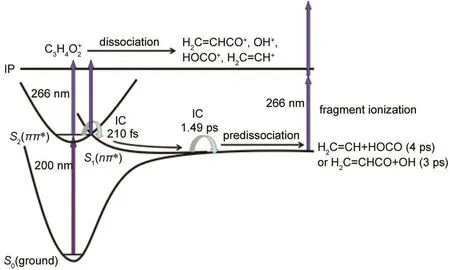
Fig.5 Aschematic diagram of ultrafast dynamic processes involved in the excitation of acrylic acid with 200 nm pump and 266 nm probe
where d[X]/dt(X=ionf,ionp,and S0)is the signal of fragment ion,parent ion,and ion on the vibrationally hot ground state surface changing over delay time,respectively.P2is the intensity ratio of two components,d[S0]/dt and d[ionp]/dt.Since d[ionp]/dt is a known quantity,using its fitting results shown in Fig.3 and according to the kinetic equation(6),the time-resolved profiles of fragment ions(Fig.2(b-d))can be easily fitted and the results are displayed in Fig.4.Open circles are the experimental results and solid lines are the fitting results.
As shown in Fig.4,the kinetic fitting results of each fragment ion signal consists of three components,the two of which (red and purple components)are the same as parent ion,corresponding to the internal conversion from S2to S1state(210 fs) and the internal conversion from S1to vibrationally hot S0state (1.49 ps).The third blue component shows an exponential increase(τrise)followed by a long-life part labeled as F,which reflects the dissociation of acrylic acid on the vibrationally hot S0state and the ionization of neutral fragments forming long-life fragment ions,respectively.τrisedescribes the predissociation lifetime and F corresponds to the lifetime of the fragment ion. At long time delays,F is the only part remaining after all other components have decayed to zero.It should be noted that the fragment ion is not stable infinitely.It only means that it does not decay appreciably on the time scale of our experiment(i.e., approximately hundreds of picoseconds).According to our kinetic fitting results in Fig.4,the time constant of predissociation for C-C bond cleavage to H2C=CH and HOCO is approximately 4 ps,while for C-O bond cleavage to H2C=CHCO and OH is about 3 ps.The values of P2are also determined in our kinetic fitting,which are approximately 0.02,0.02,and 0.05 for H2C=CHCO+,HOCO+,and H2C=CH+,respectively.
Summarizing the above analyses,the involved ultrafast dynamic processes of acrylic acid with the pump pulse of 200 nm and the probe pulse of 266 nm are revealed and shown in
Fig.5.Acrylic acid is excited to the optically bright S2(ππ*)by absorbing one 200 nm photon,the S2state rapidly decays via internal conversion(IC)to the lower lying repulsive S1(nπ*) state in 210 fs followed by another IC to the vibrationally hot S0state in 1.49 ps.The molecule populated on the S2and S1excited states can be ionized forming parent ion by absorbing 266 nm probe light.Then cleavage of C-C and C-O bonds happened in the parent ion,generating fragment ions of H2C=CHCO+,HOCO+,H2C=CH+,and OH+.On the vibrationally hot S0surface,the populated molecule eventually via predissociation mechanism dissociating to neutral fragments H2C=CH and HOCO through C-C bond cleavage and H2C=CHCO and OH through C-O bond cleavage.The predissociation time constant is about 4 ps for C-C bond fission and 3 ps for C-O bond fission.Afterwards,the neutral fragments can be easily ionized by absorbing probe photons forming the long lifetime fragment ions.
4 Conclusions
We have demonstrated the use of femtosecond pump-probe technique combining with time-of-flight mass spectroscopy to study the ultrafast predissociation dynamics of acrylic acid. The predissociation channel was observed when acrylic acid was excited to the optically bright S2state by absorption of one 200 nm photon.The molecule populated on the S2excited state rapidly decays via internal conversion to the lower lying S1state in 210 fs followed by another internal conversion to the vibrationally hot S0state in 1.49 ps,and from where the molecule subsequently dissociates to neutral fragments H2C=CH and HOCO in approximately 4 ps,H2C=CHCO and OH in about 3 ps.Two originations of the fragment ions were clarified,which were from the dissociation of parent ion and from the ionization of neutral fragments via predissociation mechanism.
(1) Zewail,A.H.J.Phys.Chem.A 2000,104,5660.
(2) Bernardi,F.;Olivucci,M.M.;Robb,M.A.Chem.Soc.Rev. 1996,25,321.
(3) Schwalb,N.K.;Temps,F.J.Am.Chem.Soc.2007,129,9272.
(4) Kwok,W.M.;Ma,C.S.;Phillips,D.L.J.Am.Chem.Soc. 2008,130,5131.
(5) Fork,R.L.;Greene,B.I.;Shank,C.V.Appl.Phys.Lett.1981, 38,671.
(6) Zewail,A.H.Angew.Chem.Int.Edit.2000,39,2586.
(7) Domcke,W.;Stock,G.Adv.Chem.Phys.1997,100,1.
(8) Lee,A.M.D.;Coe,J.D.;Ullrich,S.;Ho,M.L.;Lee,S.J.; Cheng,B.M.;Zgierski,M.Z.;Chen,I.C.;Martinez,T.J.; Stolow,A.J.Phys.Chem.A 2007,111,11948.
(9)Osborne,M.C.;Li,Q.;Smith,I.W.M.Phys.Chem.Chem. Phys.1999,1,1447.
(10)Arendt,M.F.;Browning,P.W.;Butler,L.J.J.Chem.Phys. 1995,103,5877.
(11) Singleton,D.L.;Paraskevopoulos,G.;Irwin,R.S.J.Phys. Chem.1990,94,695.
(12) Rosenfeld,R.N.;Weiner,B.R.J.Am.Chem.Soc.1983,105, 6233.
(13) Upadhyaya,H.P.;Kumar,A.;Naik,P.D.;Sapre,A.V.;Mittal, J.P.J.Chem.Phys.2002,117,10097.
(14) Reguero,M.;Olivucci,M.;Bernardi,F.;Robb,M.A.J.Am. Chem.Soc.1994,116,2103.
(15) Fang,W.H.J.Am.Chem.Soc.1999,121,8376.
(16)Aquilante,F.;Barone,V.;Roos,B.O.J.Chem.Phys.2003,119, 12323.
(17) Kitchen,D.C.;Forde,N.R.;Butler,L.J.J.Phys.Chem.A 1997,101,6603.
(18) Forman,R.L.;MacKinnon,H.M.;Ritchie,P.D.J.Chem.Soc. C 1968,2013.
(19) Miyoshi,A.;Matsui,H.;Washida,N.J.Chem.Phys.1994,100, 3532.
(20) Ruelle,P.J.Comput.Chem.1987,8,158.
(21) Fang,W.H.Chem.Phys.Lett.2000,325,683.
(22) Fang,W.H.;Liu,R.Z.J.Am.Chem.Soc.2000,122,10886.
(23)Wei,Z.R.;Zhang,F.;Wang,Y.M.;Zhang,B.Chin.J.Chem. Phys.2007,20,419.[魏振荣,张 峰,王艳梅,张 冰.化学物理学报,2007,20,419.]
(24) Chen,Y.;Zhang,C.H.;Cao,Z.Z.;Zhang,B.Acta Phys.-Chim. Sin.2008,24,844.[陈 荫,张昌华,曹振洲,张 冰.物理化学学报,2008,24,844.]
(25) Zhang,F.;Cao,Z.Z.;Qin,X.;Liu,Y.Z.;Wang,Y.M.;Zhang, B.Acta Phys.-Chim.Sin.2008,24,1335.[张 锋,曹振洲,覃 晓,刘玉柱,王艳梅,张 冰.物理化学学报,2008,24, 1335.]
(26)Wang,Y.M.;Zhang,S.;Wei,Z.R.;Zhang,B.Chem.Phys.Lett. 2009,468,14.
(27) Katrib,A.;Rabalais,J.W.J.Phys.Chem.1973,77,2358.
(28) Turro,N.J.Modern Molecular Photochemistry;Benjamin/ Cummings:Menlo Park,1978.
November 23,2011;Revised:December 29,2011;Published on Web:January 12,2012.
Ultrafast Predissociation Dynamics of Excited State of Acrylic Acid
ZHANG Rong-Rong QIN Chao-Chao LONG Jin-You YANG Ming-Hui ZHANG Bing*
(State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics,Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences,Wuhan 430071,P.R.China; Graduate University of Chinese Academy of Sciences, Beijing 100049,P.R.China)
The ultrafast predissociation dynamics of acrylic acid after excitation to the second electronically excited state(S2)with a 200 nm pump pulse were studied using a femtosecond pump-probe technique combined with time-of-flight mass spectroscopy(TOF-MS).The time-resolved mass spectra signals of the parent ion and fragment ions were collected.By using the kinetic equations to fit and analyze the time-resolved mass spectra ion signals,the existence of the predissociation channel was revealed.The excited molecule populated in the S2state decayed to the first electronically excited state(S1)through a fast internal conversion process over a period of 210 fs.The excited molecule populated on the S1state then decayed to the vibrationally hot ground state(S0)through another internal conversion process over a period of 1.49 ps.Finally,on the vibrationally hot ground state surface,the molecule dissociated to the neutral fragments,H2C=CH and HOCO,H2C=CHCO and OH via C-C bond fission and C-O bond fission, respectively.The corresponding predissociation time constants were determined to be approximately 4 and 3 ps,respectively.The generation of fragment ions can occur in two ways,both from the dissociation of the parent ion and the ionization of the neutral fragments on the vibrationally hot ground state surface.
Time-resolved mass spectrum;Ultrafast dynamics;Pump-probe;Time-of-flight mass spectroscopy;Internal conversion
10.3866/PKU.WHXB201201122
O644
∗Corresponding author.Email:bzhang@wipm.ac.cn;Tel:+86-27-87197441;Fax:+86-27-87198491.
The project was supported by the National Natural Science Foundation of China(20903116)and Knowledge Innovation Foundation of Chinese Academy of Science(KJCX1-YW-N30).
国家自然科学基金(20903116)和中国科学院知识创新基金(KJCX1-YW-N30)资助项目

