高度分散二氧化锰纳米纤维的水热合成和对双氧水电流检测
何赛男 胡彩园 肖 鸽 郑华均,*
(1浙江大学医学院附属妇产科医院,杭州310006;2浙江工业大学化学工程与材料学院,杭州310014)
高度分散二氧化锰纳米纤维的水热合成和对双氧水电流检测
何赛男1胡彩园2肖 鸽2郑华均2,*
(1浙江大学医学院附属妇产科医院,杭州310006;2浙江工业大学化学工程与材料学院,杭州310014)
采用水热还原氧化法合成了高度分散的具有纳米纤维结构的钾矿型二氧化锰,并将其用来制作检测双氧水浓度的传感器.运用X射线衍射(XRD)仪、电子扫描显微镜(SEM)、透射电子显微镜(TEM)和比表面积(BET)及孔隙度分析仪观察和表征二氧化锰纳米纤维的结构和表面形貌;用电化学工作站(EW)检测其传感性能.结果表明:在pH为7.4的磷酸缓冲溶液中,开路电压为0.2 V的条件下对0.1%(w,质量分数)的二氧化锰纳米纤维修饰的玻碳电极(GCE)进行测试,测试结果为随着双氧水的浓度每增加0.1 mmol·L-1,响应电流的峰值就增加约1.3 μA,在双氧水的浓度在0.1-1.5 mmol·L-1范围内得到的线性相关系数为0.996,这种电极的高灵敏度和优异的电化学活性可能归因于钾矿型二氧化锰纳米纤维的特殊纳米结构.这种传感器有很高的灵敏度和很好的重现性.综上说明这种廉价并且有很好的电化学活性的材料为设计新型电极生物传感器提供了更大可能.
双氧水;钾矿型二氧化锰;玻碳电极;磷酸缓冲溶液;生物传感器
1 Introduction
Recently,considerable attention has been put on the design and development of new biosensor devices as a response to the increasing demand for monitoring systems for life quality improvement.In particular,owing to the important progress in fields of material science and electrochemical engineering, electrochemical sensors appeared very appealing because of their simplicity and interesting performances among the different transduction possibilities.1-3Although great achievements and various electrodes developed for sensing ions in solution,a great amount of research work is still needed to particularly improve their electrochemical activity,and to better understand the equilibrium phenomena leading to the responses.4,5
Manganese dioxide(MnO2)is very attractive due to its distinctive structures and wide applications in catalysts,ionsieves,and rechargeable batteries,as well as environmental compatibility and cost effectiveness.Recently,research efforts have been made to prepare nanocrystalline MnO2because of its intriguing properties and promising applications.6-11Nanocrystalline MnO2exhibits high electrochemical catalytic activity because of its small size effect and surface effect better than commercial MnO2.Nanostructured MnO2exhibits outstanding catalytic abilities in various oxidation and reduction reactions, and its activity for the oxidation of H2O2was found to have several applications as sensors.In such cases,H2O2is oxidized catalytically on an electrode,leading to the formation of current flow depending on the concentration of H2O2.12-14H2O2is one of the products in a variety of biochemical reactions catalyzed by oxidases such as glucose,choline,and lactic acid.And the detection of H2O2is of interest to many fields,such as clinic, food,pharmaceutical,environmental analyses,and biosensing. Glucose and choline biosensors based on MnO2having activity for the oxidation of H2O2were reported.15-18However,some of the sensors have displayed the drawbacks of low sensitivity, poor selectivity,high cost,and complex procedure.
Hollandite is microporous and mixed-valence manganese oxides with tunnel structures,and their basic unit structure is made of sheets of MnO6edge-sharing octahedron,so-called octahedral molecular sieve(OMS)materials.Suib and colleagues19-22did excellent and extensive research on these materials.The K+form of hollandite is known as cryptomelane,and it is also a mixed valence.Scientists are particularly interested in the application of OMS materials in electrocatalysis because of the unique tunnel structure and mixed-valence manganese, which results in outstanding catalytic properties for H2O2oxidation if employed as a mediator.
In this paper,we report a facile hydrothermal method for synthesizing cryptomelane-type α-MnO2nanofibers.We compare the electrocatalytic activity towards the oxidation of H2O2of the cryptomelane-type α-MnO2modified electrodes with the commercial MnO2modified electrodes.The linear range,response time,detection limit,and effect of the content of cryptomelane-type α-MnO2were studied.
2 Experimental
2.1 Reagents and apparatus
KMnO4,MnSO4·H2O,H2O2,MnO2powder,and gelatin were purchased from Sigma-Aldrich.All chemicals and reagents were of analytical grade and used without further purification. Double-distilled water was used for preparation of buffer and standard solutions.H2O2was freshly prepared daily.
Crystallographic information of the as-prepared sample was investigated with X-ray diffraction(XRD)(XʹPert Pro,Holland)with Cu Kαradiation.The structural morphology of the synthesized materials was observed by scanning electron microscopy(SEM,JEOL JSM-6700,Japan)and transmission electron microscopy(TEM,Tecnai G2 F30,Holland).The presence and the contents of potassium ion were confirmed by energy dispersive spectrometer(EDS)(Noran VANTAGE-ES, USA).A Micromeritics ASAP 2010 analyzer was used to measure the N2adsorption isotherms of the samples at liquid N2temperature(-196°C).Prior to the measurement of the surface area,the sample was degassed in vacuum at 250°C for at least 4 h.The BET surface area was determined by the multipoint BET method with the adsorption data in the relative pressure(p/p0)range of 0.0-1.0.Electrochemical measurements were performed with CHI 620B electrochemical workstation (Shanghai Chenhua Instrumental Co.Ltd.).Experiments were carried out at room temperature((25±2)°C).
2.2 Synthesis of MnO2nanofiber
KMnO4(1.58 g)and MnSO4·H2O(0.676 g)powders(the mole ratio is 2.5:1)were dissolved in 80 mL distilled water for 8 h with strongly stirring at room temperature.Then,the reaction mixture was loaded into a 100 mL Teflon-lined stainless steel autoclave.The autoclave was sealed and heated in an oven at 150°C for 2 h.When cooled to room temperature,a resulting brown-black precipitate was filtered and rinsed with distilled water,and dried at 100°C overnight.
2.3 Electrode modification
Before modification,the bare GCE was polished to mirror smooth with 0.05µm Al2O3slurry,rinsed with water,and then ultrasonicated in water bath.Gelatin was used as a binder and dissolved in 50°C water,mixed with the as-prepared MnO2, which was dispersed in water with the aid of ultrasonic agitation.The contents of MnO2and gelatin in the trim were 0.1% (w)and 1%(w),respectively.After 2 μL of the trim was pipetted to the surface of GCE with microliter syringe,the solvent was evaporated at room temperature.The obtained electrode is denoted as MnO2nanofiber-gelatin/GCE,which was kept in buffer solution for 1 h before use.For comparison,the commercial MnO2powder modified electrodes were also prepared under the same conditions.
2.4 Amperometric measurement of H2O2
A conventional three-electrode setup was used.The working electrode was a bare GCE or modified GCE,the auxiliary electrode was a platinum sheet,and the reference electrode was a saturated calomel electrode(SCE).Amperometric experiment was carried out in a H2O2solution holding 50 mL of 0.1 mol· L-1phosphate buffer(pH=7.4,in accordance with acidity versus alkalinity in the physiological environment)with stirring for providing the convective transport.Freshly prepared H2O2was injected by micro-syringe according to test demand.The background current was allowed to decay to a constant value before H2O2solution was added to the cell.The calibration curve was obtained by amperometric responses when adding the same amount of H2O2standard solution into cell.
3 Results and discussion
3.1 Characterization of the as-prepared manganese dioxide
Fig.1 shows SEM and TEM images of the cryptomelanetype manganese dioxide.From Fig.1,a nanosized fibrous morphology can be observed.Fig.1b shows that the as-prepared manganese dioxide is nanofiber with diameters of 30-50 nm and lengths in the range of 2-5 μm.The product has favorable dispersibility,which may be due to stirring for 8 h before loading into autoclave.It can be seen from Fig.1a that some of the nanofibers have shown the tendency to curl,and some of them have grown into a clubbed structure.Fig.2 shows XRD pattern of the as-prepared manganese oxide,and the diffractions of very strong peaks at 2θ of 12.6°,17.9°,28.7°,37.5°,41.9°, 49.9°,60.1°.All the reflection peaks of the products can be indexed as a cryptomelane-type α-MnO2,which is in good agreement with the literature values(JCPDS No.42-1348).The final product is α-MnO2corresponding to the studies reported by Li et al.,23in which they proposed that the proportion of KMnO4to MnSO4influenced the structure and morphology of final product,and concluded that the final product was α-MnO2when the mole ratio of KMnO4to MnSO4was 2.5:1.
Elemental analysis reveals that the molar compositions for K and Mn cations are 7.18%and 92.82%,respectively,and it is a typical characterization of cryptomelane-type manganese oxide.The specific surface area of the material was determined to be 106 m2·g-1by the BET technique,making it possible that the α-MnO2will have remarkable catalytic performance.
3.2 Electrochemical characteristics of the modified electrode
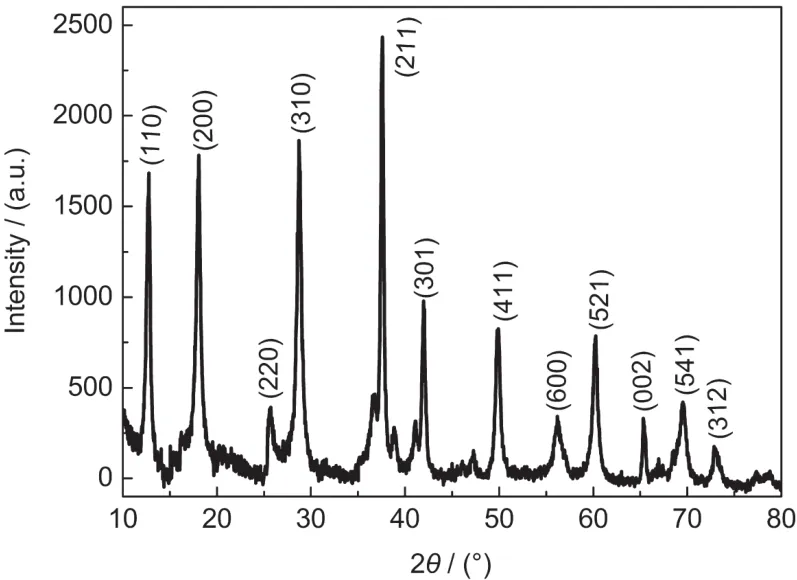
Fig.2 X-ray powder diffraction pattern of cryptomelane-type manganese dioxide
Fig.3 shows the cyclic voltammograms for hydrogen peroxide with the MnO2nanofiber-gelatin/GCE and MnO2-gelatin/ GCE in different solutions.With bare GCE,no oxidation peak was observed within the applied potential range for 0.1 mmol· L-1H2O2in phosphate buffer solution(curve a).While for MnO2nanofiber-gelatin/GCE and commercial MnO2-gelatin/ GCE in phosphate buffer solution,there is a pair of broad but weak peaks between 0.2 and 0.8 V(curves b and d).It may be assigned to the reduction of MnO2to Mn(II,III)and the reoxidation of Mn(II,III)back to MnO2.24,25It is can be observed that as for the MnO2nanofiber-gelatin/GCE in the presence of 0.1 mmol·L-1H2O2in phosphate buffer solution,the cyclic voltammogram displays a sensitive and high oxidative peak at around 0.7 V(curve c),while the commercial MnO2-gelatin/ GCE displays a low oxidative peak(curve e).The characteristic shape of the cyclic voltammogram within this potential region indicates that the signal is probably due to a parallel catalytic reaction.As soon as MnO2is reduced to lower states by H2O2,it is electro-oxidized back to MnO2at the electrode surface.24Fig.4 shows the cyclic voltammograms of the MnO2nanofiber-gelatin/GCE in phosphate buffer solution(0.1 mol· L-1,pH=7.4)containing different concentrations of H2O2(0.1-0.4 mmol·L-1).It can be seen clearly that with addition of H2O2,the oxidation peak currents gradually increase with 1.3 μA,which implies that cryptomelane-type manganese dioxide would be an excellent material to fabricate sensitive H2O2sensor.
3.3 Detection of hydrogen peroxide

Fig.1 SEM(a,b)and TEM(c)images of cryptomelane-type manganese dioxide
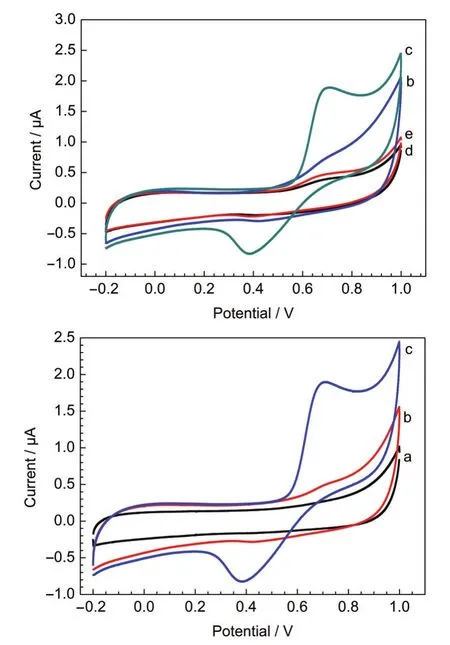
Fig.3 Cyclic voltammograms of different electrodes in different solutions(a)bare GCE with 0.1 mmol·L-1H2O2in phosphate buffer solution;(b)the MnO2nanofiber-gelatin/GCE electrode in phosphate buffer solution(pH 7.4); (c)the MnO2nanofiber-gelatin/GCE electrode with 0.1 mmol·L-1H2O2in phosphate buffer solution;(d)the commercial MnO2-gelatin/GCE electrode in phosphate buffer solution(pH 7.4);(e)the commercial MnO2-gelatin/GCE electrode with 0.1 mmol·L-1H2O2in phosphate buffer solution. The scan rate is 20 mV·s-1.
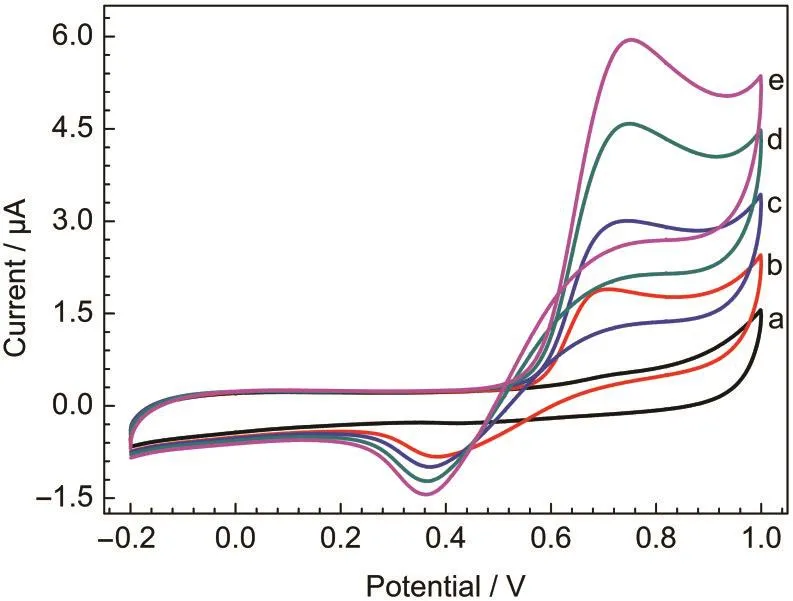
Fig.4 Cyclic voltammograms of MnO2nanofiber-gelatin/GCE in phosphate buffer solution(0.1 mol·L-1,pH 7.4)containing different concentrations of H2O2scan rate:20 mV·s-1;c(H2O2)/(mmol·L-1):(a)0,(b)0.1,(c)0.2,(d)0.3,(e)0.4
The relationship between the oxidation current and the concentration of H2O2was examined in a phosphate buffer solution(pH=7.4).The solution was stirred to ensure uniform distribution of H2O2in the cell.Fig.5(a,b)displays the amperometric response of MnO2nanofiber-gelatin/GCE and MnO2powder-gelatin/GCE to successive 0.1 mmol·L-1of H2O2at the open circuit potential of 0.2 V.When the same amount of H2O2was injected into the cell,the current was recorded instantly.It can be clearly seen that the MnO2nanofiber electrode exhibited a fast and well-defined current response over a broad concentration range,while the MnO2powder electrode exhibited a low and instable current response.It can be seen that the rising of response current is decreasing when the concentration of H2O2is in the range of 1.7-2.0 mmol·L-1in Fig.5(b),which can be attributed to reaction consumption of H2O2in the cell. Fig.5(c)represents the calibration curve for the determination of H2O2at the MnO2nanofiber-gelatin/GCE.The measured peak current was found to be linearly proportional to the concentration of H2O2in the range of 0.1-1.5 mmol·L-1in the solution with a correlation coefficient of 0.996.Fig.5(b)shows the comparison of current-time recordings of MnO2nanofibergelatin/GCE and MnO2powder-gelatin/GCE.We can conclude that the cryptomelane-type manganese dioxide nanofiber displays high electrocatalytic activity towards the oxidation of H2O2.While the content of cryptomelane-type manganese dioxide also has a very profound effect upon its amperometric response behavior.Fig.6 shows the amperometric response for H2O2with different percentages of cryptomelane-type manganese dioxide in modified GCEs(0.01%,0.05%,0.10%(mass fraction)).The amperometric response of electrode gradually increases with addition of the nanostructured cryptomelanetype manganese dioxide in mixture of MnO2and gelatin and also displays excellent electrocatalytic oxidative activity of the as-prepared cryptomelane-type manganese dioxide compared with some other research.12

Fig.6 Current-time recording obtained on increasing the H2O2 concentration in 0.1 mmol·L-1step at different percentages of cryptomelane-type manganese oxides modified GCEs at an operating potential of 0.2 VThe scan rate is 20 mV·s-1.The stirring rate is 500 r·min-1.
3.4 Detection limit,sensibility,reproducibility and stability
The sensitivity of the sensor to H2O2was calculated to be 39.4 μA·cm-2·mmol-1·L by the slope of Fig.5(c),which is higher than the results in other papers.13,15The detection limit of the sensor was estimated to be 5×10-6mol·L-1by DL=3.3s/k(s is standard deviation,k is the sensitivity,signal to noise ratio (S/N)=3).The response time was 10 s.The reproducibility of the sensor was measured in 0.1 mol·L-1phosphate buffer(pH 7.4)and the relative standard deviation(RSD)of the sensor response to 0.1 mmol·L-1H2O2was 2.5%for seven successive measurements.The sensor was stored dry at 4°C and measured at intervals of one week,it remained about 95%of its original response after one month,which shows a high stability.
4 Conclusions
In this paper,we obtained a nanostructured cryptomelanetype manganese dioxide with diameters of 30-50 nm and lengths in the range 2-5 μm through a hydrothermal reduction route and had super dispersibility.We demonstrated that the GCE modified with the nanostructured cryptomelane-type manganese dioxide can offer low-potential amperometric detection for H2O2.The oxidation peak current increases 1.3 μA with addition of 0.1 mmol·L-1H2O2based on the MnO2-gelatin/GCE electrode,in which the percentage of cryptomelane-type manganese dioxide is 0.1%,which is attributed to well-structured of cryptomelane-type manganese oxide.Its low-potential detection and applicability in neutral solution indicate great promise for the design of amperometric biosensors.In view of its sensitivity,low detection limit,simplicity,and low cost of construction,theGCE modified with thenanostructured cryptomelane-type manganese dioxide exhibits great prospects for future biosensor work.
(1) Eftekhari,A.Microchim.Acta 2003,141,15.
(2) Huo,H.Y.;Luo,H.Q.;Li,N.B.Microchim.Acta 2009,167, 195.
(3) Zhang,Y.;Kang,T.F.;Wan,Y.W.;Chen,S.Y.Microchim.Acta 2009,165,307.
(4) Martinez,M.T.;Lima,A.S.;Bocchi,N.;Teixeira,M.F.S. Talanta 2009,80,519.
(5) Teixeira,M.F.D.S.;Fatibello-Filho,O.;Ferracin,L.C.; Rocha-Filho,R.C.;Bocchib,N.Sensors and Actuators B 2000, 67,96.
(6) Xiao,T.D.;Strutt,P.R.;Benaissa,M.;Chen,H.;Kear,B.H. Nanostruct.Mater.1998,10,1051.
(7)Wang,X.;Li,Y.D.J.Am.Chem.Soc.2001,124,2880.
(8)Wang,X.;Li,Y.D.Chem.Commun.2002,764.
(9) Xiong,Y.J.;Xie,Y.;Li,Z.Q.;Wu,C.Z.Chem.Eur.J.2003,9, 1645.
(10) Han,L.;Ni,J.P.;Zhang,L.M.;Yue,B.H.;Shen,S.S.;Zhang, H.;Lu,W.C.Acta Phys.-Chim.Sin.2011,27,743.[韩 玲,倪纪朋,张良苗,岳宝华,申杉杉,张 浩,陆文聪.物理化学学报,2011,27,743.]
(11) Sun,Z.;Liu,K.Y.;Zhang,H.F.;Li,A.S.;Xu,X.C.Acta Phys.-Chim.Sin.2009,25,1991.[孙 哲,刘开宇,张海峰,李傲生,徐小存.物理化学学报,2009,25,1991.]
(12) Lin,Y.H.;Cui,X.L.;Li,L.Y.Electrochem.Commun.2004,7, 166.
(13) Yao,S.J.;Yuan,S.;Xu,J.H.;Wang,Y.;Luo,J.L.;Hu,S.S. Appl.Clay Sci.2006,33,35.
(14) Šljukiç,B.;Compton,R.G.Electroanalysis 2007,19,1275.
(15) Cui,X.L.;Liu,G.D.;Lin,Y.H.Nanomedicine 2005,1,130.
(16) Hocevar,S.B.;Ogorevc,B.;Schachl,K.;Kalcher,K. Electroanalysis 2004,16,20.
(17) Chen,J.;Zhang,W.D.;Ye,J.S.Electrochem.Commun.2008, 10,1268.
(18) Bai,Y.H.;Du,Y.;Xu,J.J.;Chen,H.Y.Electrochem.Commun. 2007,9,2611.
(19) Tian,Z.;Tong,W.;Wang,J.;Duan,N.;Krishnan,V.V.;Suib,S. L.Science 1999,276,926.
(20) Xia,G.G.;Yin,Y.G.;Willis,W.S.;Wang,J.Y.;Suib,S.L. J.Catal.1999,185,91.
(21) Son,Y.C.;Makwana,V.D.;Howell,A.R.;Suib,S.L.Angew. Chem.Int.Edit.2001,40,4280.
(22) Liu,J.;Makwana,V.;Cai,J.;Suib,S.L.;Aindow,M.J.Phys. Chem.B 2003,107,9185.
(23)Wang,X.;Li,Y.D.Chem.Eur.J.2003,9,306.
(24) Emir,T.;Kalcher,K.;Schachl,K.;Komersova,A.;Bartos,M.; Moderegg,H.;Svancara,I.;Vytras,K.Anal.Lett.2001,34, 2633.
(25) Yin,L.;Chou,J.;Chung,W.;Sun,T.;Hsiung,K.;Hsiung,S. Sensors and Actuators B 2001,76,187.
October 14,2011;Revised:December 6,2011;Published on Web:December 21,2011.∗
.Email:zhenghj@zjut.edu.cn;Tel:+86-13957175665.
Hydrothermal Synthesis and Amperometric Determination of Hydrogen Peroxide of Highly-Dispersed MnO2Nanofibers
HE Sai-Nan1HU Cai-Yuan2XIAO Ge2ZHENG Hua-Jun2,*
(1Womenʹs Hospital,School of Medicine,Zhejiang University,Hangzhou 310006,P.R.China;2College of Chemical Engineering and Materials Science,Zhejiang University of Technology,Hangzhou 310014,P.R.China)
A high dispersed nanofiber cryptomelane-type manganese dioxide was synthesized by a facile hydrothermal reduction route.The morphological characterization was examined by scanning electron microscopy(SEM)and transmission electron microscopy(TEM).The structure and electrochemical properties of the synthesized manganese dioxide were characterized by X-ray diffraction(XRD),Brunauer-Emmett-Teller(BET)surface area analyses,and an electrochemical workstation(EW).A glassy carbon electrode(GCE)modified with the nanostructured cryptomelane-type manganese dioxide was investigated for amperometric detection of hydrogen peroxide(H2O2)in phosphate buffer solution with a pH 7.4 at an open circuit potential of 0.2 V.The oxidation peak current was found to increase by 1.3 μA with the addition of 0.1 mmol·L-1H2O2based on a MnO2nanofiber-gelatin/GCE electrode.The amperometric signals are linearly proportional to the H2O2concentration in the range 0.1-1.5 mmol·L-1with a correlation coefficient of 0.996 using the GCE modified with 0.1%(w,mass fraction)cryptomelane-type manganese oxides.The sensor is sensitive and its significant electrocatalytic activity results from the nanostructure of the cryptomelane-type manganese oxides.In addition,the sensor has a good reproducibility,a low detection limit,simplicity,and a low cost of construction.These results demonstrate that this material with high electrocatalytic activity offers great promise as a new class ofnanostructured electrodes forbiosensors.
Hydrogen peroxide;Cryptomelane-type manganese dioxide;Glassy carbon electrode; Phosphate buffer solution;Biosensor
10.3866/PKU.WHXB201112214
O646
The project was supported by the National Natural Science Foundation of China(20973156).
国家自然科学基金(20973156)资助项目

