高脂血症对急性坏死性胰腺炎大鼠胰腺NF-κB活化及腺泡细胞凋亡的影响
孙娟 林志辉
·论著·
高脂血症对急性坏死性胰腺炎大鼠胰腺NF-κB活化及腺泡细胞凋亡的影响
孙娟 林志辉
目的探讨高脂血症对急性坏死性胰腺炎(ANP)大鼠胰腺组织病理损伤程度、NF-κB活化及胰腺腺泡细胞凋亡的影响。方法50只雄性SD大鼠随机分成对照组、高脂血症组(HL组)、ANP组 及HL+ANP组。对照组予均衡饲料喂养2周,仅开、关腹;HL组脂肪乳灌胃2周后开、关腹;ANP组予均衡饲料喂养2周后采用胰胆管注射牛磺胆酸钠制备ANP模型;HL+ANP组在脂肪乳灌胃2周后制备ANP模型。免疫组化法检测胰腺组织NF-κBp65及Fas、FasL蛋白表达,TUNEL法检测胰腺腺泡细胞凋亡。结果HL组和HL+ANP组大鼠的血脂明显升高。HL组胰腺见部分细胞有脂质空泡形成,中等量炎症细胞浸润;NF-κB活化轻度增强;凋亡蛋白Fas、FasL表达也有所增强;凋亡指数从(0.62±0.28)%增加到(3.35±1.12)%。ANP组胰腺大片坏死,大量炎症细胞浸润;大量腺胞细胞核有NF-κB p65表达;Fas与FasL表达亦明显增强;凋亡指数为(2.20±1.78)%。HL+ANP组的胰腺坏死较ANP组更严重(3.4±0.7比2.4±1.1,P<0.05),NF-κB p65的表达阳性率及强度较ANP组更高;Fas与FasL的表达较ANP组有所减弱;凋亡指数为(0.93±0.87)%,较ANP组明显降低(P<0.05)。结论高血脂能增强ANP大鼠胰腺NF-κB的活化,抑制腺泡细胞的凋亡,减少Fas与FasL的表达,加重胰腺组织坏死,故必须控制高血脂以减轻胰腺炎的损伤程度。
胰腺炎,急性坏死性; 高脂血症; 核因子-κB; 细胞凋亡
高三酰甘油(hyperlipidemia, HL)血症会导致或加重重症急性胰腺炎(SAP)的发生,这一观点已得到人们的广泛认可,但其确切机制仍有待进一步研究。目前国内外大部分对HL与急性胰腺炎(AP)关系的研究仅局限于HL对AP的病情及胰腺组织病理损伤程度的影响。本实验观察HL对急性坏死性胰腺炎(ANP)大鼠胰腺组织NF-κB活化及胰腺腺泡细胞凋亡的影响,为临床治疗提供实验依据。
材料和方法
一、实验动物及分组
50只雄性SD大鼠,清洁级,体重200~220 g,按数字表法随机分成对照组、高脂血症组(HL组)、ANP组 及HL+ANP组。前2组各10只,后2组各15只。应用自制脂肪乳剂每日1次灌胃、持续2周的方法建立大鼠高脂血症模型;以胰胆管逆行注射3.5%牛磺胆酸钠(Sigma公司)1 ml/kg体重的方法建立大鼠ANP模型。对照组予均衡饲料喂养2周,开腹轻翻胰腺后关腹;HL组予脂肪乳灌胃2周,开腹轻翻胰腺后关腹;ANP组经均衡饲料喂养2周后制备ANP模型;HL+ANP组在脂肪乳灌胃2周后制备ANP模型。
二、观察指标
1.血三酰甘油、胆固醇、淀粉酶检测:制模后6 h处死大鼠,腹主动脉采血,离心分离血清,由实验诊断科常规检测血三酰甘油、胆固醇、淀粉酶水平。
2.胰腺病理检查:处死大鼠后观察胰腺、腹腔等变化,取胰腺组织常规病理检查,由病理科医师盲法阅片,并根据Grewal等[1]标准对胰腺组织损伤进行定量评估。
3.胰腺组织NF-κB p65、Fas、FasL表达检测:应
用免疫组化二步法检测胰腺组织NF-κB p65、Fas、FasL表达。兔抗鼠NF-κB p65、Fas、FasL多抗均购自美国Santa Cruze公司。免疫组化试剂盒购自北京中杉公司,按说明书操作。细胞表达强度判断:“-”未着色,“+”浅黄色,“++”黄色,“+++”棕黄色。每张切片高倍镜下随机观察100个细胞,共4个视野,计算阳性细胞占总细胞的百分率,取均值。
4.胰腺腺泡细胞凋亡检测:应用TUNEL法检测。试剂盒购自美国Roche公司,按说明书操作。每张切片随机选取5个凋亡细胞数最多的高倍视野,计算500个腺泡细胞中凋亡细胞所占的百分比为凋亡指数(AI)。
三、统计学处理
结 果
一、大鼠血三酰甘油、胆固醇及淀粉酶水平
HL组大鼠血三酰甘油、胆固醇水平均较对照组明显升高,淀粉酶水平与对照组无显著差异。ANP组血三酰甘油、淀粉酶水平较对照组明显升高(P值均<0.01),但胆固醇水平与对照组无显著差异。HL+ANP组血甘油三酯、胆固醇、淀粉酶水平均较对照组明显升高(P值均<0.01);三酰甘油水平较HL组明显下降,胆固醇较HL组明显升高(P值均<0.01);胆固醇水平较ANP组明显升高,淀粉酶水平较ANP组明显下降(P值均<0.01,表1)。
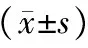
表1 各组大鼠血清三酰甘油、胆固醇及淀粉酶水平
注:与对照组比较,aP<0.01;与HL组比较,bP<0.01;与ANP组比较,cP<0.01
二、胰腺组织病理改变
对照组胰腺组织无明显病理改变。HL组肉眼观察未见异常;镜下见部分细胞有脂质空泡形成,中等量炎症细胞浸润,未见明显出血及坏死。ANP组与HL+ANP组肉眼均见腹腔内大量血性腹水,胰腺呈现体积增大、包膜紧张、表面显著充血、切面见出血和坏死等改变;ANP组镜下见叶间隙及腺泡间隔显著扩张,大量炎症细胞浸润及较多红细胞,有面积不等的腺细胞坏死;HL+ANP组同样出现水肿、炎症细胞浸润、出血、坏死等改变,尤以坏死更为严重。各组胰腺组织病理评分见表2。
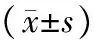
表2 各组胰腺组织病理损伤评分
注:与对照组比较,aP<0.01;与ANP组比较,bP<0.05
三、胰腺组织NF-κB p65、Fas、FasL表达
对照组胰腺组织几乎无NF-κB p65的表达。HL组胰腺仅见少数腺泡细胞核中NF-κB p65表达,偶见胞质表达,阳性表达率与对照组相当,但表达强度略高于对照组。ANP组与HL+ANP组均见大量腺胞细胞核中NF-κB p65表达,胞质中也有表达,表达阳性率及强度均高于对照组,且HL+ANP组NF-κB p65表达阳性率及强度更高(表3,图1)。
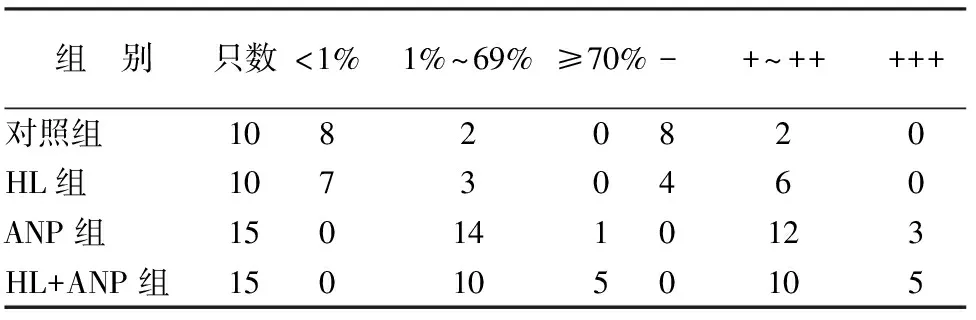
表3 各组胰腺组织NF-κB p65的表达阳性率及强度(只)
注:各组两两比较,P值均<0.05
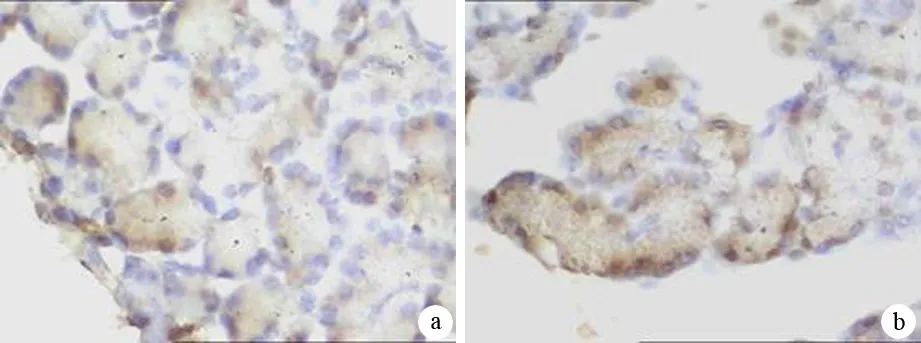
图1ANP组(a)、HL+ANP组(b)大鼠胰腺组织NF-κB p65表达(免疫组化 ×400)
对照组胰腺几乎无Fas表达。HL组胰腺Fas弱阳性表达,部分胞质染成浅黄色。对照组与HL组胰腺均有FasL表达,几乎所有胞质均被染成浅黄色,也有少量胞膜着色。ANP组胰腺Fas与FasL的表达均较对照组明显增强,以炎症浸润区和坏死区最为显著。HL+ANP组胰腺Fas与FasL的表达较ANP组有所减弱(图2)。
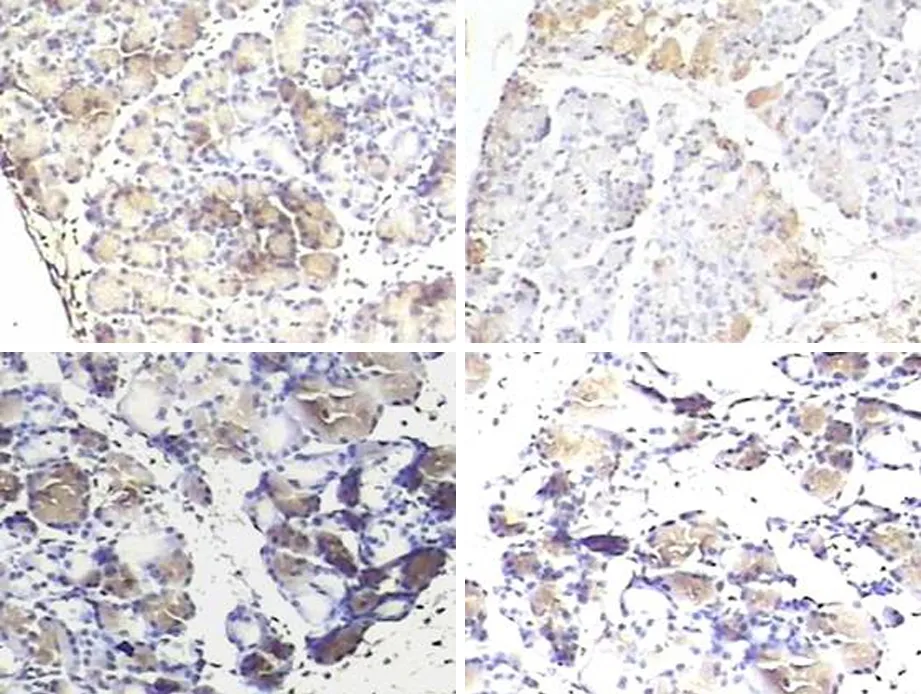
图2ANP组(左列)、HL+ANP组(右列)大鼠胰腺组织Fas(上)表达、FasL(下)表达(免疫组化 ×400)
四、胰腺腺泡细胞凋亡指数
对照组、HL组、ANP组、HL+ANP组胰腺腺泡细胞AI分别为(0.62±0.28)%、(3.35±1.12)%、(2.20±1.78)%、(0.93±0.87)%。HL组和ANP组胰腺腺泡AI均较对照组明显升高;而HL+ANP组AI较ANP组下降(图3)。
讨 论
近年来, 对NF-κB在AP尤其是SAP中作用的研究已取得重大进展[2-3]。我们的实验发现,发生ANP的两组大鼠中NF-κBp65的表达阳性率和强度都比对照组明显增强,这与国内外研究结果相同。我们的研究还发现,HL大鼠发生ANP时,其胰腺组织中NF-κBp65比血脂正常的大鼠表达增强,这可能是HL大鼠发生ANP时胰腺组织坏死更为严重的原因之一。
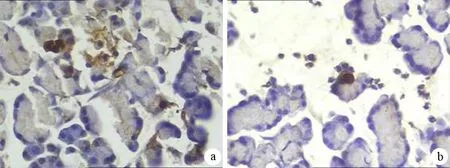
图3ANP组(a)、HL+ANP组(b)大鼠胰腺腺泡细胞凋亡(TUNEL ×400)
本实验结果显示,对照组胰腺组织中偶见腺泡细胞发生凋亡,其Fas与FasL的表达呈阴性或弱阳性,在发生ANP后,胰腺腺泡细胞的凋亡指数显著升高,Fas与FasL的表达也显著增强。而HL+ANP组的腺泡细胞凋亡指数较ANP组降低,Fas与FasL的表达强度也明显减弱,表明与正常的大鼠相比,HL大鼠发生ANP时腺泡细胞凋亡较少,这可能是HL大鼠发生ANP时胰腺组织坏死更为严重的又一原因。
另外,本实验显示,HL+ANP组大鼠胰腺病理损伤中,水肿、炎症、出血评分和ANP组比较没有显著性差异,但坏死的评分显著增高,说明HL大鼠发生ANP时胰腺坏死程度比血脂正常的大鼠严重,这与Kimura等[4]、Hofbauer等[5]和尤和谊等[6]的研究结果相符。本结果还显示,ANP大鼠的血三酰甘油水平比正常大鼠明显升高,提示不但血三酰甘油水平升高可加重ANP,ANP也会引起血三酰甘油水平的升高,二者互相影响,互为因果,造成恶性循环。
本实验HL+ANP组大鼠的血清淀粉酶水平较ANP组的降低。这种现象在临床上也有发现,约有50%的HL性AP患者的血、尿淀粉酶水平正常或只是稍高于正常水平,这给诊断带来了一定的困难[7]。其原因可能是由于这类患者的血浆中存在一种抑制血淀粉酶活性的因子,这种非脂类抑制因子还能通过肾脏进入尿液,抑制尿淀粉酶的活性。当然也不排除由于胰腺损伤太严重导致血清淀粉酶反而下降的可能。
Hofbauer等[5]的动物实验显示单纯高三酰甘油血症组大鼠的胰腺存在轻度水肿。而本实验显示,HL组大鼠胰腺组织的水肿程度与对照组无显著性差异,但炎症评分显著增高。免疫组化结果还显示,HL组大鼠的胰腺组织中NF-κB p65的表达阳性率较对照组虽然没有显著性差异,但表达强度却明显增强。另外,用TUNEL测定其腺泡细胞凋亡指数也显著增高,凋亡调控蛋白Fas、FasL的表达也相应增强。表明血脂增高的大鼠的胰腺组织中已有NF-κB的活化,可引起轻度炎症反应,同时启动腺泡细胞凋亡这一机体自我保护机制来抑制和局限炎症反应。但随着血脂升高,血浆中游离脂肪酸进一步增多,血粘度升高,血栓素A2/前列腺素I2失衡加重,各种因素刺激NF-κB大量活化,引起炎症介质瀑布样激活,导致炎症失控,发生AP甚至SAP,因此HL可加重或引起AP的发生。
[1] Grewal HP,Moheyel Din A,Gaber L,et al.Amelioration of the physiologic and biochemical changes of acute pancreatitis using an anti-TNF-alpha polyclonal antibody.Am J Surg,1994,167:214-219.
[2] Gukovsky I,Gukovskaya AS,Blinman TA,et al.Early NF-kappaB activation is associated with hormone induced pancreatitis.Am J Physiol,1998,275:G1402-G1414.
[3] Altavilla D,Famulari C,Passaniti M,et al.Attenuated cerulein-induced pancreatitis in nuclear factor-kappaB-deficient mice.Lab Invest,2003,83:1723-1732.
[4] Kimura W,Mossner J.Role of hypertriglyceridemia in the pathogenesis of experimental acute pancr eatitis in rats.Int J Pacreatol,1996,20:177-184.
[5] Hofbauer B,Friess H,Weber A,et al.Hyperlipaemia intensifies the course of acute oedematous and acute necrotising pancreatitis in the rat.Gut,1996,38:753-758.
[6] 尤和谊,蔡端.高脂血症对大鼠急性胰腺炎发生的影响.肝胆胰外科杂志,2005,17:26-28.
[7] Yadav D,Pitchumoni CS.Issues in hyperlipidemic pancreatitis.Clin J Gastroenterol,2003,36: 54-62.
2010-06-07)
(本文编辑:屠振兴)
ActivationofNF-κBandacinarcellapoptosisinhyperlipidemicratswithsevereacutepancreatitis
SUNJuan,LINZhi-hui.
DepartmentofGastroenterology,FuzhouFirstHospital,Fuzhou350009,China
Correspondingauthor:LINZhi-hui,Email:wind8864gy@163.com
ObjectiveTo investigate the effect of hyperlipidemia (HL) on the pancreatic injuries during acute necrotizing pancreatitis (ANP) as well as the activation of nuclear factor kappa B (NF-κB) and pancreatic acinar cell apoptosis.MethodsFifty SD male rats were randomly divided into control group, hyperlipidemia (HL) group, ANP group and HL+ANP group. Rats in control group were fed with balanced diet for 2 weeks, and underwent opening and closing of the abdomen. Rats in HL group
fat emulsion lavage, and then underwent opening and closing of the abdomen. Rats in ANP group were fed with balanced diet for 2 weeks, and were induced by retrograde injection of sodium taurocholate into the bili-pancreatic duct to establish the ANP model. Rats in HL+ANP group received fat emulsion lavage for 2 weeks, and were induced by retrograde injection of sodium taurocholate into the bili-pancreatic duct to establish the ANP model. NF-κB p65 and Fas, FasL protein expression were determined by immunohistochemical method and the apoptosis in pancreatic acinar cell were detected by TUNEL.ResultsTwo weeks after fat emulsion lavage, rats in HL and HL+ANP group had significantly higher serum level of lipid. Lipid vacuoles were present in some pancreatic cells in the rats of HL group, and middle number of inflammatory cells infiltration was found, the activation of NF-κB was slightly enhanced; and the expression of Fas, FasL was also enhanced. Apoptosis index increased from (0.62±0.28)% to (3.35±1.12)%. Massive pancreatic tissue necrosis, large amount of inflammatory cells infiltration was found in ANP group. NF-κB p65 was present in large number of pancreatic nucleus. The expression of Fas and FasL was also increased; the apoptosis index was (2.20±1.78)%. The degree of pancreatic necrosis was greater in HL+ANP group than that in ANP group (3.4±0.7vs. 2.4±1.1,P<0.05). The positive rate and density of NF-κB expression was higher than that in ANP group; and the expression of Fas and FasL was decreased more than that in ANP group; the apoptosis index was (0.93±0.87)%, which was significantly lower than that in ANP group (P<0.05).ConclusionsHigh level of lipid can increase the activation of NF-κB and inhibit the acinar cell apoptosis, and decrease the expression of Fas and FasL, increase the pancreatic tissue necrosis, therefore in order to attenuate the injury of pancreas, it is essential to control blood lipid.
Pancreatitis,acute necrotizing; Hyperlipidemia; NF-κB; Apoptosis
10.3760/cma.j.issn.1674-1935.2011.03.019
350009 福州,福建福州市第一医院消化内科(孙娟);福建省立医院消化内科(林志辉)
林志辉,Email:wind8864gy@163.com

