SHP-2酪氨酸磷酸酶激活突变导致小鼠髓系异常增殖*
张 薇, 杨金莲, 胡中倩, 卢艳敏, 储著朗, 余科科, 瞿成奎,3, 汪思应△
(1安徽医科大学病理生理学教研室,安徽 合肥 230032;2安徽医学高等专科学校病理生理学教研室,安徽 合肥 230601;3美国凯斯西储大学,克里夫兰 44106)
SHP-2酪氨酸磷酸酶激活突变导致小鼠髓系异常增殖*
张 薇1,2▲, 杨金莲1▲, 胡中倩1, 卢艳敏1, 储著朗1, 余科科1, 瞿成奎1,3, 汪思应1△
(1安徽医科大学病理生理学教研室,安徽 合肥 230032;2安徽医学高等专科学校病理生理学教研室,安徽 合肥 230601;3美国凯斯西储大学,克里夫兰 44106)
目的观察激活突变SHP-2酪氨酸磷酸酶是否参与髓系异常增殖的发生。方法以野生型(WT)和SHP-2D61G/+突变型C57BL/6小鼠为研究对象,计数外周血白细胞,比较脾大小,流式细胞术检测外周血及骨髓髓系来源细胞表面标志分子(Mac-1、Gr-1),并统计外周血Mac-1和Gr-1阳性细胞率及骨髓细胞中红系 (Ter119)、髓系 (Mac-1、Gr-1)、T(CD3)、B(B220)淋巴细胞系的阳性细胞率,观察骨髓造血干/祖细胞的集落形成(CFU)能力,Western blotting检测外周血白细胞经白细胞介素 3(IL-3)和5 μg/L,刺激后磷酸化的丝氨酸/苏氨酸蛋白激酶B(p-Akt)和磷酸化的细胞外信号调节激酶(p-ERK)表达水平。结果SHP-2D61G/+突变16周龄组小鼠较WT组外周血白细胞数增多(Plt;0.05),脾明显增大,同时外周血白细胞的Mac-1和Gr-1阳性细胞率增加(Plt;0.05),骨髓细胞中Mac-1和Gr-1的阳性细胞率也增多,但红系、淋巴细胞系的变化不明显。同时骨髓中粒-单核细胞集落形成单位(CFU-GM)较正常对照组明显增加,白细胞经IL-3刺激后Akt和ERK蛋白磷酸化水平升高。结论SHP-2D61G/+突变可能通过MAPK及PI3K的活化而导致小鼠髓系异常增殖。
SHP-2酪氨酸磷酸酶; 激活突变; 小鼠; 髓系增殖
SHP-2酪氨酸磷酸酶(SHP-2 tyrosine phosphatase)是蛋白酪氨酸磷酸酶家族中的一员,广泛表达于各组织的细胞中。SHP-2在N末端含有2个SH2(即Src同源体2)结构域,C末端上含有1个蛋白酪氨酸磷酸酶(protein tyrosine phosphatases,PTP)结构域及2个酪氨酸残基(Tyr542和Tyr580)[1]。正常基础状态下,N-末端的SH2结构域与PTP结构域结合抑制了SHP-2的催化活性[2,3]。当SHP-2经特定的生长因子如血小板源性生长因子(platelet-derived growth factor,PDGF)、表皮生长因子(epidermal growth factor,EGF)、促生长因子(insulin-like growth factor, IGF)[4]或细胞因子如白细胞介素3(interleukin-3,IL-3)、粒细胞-巨噬细胞集落刺激因子(granulocyte-macrophage colony-stimulating factor,GM-CSF)和促红细胞生成素(erythropoietin,EPO)以及胰岛素和干扰素刺激,以及N-SH2结构域连接酪氨酸磷酸化的对接蛋白如Gab1、Gab2等,可解除自身抑制状态,激活SHP-2的磷酸酶活性[5];或者SHP-2的PTP结构域与N-SH2结构的突变导致PTP被部分或全部地解离出来,从而使SHP-2磷酸酶活性被不同程度地激活。而羧基端PTP结构域是对SHP-2起调节功能的关键部位[6],其功能主要是在受体或胞浆酪氨酸蛋白激酶(protein tyrosine kinases,PTKs)介导的信号途径中起促进作用,同时还发现SHP-2具有磷酸酶非依赖性接头蛋白功能[7-11]。
2001年以来陆续在Noonan综合征(伴髓系增生症)、幼年型粒单细胞白血病、儿童骨髓增生异常综合症和骨髓增生异常、B细胞急性淋巴细胞白血病、急性髓系白血病和一些实体瘤中发现有PTPN11(编码SHP-2的基因)激活突变[12-17]。这些突变主要集中在SHP-2的N-SH2或PTP结构域内,其中N-SH2结构域内D61G/Y或E76K突变被认为与白血病等肿瘤密切相关。但PTPN11突变引起疾病的机制尚不清楚。为此,Neel实验室建立了SHP-2 D61G基因敲入小鼠模型,但在该模型小鼠中没有发现白血病,仅出现Noonan综合征表现[18]。本实验中采用已建立的SHP-2D61G/+模型小鼠及正常对照组C57BL/6小鼠为研究对象,观察SHP-2激活突变后对小鼠髓系增殖及相关信号通路的影响,以确定SHP-2激活突变后是否参与白血病早期的典型表型-髓系细胞异常增殖发生,为进一步研究白血病提供新的线索。
材 料 和 方 法
1材料
1.1药品与试剂 生理盐水购于北京双鹤药业,胎牛血清、RPMI-1640购于Gibco,LTS1093小鼠白细胞分离液购于上海欧韦达公司, FITC(fluorescein isothiocyanate)标记的Mac-1、Gr-1、CD3等抗体购于北京博奥森生物公司, Base methylcellulose medium(Methocult M 3134) 购于Stem Cell。Western 所用ERK、Akt、p-ERK,p-Akt等抗体购于Santa Cruz。
1.2动物 野生型(wild type,WT)小鼠采用C57BL/6小鼠,雌雄各半,8周龄及16周龄, SPF级,由安徽医科大学动物中心提供,为WT组。C57BL/6品系SHP-2D61G/+小鼠由美国Case Western Reserve大学瞿成奎教授提供,为SHP-2D61G/+突变组。分别取8周龄(WT8,SHP-2D61G/+8)和16周龄小鼠(WT16,SHP-2D61G/+16)共分为4组进行实验,每组8只。
2方法
2.1外周血白细胞(white blood cell, WBC)计数 分别取WT组和SHP-2D61G/+突变组小鼠尾静脉血约300 μL于2% EDTA-K2抗凝剂的采血管中,红细胞破坏法(3%乙酸溶液∶血液=19∶1)获得白细胞,应用F2800微细胞仪计数小鼠白细胞。
2.2骨髓细胞的建立 将小鼠脱颈椎处死后.无菌分离双侧股骨和胫骨并剪去骨端,用3 mL IMDM (Gibco)培养液的注射器冲出骨髓,再以22号针头抽吸吹打3次制成单细胞悬液,用白细胞稀释液稀释,作骨髓有核细胞计数后备用。
2.3脾指数测定 分别对2组小鼠称重,麻醉小鼠摘眼球取血后脱颈处死,无菌条件下常规分离,取脾分别称重。计算脾指数(spleen index,SI)= 脾重量(mg)/小鼠体重(g)值。
2.4小鼠白细胞分离培养 分离外周血单个核细胞的分层液比重是 1.077±0.001(骨髓中单个核细胞的分层液比重是1.120±0.001)的聚蔗糖(Ficoll)-泛影葡胺(Urografin)(F/H)分层液。无菌采取小鼠抗凝血1 mL,与等量Hanks液充分混匀;用滴管沿管壁缓慢叠加于2 mL分层液面上。2 000 r/min离心20 min;离心后管内分为3层,上层为血浆和Hanks液,下层主要为红细胞和粒细胞,中层为淋巴细胞分离液,在上、中层界面处有一以单个核细胞为主的白色云雾层狭窄带,单个核细胞包括淋巴细胞和单核细胞;用毛细血管插到云雾层,吸取单个核细胞。置入另一短管中,加入5倍体积的Hanks液,1 500 r/min离心10 min,洗涤细胞2次,弃上清,加入含有10%胎牛血清的RPMI-1640,0.5 μg/L IL-3,重悬细胞,常规培养。
2.5流式细胞术检测细胞表面标记表达 外周血裂解红细胞后或冲洗小鼠骨髓细胞,离心洗涤后,用含2%BSA的PBS制备成细胞悬液,加2 ng Fc受体阻断抗体,室温孵育15 min,离心洗涤1次,按1×106细胞/100μL,每管分别加FITC标记的相关CD3等抗原抗体,冰浴30 min,加3 mL冰PBS后,离心洗涤,每管加500 μL PBS(1%BSA)重悬细胞,流式细胞仪检测分析。
2.6骨髓粒-单核细胞集落生成单位的集落培养(colony-forming unit-granulocyte and macrophage ,CFU-GM) 用甲基纤维素半固体培养系统[19],加入小鼠干细胞因子(mSCF) 50 μg/L、小鼠IL-3(mIL-3) 20 μg/L、人IL-6(hIL-6) 50 μg/L和人EPO(hEPO) 3×103U/L, 接种2×107细胞于35 mm培养皿中,常规条件培养14 d后倒置显微镜下观察计数集落数目(细胞数gt;50为1个集落),并根据细胞形态分类:来源于红系造血祖细胞的为CFU-E,来源于粒单系的为CFU-GM,红粒系混合的为CFU-GEM。
2.7外周血白细胞总蛋白的提取及Western blotting检测磷酸化的细胞外信号调节激酶(phosphorylated- extracellular signal-regulated kinase,p-ERK)和磷酸化的丝氨酸/苏氨酸蛋白激酶(phosphorylated -serine-threonine protein kinase B,p-Akt)的表达水平 正常生长状态的白细胞饥饿8 h,IL-3 5 μg/L刺激0、5、15 min,细胞蛋白经SDS-PAGE电泳,电转移至NC膜上,用含2%BSA/TBST封闭液封闭2 h,加p-ERK和p-Akt抗体过夜(4 ℃),TBST洗3次,每次10 min,再加经辣根过氧化物酶标记的相应的Ⅱ抗(用封闭液1∶2 000稀释),摇床上缓慢摇动1 h,用TBST洗3次,每次10 min;用ECL系统进行检测,压片,曝光。
3统计学处理
结 果
1SHP-2D61G/+突变小鼠外周血白细胞数增加
对SHP-2D61G/+突变小鼠及WT组小鼠的外周血白细胞计数观察发现,16周龄SHP-2D61G/+突变的外周血白细胞数明显比同龄WT组高(Plt;0.01),见图1,提示SHP-2D61G/+突变导致了外周血白细胞的增殖。

Figure 1. Increasing peripheral white blood cells in the mice with gain-of -function mutant of SHP-2±s.**Plt;0.01 vs WT16.
2SHP-2D61G/+突变小鼠脾明显增大
脾脏增大是髓系异常增殖的显著特征之一。实验中我们发现SHP-2D61G/+突变组小鼠的脾明显较同龄的WT组增大、充血(图2照片展示16周龄WT及SHP-2激活突变小鼠脾脏),脾指数检测也发现SHP-2D61G/+突变小鼠脾的相对增大程度较同龄WT组升高 (Plt;0.01),见图2。以高龄鼠最为明显。
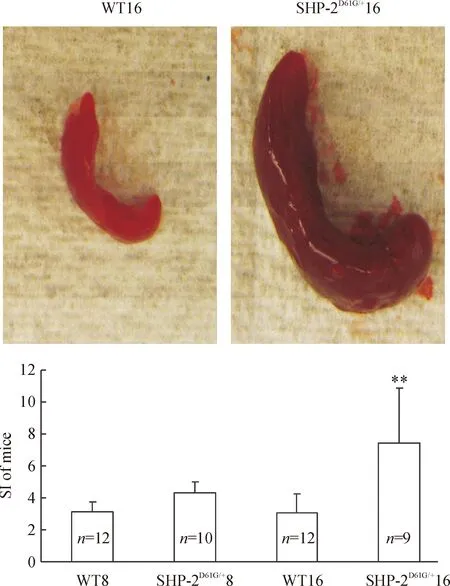
Figure 2. Enlarged spleen in the mice with gain-of-function mutant of SHP-2±s.**Plt;0.01 vs WT16.
3SHP-2D61G/+突变小鼠外周血及骨髓中髓系细胞比例升高
图3结果显示,SHP-2D61G/+突变组外周血增加的白细胞中,髓系来源(Mac-1和Gr-1阳性)比例高于对照正常小鼠,以高龄小鼠最为明显。骨髓细胞表面标记检测也发现相同结果,SHP-2D61G/+突变组Mac-1和Gr-1阳性骨髓细胞比例明显高于对照组(Plt;0.05),见图4,提示小鼠髓系增殖异常。
4骨髓祖细胞集落形成实验
取8周龄小鼠骨髓细胞,用甲基纤维素半固体培养系统检测在多种细胞因子诱导下,不同造血祖细胞集落形成能力,结果发现SHP-2D61G/+突变组髓系来源祖细胞集落形成不仅数量明显高于对照组(Plt;0.01),见图5。从形态学上比较,突变组的集落亦大于对照组(结果未展示)。
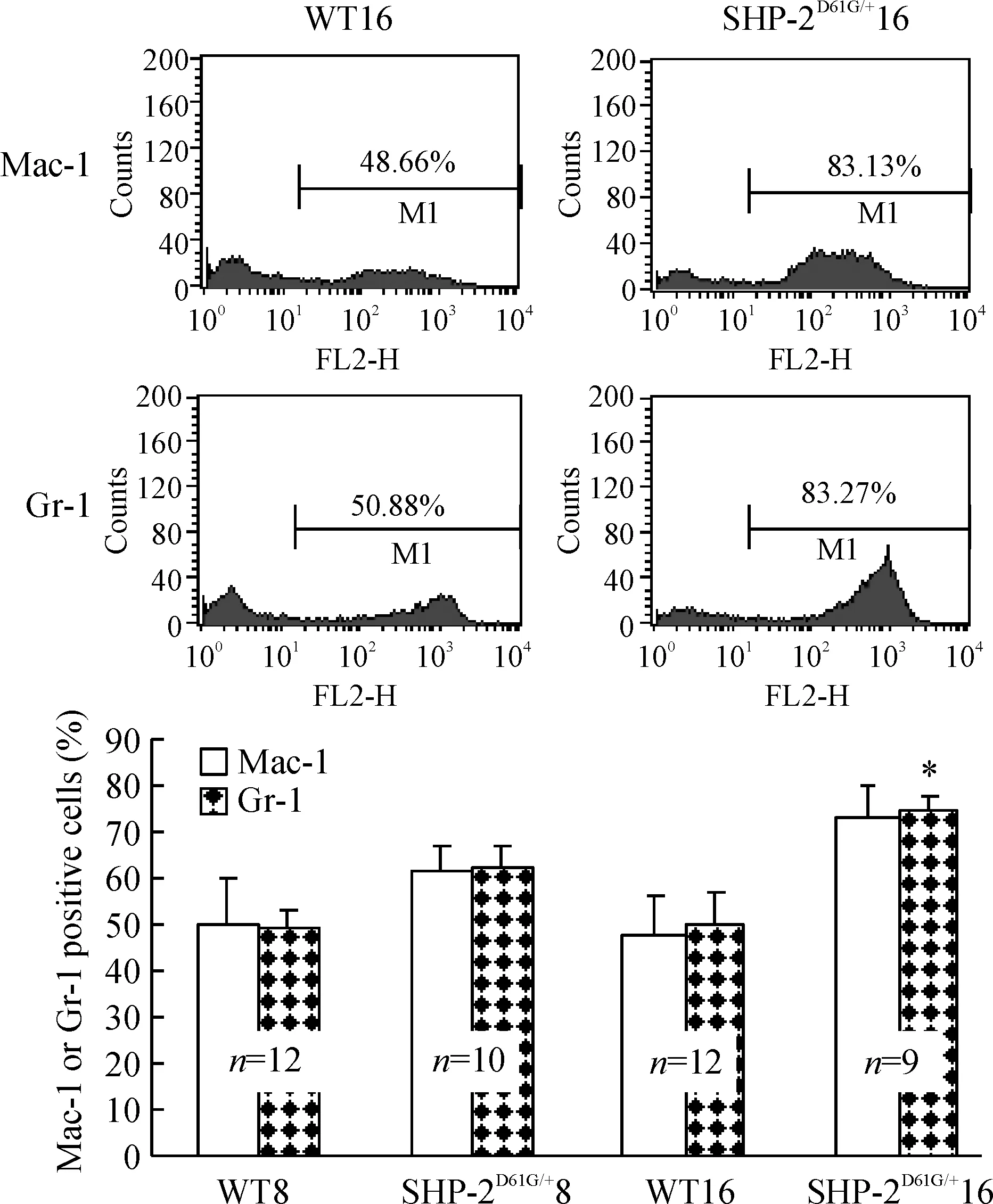
Figure 3. Percentage of myeloid cells is increased in the mice with gain-of -function mutant of SHP-2±s.*Plt;0.05 vs WT16.
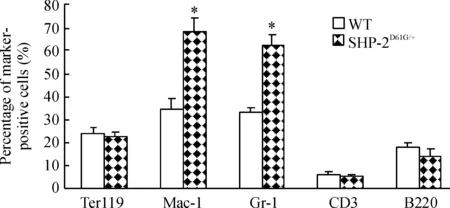
Figure 4. Percentage of myeloid cells is increased in the bone marrow of mice with gain-of-function mutant of SHP-2±s.n=5.*Plt;0.05 vs Mac-1.
5Westernblotting检测p-ERK和p-Akt的表达水平
从图6可以看出,经IL-3 5 μg/L刺激后,SHP-2 D61G突变小鼠白细胞p-ERK和p-Akt的表达水平均有明显上调,提示MAPK和PI3K途径被明显激活。
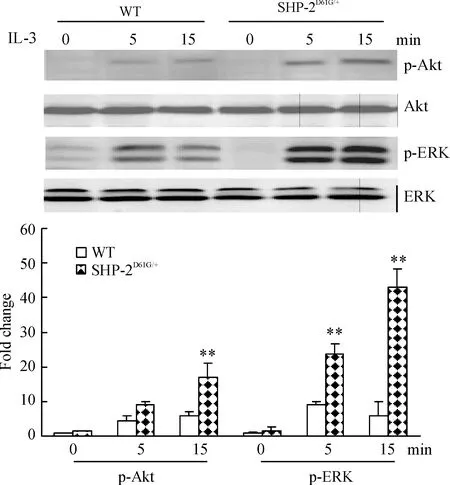
Figure 6. Phosphorylation of ERK and Akt was increased in the WBC of mice with gain-of-function mutant of SHP-2 induced by IL-3±s.n=5.**Plt;0.01 vs WT.
讨 论
髓系异常增殖是白血病前期典型表现之一,研究髓系异常增殖应该能为白血病发病的研究提供线索。临床髓系异常增殖病可以单发,也可以伴发于其他疾病如Noonan综合征之后或白血病前期。目前机制不清。研究发现10%的髓系增殖病患者体内存在SHP-2激活突变,SHP-2是一个在细胞内广泛存在的酪氨酸磷酸酶,其在细胞生长、分化、迁移、凋亡的信号调控中起着重要的作用,尤其对胚胎干细胞的分化和造血细胞的分化也起着关键作用[1,4,20]。Neel等在研究SHP-2蛋白结构时,利用计算机模拟技术认为如果SHP-2 N-SH2结构域内第61位的天冬氨酸(D)突变成甘氨酸(G),则N-SH2结构域与其PTP结构域的自身抑制作用将降低,SHP-2磷酸酶活性将升高。为进一步研究SHP-2 D61G突变的功能,他们建立了SHP-2 D61G基因敲入小鼠模型,SHP-2 D61G纯合子突变(SHP-2D61G/D61G)小鼠死于胚胎期,约50%杂合子突变(SHP-2D61G/+)小鼠生长发育成活,表现Noonan综合征症状,但无白血病出现[18]。临床Noonan综合征患者常伴发髓系增殖,后期可能发生白血病。因此。我们在获赠该小鼠后,观察SHP-2D61G/+小鼠是否发生了髓系异常增殖及其机制。
实验中我们发现SHP-2D61G/+突变组小鼠随着年龄增大外周血白细胞数明显较同周龄WT组增高,同时脾明显增大, 16周龄时小鼠差异显著和流式显示外周血白细胞中髓系来源的细胞即Mac-1和Gr-1阳性细胞率在SHP-2D61G/+突变组也明显高于WT组;同时骨髓细胞中髓系细胞(Mac-1、Gr-1)比例明显增高,而红细胞(Ter119)、T细胞(CD3)和B细胞(B220)淋巴细胞系变化不明显;提示SHP-2 激活突变小鼠髓系发生异常增殖。机制研究时发现:SHP-2 激活突变小鼠骨髓造血祖细胞形成集落中CFU-GM比例明显高于对照正常小鼠,并且集落明显增大,提示髓系祖细胞对细胞因子反应性增强。进一步实验结果发现SHP-2激活突变小鼠白细胞后经IL-3刺激后磷酸化ERK及AKT水平均明显高于对照组细胞,提示髓系异常增殖可能与其增强的MAPK及PI3K信号途径有关。但SHP-2是酪氨酸磷酸酶,SHP-2激活突变往往磷酸酶活性升高,应该导致下游蛋白磷酸化水平降低,为何经细胞因子刺激后,突变细胞内p-ERK及p-Akt水平升高呢,可能与SHP-2除了磷酸酶功能外,还具备接头蛋白功能[21]。我们前期研究也发现SHP-2 D61G突变的肥大细胞对细胞因子IL-3反应性增强,且可能是突变SHP-2与Gab2结合增多有关[22,23]。Gab的活化是MAPK途径活化所必须的[21]。但详细的机制以及与白血病发病的关系还有待进一步研究阐明。
[1] Qu CK. The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions[J]. Cell Res,2000,10(4):279-288.
[2] Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2[J]. Structure,1998, 6(3):249-254.
[3] Hof P, Pluskey S, Dhe-Paganon S, et a1. Crystal structure of the tyrosine phosphatase SHP-2[J]. Cell,1998,92(4):441-450.
[4] Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction[J]. Curr Opin Cell Biol,1997,9(2): 193 - 204.
[5] Mohi MG, Neel BG. The role of Shp2 (PTPN11) in cancer[J]. Curr Opin Genet Dev,2007,17(1): 23-30.
[6] Araki T, Nawa H , Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors[J]. J Biol Chem,2003,278(43): 41677-41684 .
[7] Keilhack H, David FS, Mcgregor M, et al. Diverse biochemical properties of Shp-2 mutants. Implications for disease phenotypes[J]. J Biol Chem,2005, 280(35):30984-30993.
[8] Yuan L, Yu WM, Xu M, et al. SHP-2 phosphatase regulates DNA damage-induced apoptosis and G2/M arrest in catalytically dependent and independent manners, respectively[J]. J Biol Chem,2005, 280(52):42701-42706.
[9] Yang W, Klaman LD, Chen B, et al. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival[J]. Dev Cell, 2006, 10(3):317-327.
[10]Loh ML, Sakai DS, Flotho C, et al.Mutations in CBL occur frequently in juvenile myelomonocytic leukemia[J]. Blood,2009, 114 (9): 1859-1863.
[11]Loh ML, Reynolds MG, Vattikuti S, et al.PTPN11 mutations in pediatric patients with acute myeloid leukemia:results from the Children’s Cancer Group[J]. Leukemia,2004, 18(11):1831-1834.
[12]Bouyain S,Watkins DJ. Identification of tyrosine phosphatase ligands for contactin cell adhesion molecules[J]. Commun Integr Biol, 2010, 3(3):284-286.
[13]Batz C, Hasle H, Bergsträsser E,et al. DoesSPRED1 contribute to leukemogenesis in juvenile myelomonocytic leukemia (JMML)?[J].Blood,2010, 115(12): 2557-2558.
[14]Tartaglia M,Niemeyer CM,Fragale A,et al.Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia,myelodysplastic syndromes and acute myeloid leukemia[J].Nat Genet,2003,34(2):148-150.
[15]Bentires-Alj M, Paez JG, David FS, et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia[J]. Cancer Research,2004, 64(24): 8816-8820.
[16]Chan G,Kalaizidis D,Neel BG.The tyrosine phosphatase Shp2 (PTPN11) in cancer[J].Cancer Metast asis Rev,2007, 27(2):179-192.
[17]Wang S, Yu WM, Zhang W, et al.Noonan syndrome/leukemia-associated gain-of-function mutations in SHP-2 phosphatase (PTPN11) enhance cell migration and angiogenesis[J]. J Biol Chem, 2009,284(2):913-920.
[18]Araki T, Mohi MG, Ismat FA, et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects ofPtpn11 mutation[J]. Nat Med, 2004, 10(8):849-857.
[19]Miyamoto T, Nagafuji K, Akashi K, et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia[J]. Blood,1996, 87(11):4789-4796.
[20]Cha Y,Park KS. SHP2 is a downstream target of ZAP70 to regulate JAK1/STAT3 and ERK signaling pathways in mouse embryonic stem cells[J]. FEBS Lett,2010, 584(19):4241-4246.
[21]Yu WM, Hawley TS, Hawley RG, et al. Catalytic-dependent and -independent roles of SHP-2 tyrosine phosphatase in interleukin-3 signaling[J]. Oncogene, 2003, 22(38):5995-6004.
[22]Xu D, Wang S, Yu WM,et al. A germline gain-of-function mutation inPtpn11 (Shp-2) phosphatase induces myeloproliferative disease by aberrant activation of hematopoietic stem cells[J]. Blood,2010, 116(18):3611-3621.
[23]霍寅萍,储著朗,余科科,等.SHP-2酪氨酸磷酸酶激活突变的肥大细胞对IL3呈高增殖敏感性[J].安徽医科大学学报,2010,45(5):593-596.
AnactivatedmutantofSHP-2tyrosinephosphatasecausesmurinemyeloidabnormalproliferation
ZHANG Wei1,2, YANG Jin-lian1, HU Zhong-qian1, LU Yan-min1, CHU Zhu-lang1, YU Ke-ke1, QU Cheng-kui1, 3, WANG Si-ying1
(1DepartmentofPathophysiology,AnhuiMedicalUniversity,Hefei230032,China;2DepartmentofPathophysiology,AnhuiMedicalCollege,Hefei230601,China;3CaseWesternReserveUniversity,Cleveland44106,USA.E-mail:sywang@ahmu.edu.cn)
AIM: To investigate whether an activated mutant of SHP-2 tyrosine phosphatase is involved in abnormal proliferation of murine myeloid.METHODSWild-type (WT) and SHP-2D61G/+mutant mice aged 8 weeks and 16 weeks were used. The number of peripheral blood leukocytes and the spleen sizes were measured by cell counting and weighing methods,respectively. The surface markers (Mac-1 and Gr-1 for myeloid, Ter119 for erythroid, CD3 for T-lymphocyte and B220 for B-lymphocyte) of hematopoitic cells in peripheral blood and bone marrow were detected by flow cytometry. The rate of Mac-1 or Gr-1 positive cells in the peripheral blood and the rate of Mac-1, Gr-1, Ter119, CD3 or B220 positive cells in bone marrow were analyzed. The ability of colony formation unit (CFU) of the bone marrow was also observed by CFU assay. Finally, the expression of p-Akt and p-ERK in the peripheral blood leukocytes induced by interleukin-3 (IL-3, 5 μg/L) was detected by Western blotting.RESULTSThe number of leukocytes in peripheral blood of 16-week-old mice was more (Plt;0.01) and the spleens were bigger in mutant SHP-2D61G/+mice than those in WT mice. The rate of Mac-1 and Gr-1 positive cells in peripheral blood leukocytes of 16-week-old SHP-2D61G/+mice were dramatically increased (Plt;0.05). Mac-1 and Gr-1 positive cell rates in bone marrow of SHP-2D61G / +mice were much higher (Plt;0.05) than those in WT mice and no statistic significance was found in the erythroid or lymphocyte cells. The number of CFU-GM (represents myeloid) was increased in mutant mice. The expression of p-Akt and p-ERK in peripheral blood leukocytes of mutant mice was significantly enhanced after stimulated with IL-3.CONCLUSIONThese results suggest that activated mutant SHP-2 results in the disorder of mouse myeloid proliferation via MAPK and PI3K activation.
SHP-2 tyrosine phosphatase; Activated mutant; Mice; Myeloid proliferation
1000-4718(2011)04-0682-06
R363
A
10.3969/j.issn.1000-4718.2011.04.012
2010-12-15
2011-02-25
国家自然科学基金资助项目(No. 30873046);安徽省优秀留学人才基金资助项目(No.2009-2010)
△通讯作者 Tel:0551-5167706;E-mail: sywang@ahmu.edu.cn
▲并列第1作者

