流变相法制备倍率性能优异的LiFePO4/C复合材料
徐 瑞 钟本和 郭孝东 唐 艳 唐 红 方为茂*, 刘 恒
(1四川大学化学工程学院,成都 610065) (2四川大学材料科学与工程学院,成都 610065)
流变相法制备倍率性能优异的LiFePO4/C复合材料
徐 瑞1钟本和1郭孝东1唐 艳1唐 红1方为茂*,1刘 恒2
(1四川大学化学工程学院,成都 610065) (2四川大学材料科学与工程学院,成都 610065)
以月桂酸为碳源和表面活性剂,氢氧化锂、碳酸锂和醋酸锂为锂源,采用流变相法制备LiFePO4/C复合材料。运用X射线衍射(XRD)、扫描电子显微镜(SEM)、粒度分析、恒流充放电测试、循环伏安以及交流阻抗测试等方法对复合材料进行表征。结果表明,不同的锂源对LiFePO4/C复合材料的结构和电化学性能均有很大影响,以氢氧化锂为锂源合成的LiFePO4/C材料展示出最佳的循环性能和倍率性能。该材料在0.1C下放电比容量为153.4mAh·g-1,在大倍率10 C下,容量保持率仍可达76%,甚至10C下循环800次后,容量衰减率仅有4%,SEM结果显示该材料具有较小的粒径(~200 nm),且分布集中,有效提高了电子迁移速率,从而改进了LiFePO4/C的倍率性能。
LiFePO4/C;流变相法;锂源;高倍率
After the discovery of olivine-structured LiFePO4in 1997 by Goodenough and co-workers[1],thematerial has always been one of the hottest cathode active materials for Lithium-ion batteries.Recently,rapidly capacity fading of LiFePO4at high current density has been a bottleneck problem, which limits theapplication in large electric drives.Padhietal[1]showed that discharging would be ended when the discharging current increased and the lithium-ions of LiFePO4/ Li1-xFePO4interface were not enough to preserve the current of discharge,so it is an usefulway to improve the specific capacity at high rate by enhancing the diffusion rate of Li+.Some references[2-5]reported that smaller particle size of LiFePO4could shorten the diffusion distance of Li+,which is beneficial to the enhancement of diffusion rate;uniformly distributed carbon layer on the surface of active powder can make sure the homogeneous insertion/deinsertion of Li+.So via reducing the particle size and improving the carbon coating,the bottleneck problem of LiFePO4can be achieved a breakthrough.
At present,various synthetic routes,such as solid state reaction[6-8],sol-gel reaction[9-10],co-precipitation reaction[11-12],hydrothermal reaction[13-14]and microwave heating reaction[15-16]etc,have been applied to synthesize LiFePO4.For solid state reaction,simple preparative technology and devices make it to be beneficial to industrial production,but by this route, particles obtained are usually with irregular morphology,non-uniform particle size distribution,and the higher calcining temperature and longer calcining timewould also cause tremendousenergy comsumption.Sol-gel reaction and co-precipitation reaction both belonging to liquid phase method are similar to some degree,such as the mixing of the raw materials in molecular scale,the narrower size distribution and the smaller particle size of the product.But the preparing condition of sol-gel reaction is harsh,moreover, particles are easy to agglomerate in stoving and calcination process,in view of these,it is difficult to scale up thismethod into industrialapplication.For coprecipitation reaction,differentsedimentation rates and equilibrium solubility of components affect the stoichiometric ratio of the elements in the product, which is a significant impact factor on the products performance.The hydrothermalmethod is another kind of liquid phase reaction,owning some advantages mentioned above.But the hydrothermal reaction under high temperature and high pressure is more complex and is hard to control in reactor,therefore a higher qualification is needed for equipment tomeet the harsh reaction condition.
In this work,we resolved the problem that the capacity of LiFePO4faded rapidly at high rate through controlling the particle size of LiFePO4.The rheological phase method combines the advantages of solid state reaction and liquid state reaction,such as decreasing energy consumption,reducing the particle size of the product and being suitable for industrial production.LiFePO4/C synthesized by the rheologicalphasemethod has excellent rate capability because of its smaller particle size.We also studied the effect of different lithium sourceson the performance ofsamples.
1 Experimental
1.1 Synthesis of LiFePO4/C
The LiFePO4/C composites were prepared by rheological phase method.Iron phosphate (FePO4· 2H2O,AR),lithium hydroxide(LiOH·H2O,AR),lithium carbonate(Li2CO3,AR),lithium acetate(CH3COOLi· 2H2O,AR)and lauric acid(C12H24O2,AR)were used as raw materials.LiOH·H2O,Li2CO3and CH3COOLi· 2H2Owere separatelymixed with stoichiometric amount of FePO4·2H2O and grounded for 2 h to get a solid reactantmixture,whichwas thenmixed with lauric acid dissolved in ethanoland grounded for1 h to geta solidliquid rheological body,it looked like a kind ofmushy slurry.Then,the different rheological bodies were calcined at 650℃ in a tube furnace for 6 h under nitrogen flow,then naturally cooled to room temperature to obtain the LiFePO4/C composites.The products prepared with lithium hydroxide,lithium carbonate, lithium acetate as lithium sources aremarked with A, B,C,respectively.
1.2 M aterials characterization
The crystalline structrue of each product was characterized by X-ray diffraction (XRD,Philips X′Pert PW1730)with Cu Kαradiation(λ=0.154 18 nm) at scanning angle of 10°~80°and scanning rate of 8°· min-1.The particle morphology and particle size of samples were observed by scanning electron microscopy (SEM,SPA400 Seiko Instructures).The particle size distribution was tested by JL-1155 laser particle size distribution tester.The carbon content was tested by CS-902 analytical instrument.
1.3 Electrochem ical characterization
Tomake electrode,80wt%LiFePO4/C composite, 13wt%acetylene black and 7wt%of polyvinylidene fluoride (PVDF)were mixed in N-methylpyrrolidone (NMP)as solvent.The obtained slurry was then deposited uniformly onto a thin Al foil,and dried in vacuum at 80℃ for 10 h.The dried filmed Al foil was cut into disks as cathode electrode.The electrolyte was 1 mol·L-1LiPF6dissolved in ethylene carbonate (EC),diethylene carbonate (DEC)and dimethylene carbonate(DMC)with amolar ratio of 1∶1∶1.The Li foil was used as anode and the Celgard 2400 as the separator.The coin-type cells were assembled in a glove box filling with argon.The cells were measured by galvanostatic constant current charging/discharging tests with the potential range of 2.5~4.3 V using a battery test system (Neware BTS-610)at room temperature (25 ℃ ).The cyclic voltammetry(CV)and electrochemical impedance(EIS) of the cell were conducted by a CHI660B electrochemical work station,CV tests were carried out in the potential range of 2.5~4.3 V at a scanning rate of 0.10mV·s-1.
2 Results and discussion
2.1 Structure and morphology analysis
Fig.1 shows the X-ray diffraction patterns of the LiFePO4/C synthesized with different lithium sources.Compared with the normal pattern of LiFePO4(PDF No.40-1499),the crucial diffraction peaks of three samples are well matched with the standard of an orthorhombic olivine-type structure with a space group of Pnma.All the peaks of sample A and B are sharp without any peaks of impurities,which proves that the product is well-crystallized.However,the pattern of sample C appears an obvious impurity Li3PO4, analyzed by Jade 5.0,which indicates that reaction of the raw materials are incomplete,in addition,the wide and weak peaks suggest the poor crystallinity.No residual carbon-related diffraction peak is detected here,which indicates that the residual carbon is amorphous and the carbon can not affect the crystalline structure of LiFePO4.

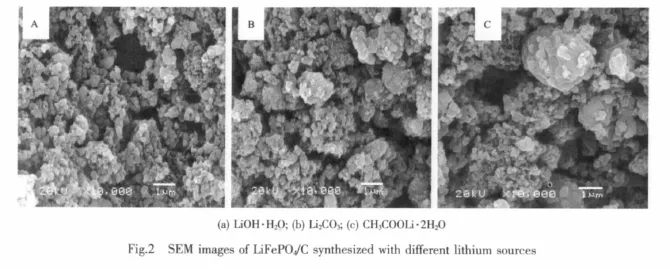
Fig.2 shows the SEM images of LiFePO4/C synthesized with different lithium sources.All the samples used lauric acid as carbon source and the carbon content is about 2.3%.Sample A has the smallest particle size of about 200 nm and uniform size distribution;the particle size of sample B is also small but the distribution is non-uniform and some primary particles seem a little agglomerated;the particle size of sample C is big and agglomerate even more seriously.Smaller particles have large specific surface area,which contribute not only to the effective contact of electrolyte and the surface of LiFePO4,but also to the diffusion of Li+,so we anticipate that the sample synthesized by lithium hydroxide based rheological phase method will have the optimal performance.It indicates that the various systems of raw materials has great effect on the morphology of products.FePO4is neutral and lauric acid is acidic, so compared with other lithium source,lithium hydroxide with strong alkaline can mix with FePO4and lauric acid well to reach the homogeneity of molecular level.At the same time,the rheological phasemethod integrates the advantages of solid-phase method and liquid-phase method,which makes the homogeneous system with certain viscosity to decrease the particle size.
2.2 Electrochem ical cycling test
Fig.3 shows the initial charge-discharge curves of LiFePO4/C synthesized with different lithium sources at 0.1C.From the curves,all the samples have stable charge-discharge platform located at 3.45/3.4 V, corresponding to the redox plateau potentials of Fe2+/ Fe3+,the charge capacity of samples A,B and C is 155.8 mAh·g-1,157.2 mAh·g-1and 113.5 mAh·g-1, the discharge capacity of samples A,B and C is 153.4 mAh·g-1,139.7 mAh·g-1and 98.4 mAh·g-1,coulomb efficiency is 98.2%,88.9%and 86.7%,resectively.It can be seen that the sample synthesized with lithium hydroxide shows the highest discharge capacity and coulomb efficiency,i.e.sample A has the best reversibility.The charge-discharge process of LiFePO4occurs in the mutual transformation process of FePO4and LiFePO4:lithium ions extract from LiFePO4and get into electrolyte when charging;on the contrary, lithium ions insert into Li1-xFePO4from electrolyte when discharging.The uniform and regular particle morphology of sample A would mean a more stable structure,so after the delithiation,nearly all the lithium ions can insert into thematerials again,which leads to high coulomb efficiency.However,there is a certain amount of impurities in sample C by XRD analysis result.The existence of impurities does not only decrease the content of electrochemical active substance,but also impedes the extraction/insertion of lithium,which causes a part of lithium ions can not join in electrochemical reaction and thus leading to lower specific capacity.
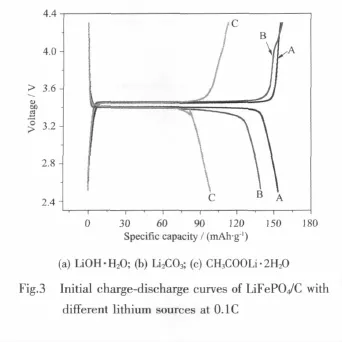
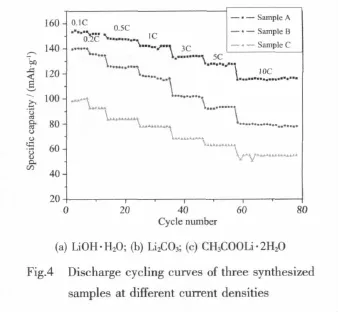
Fig.4 shows the discharge cycling curves of three samples at different current rates.Seeing from the chart,sample A shows the best cycling performance and its specific capacity decays slowly with current density increasing.At 0.1C,the specific capacity is 153.4 mAh·g-1and maintains 116.4 mAh·g-1at high rate of 10C,with capacity retention rate of 76%. However,the specific discharge capacity of sample B and C at 0.1C is 139.7 mAh·g-1,and 98.4 mAh·g-1, respectively,their capacity retention rates are both only 56%at 10C.Liu Youyong et al[17]synthesized nanospheres-LiFePO4composite by PEG based sol-gel synthesis,the specific capacity is 113 mAh·g-1at 5C; Wu Yongming et al[18]successfully developed a facile method to synthesize the hierarchical LFP/C NMs,the specific capacity of LFP/C NMs decreased from 150 to 85 mAh·g-1with an increasing current rate from a value of 0.1C to 5C.Compared with the data of the two documents,our sample displays an excellent rate capability.
Contrasting the SEM images with the electrochemical performance of samples,we can also see that there is a close connection between electrochemical performance and the morphology of the samples.The sample A has the smallest particle size of 200 nm and without blocky particles,Li+can rapidly extract/insert from LiFePO4owing to the shorter diffusion distance of lithium ions,which improves its rate capability.The reason why sample C exhibits bad rate capability is that the purity of the target product and morphology mentioned in the XRD and SEM results are not beneficial to the extraction/ insertion of Li+.Dong et al[19]considered that pH value could affect the dispersion of water system and cell performance of LiCoO2cathodes,when the pH value of the slurry was pH≤7.0 or pH≥11.6,the adhesive force between slurry covering and aluminum foil, conductivity and charge-discharge performance of LiCoO2cathodes decreased obviously.Hence,we presume that the above situation may be suitable for LiFePO4in thiswork.In otherwords,when the system of mixed materials is alkaline,it can be obtained LiFePO4electrode with fine dispersion and excellent electrochemical performance.Only thematerial system of sample A is alkaline,so its electrochemical performance is the best.
2.3 Analysis of the optim um sam p le
2.3.1 Analysis of particle size
The particle size distribution of sample A and its precursor are given in Table 1.The particle size of sample A is small,the average particle size is only 1.942μm,D10,D50,D90value and the average particle size of sample A are close to its precursor,which represents particles without growing much during calcination,the result is in accordance with SEM, which shows that the reaction system composed of LiOH·H2O,FePO4and lauric acid can suppress particle growth,thus the small particles obtained.

Table 1 Particle size distribution of sample A and its precursor
2.3.2 Cycling stability test
Fig.5 shows the cycling curve of sample A at 10 C,its specific capacity basically keeps at about 115 mAh·g-1,the capacity retention rate after 800 cycles is still 96%,which indicates that sample A has an excellent cycling stability.All the results show that the nanoparticles of sample A is stable,even at high rate,the reversibility of lithium extraction/insertion is also good.
2.3.3 Cyclic voltammetry test
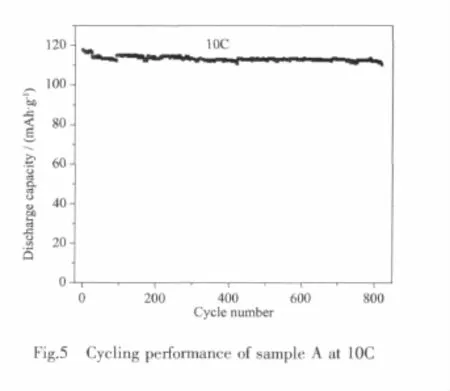
Fig.6 shows the cyclic voltammetry curve of sample A for the 1st,2nd and 5th cycles at scanning rate of 0.1 mV·s-1.The reduction and oxidation peak positions for the 1st,2nd and 5th cycles are the same, it proves that the structure of the sample A is very stable during the charge/discharge processes.Seeing from the chart,the CV curves exhibit symmetrical and sharp shape of the anodic/cathodic peaks at 3.27 V and 3.62 V,respectively,the redox potential interval is small only 0.35 V,which demonstrates the higher electrochemical reactivity and lower electrode polarization.The nearly equal area of anodic/cathodic peaks indicates well lithium insertion/extraction reversibility of the LiFePO4/C,which is in well agreement with the good performance showed in galvanostatic charge/discharge results.
2.3.4 Electrochemical Impedance analysis
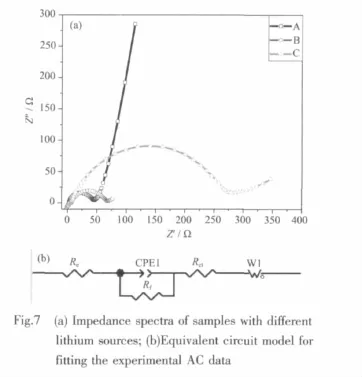
Fig.7a shows the impedance spectra of LiFePO4composites with different lithium sources.As can be seen from the figure,all spectra have an intercept at the Z′axis,a semicircle and a straight line in the high-, middle- and low-frequency regions. An intercept at the Z′axis in high frequency corresponds to the ohmic resistance, which represents the resistance of the electrolyte and LiFePO4/C thin film. Themiddle frequency semicircle indicates the chargetransfer resistance of electrochemical reaction.The slow frequency line is attributed to the diffusion of the lithium ions into the bulk of the electrode material. An equivalent circuitmodel of EISwas constructed to analyze the resistance of LiFePO4/C electrode(Fig.7b), which can explain the impedance spectra through the ohmic resistance Re,charge-transfer Rfand the diffusion resistance Rct.The parameters of the equivalent circuit by computer simulations are shown in Table 2.Seeing from the table,the resistance of the electrolyte and sample A thin film is only 2.673Ω, the charge-transfer resistance of electrochemical reaction is 41.05Ω,the diffusion resistance is 40.43 Ω.all the resistance of sample A is the lowest,which leads to the highest electrical conductivity and the optimum electrochemical performance of sample A.

3 Conclusions
In this work, LiFePO4/C composite wassynthesized by rheological phase method with lauric acid as carbon source and surfactant.It is found that all the composites are olivine-type LiFePO4with high crystallinity, and the energy consumption of calcination process decreases.Using LiOH·H2O as lithium source can get LiFePO4/C with excellent electrochemical performance and rate capability, specific capacity of 153.4mAh·g-1at 0.1C,and 116.4 mAh·g-1at high rate of 10C,The good performance is mainly due to the small particle size of only 200 nm. Through analyzing the optimum sample in detail,we found using rheological phase method with the raw material system of LiOH·H2O,FePO4and lauric acid to synthesize LiFePO4/C can restrain the particles growing and get the sample with outstanding cycling stability and reversibility at high rate.

Table 2 Numerical values of the elements from equivalent circuit
[1]Padhi A K,Nanjundaswamy K S,Goodenough J B.J. Electrochem.Soc.,1997,144(4):1188-1194
[2]Liu H,Feng Y,Wang Z H,et al.Powder Technol.,2008, 184:313-317
[3]Huang Y H,Ren H B,Peng ZH,et al.Electrochimica Acta, 2009,55:311-315
[4]WANGXiao-Juan(王小娟),LIXin-Hai(李新海),WANG Zhi-Xing(王志兴),et al.J.Funct.Mater.(Gongneng Cailiao), 2009,40(12):1996-2003.
[5]YU Hong-Ming(于红明),ZHENGWei(郑威),CAO Gao-Shao (曹高劭),et al.Acta Physico-Chimica Sinica(Wuli Huaxue Xuebao),2009,25(11):2186-2190
[6]WANG Qiu-Ming(王秋明).Thesis for the Master Degree of Harbin Institute of Technology(哈尔滨工业大学硕士论文).2008.
[7]Zhang SS,Allen JL,Xu K,et al.J.Power Sources,2005, 147:234-240
[8]Kang H C,Jun D K,Jin B,et al.J.Power Sources,2008, 179:340-346
[9]Sanchez M A E,Brito G E S,Fantini M C A,et al.Solid State Ionics,2006,177:497-500
[10]Miran G,Robert D,Marjan B,et al.Solid State Ionics, 2005,176:1801-1805
[11]Yang R,Song X P,Zhao M S,et al.J.Alloys Compd., 2009,468:365-369
[12]Park K S,Kang K T,Lee S B,et al.Mater.Res.Bull., 2004,39:1803-1810
[13]Chen J J,Wang S J,Stanley W M.J.Power Sources, 2007,174:442-448
[14]Chen J J,Stanley W M.Electrochem.Commun.,2006,8: 855-858
[15]Guo X F,Zhan H,Zhou Y H.Solid State Ionics,2009,180: 386-391
[16]Zhang Y,Feng H,Wu X B,et al.Electrochimica Acta, 2009,54:3206-3210
[17]Liu Y Y,Cao C B,Li J.Electrochimica Acta,2010,55: 3921-3926
[18]Wu Y M,Wen Z H,Li JH.Adv.Mater.,2011,23(9):1126-1129
[19]Dong Y L,Li JZ,Li ZC.Mater.Sci.Eng.,2006,38(1):1-6
LiFePO4/C Composite w ith Excellent Rate Capability Synthesized by Rheological Phase M ethod
XU Rui1ZHONG Ben-He1GUO Xiao-Dong1TANG Yan1TANG Hong1FANGWei-Mao*,1LIU Heng2
(1College of Chemical Engineering,Sichuan University,Chengdu 610065,China) (2College ofMaterials Science and Engineering,Sichuan University,Chengdu 610065,China)
LiFePO4/C was synthesized by rheological phasemethod (a soft chemicalmethod)using lauric acid as the carbon source and surfactant,lithium hydroxide,lithium carbonate and lithium acetate as the lithium source.Thematerials were characterized by X-ray diffraction(XRD),scanning electron microscopy(SEM),particle size analysis,constant-current charge/discharge test,cyclic voltammetry(CV)and electrochemical impedance spectra (EIS).The results show that the effect of the lithium source on the morphology and the electrochemical performance of LiFePO4/C composites is great.The LiFePO4/C sample with lithium hydroxide as the lithium source exhibits the best cycling performance and rate capability.It shows a specific capacity of 153.4 mAh·g-1at 0.1 C,a capacity retention rate of 76%at high current density of 10 C,and a lossing rate of 4%at 10C after 800 cycles.The excellent performance of this LiFePO4/C is attributed to the smaller size (~200 nm)and the narrower particle size distribution.
LiFePO4/C;rheological phasemethod;lithium sources;high rate
This work was supported by the National Scientific and Technical Backup Plan of China (2007BAQ01055). We also gratefully acknowledge the assistance of the Analysis and Test Center of Sichuan University.
O646;TB33;TM912.9
A
1001-4861(2012)07-1506-07
2011-12-22。收修改稿日期:2012-02-20。
国家科技支撑计划(No.2007BAQ01055),国家自然科学基金(No.50574063)和四川大学青年基金(No.2011SCU11081)资助项目。
*通讯联系人。E-mail:wmfang@scu.edu.cn

