β-环糊精与系列无机盐分子-离子加合物的粉末X射线衍射分析
党 政 宋乐新
(中国科学技术大学高分子科学与工程学系,中国科学院软物质化学重点实验室,合肥 230026)
β-Cyclodextrin(β-CD)is a cyclic oligosaccharide consisting of seven glucopyranose units,in the form of a hollow truncated cone[1-3].With hydrophilic exterior and hydrophobic interior,it can form supramolecular inclusion complexes with many kinds of guests such as organic molecules,polymers,inorganic ions, and coordination compounds by weak interaction processes[4-8]. Owing to good crystallization behavior,β-CD as well as its inclusion complexes can be analyzed by X-ray diffraction(XRD) technology[9-11].
A solid inorganic ion-CD adduct means the product formed by a CD and a certain inorganic salt,such as CaCl2[12]and CuCl2[12-13]. For small inorganic ions,they are likely to be in the form of an intercalary structure between CD molecules in their adducts with the aid of the molecule-ion encapsulation interaction[2](Fig.1).
As a whole,even in solution,the studies concerned about the formation of the molecule-ion adducts formed by CDs and inorganic salts are quite rare[1,14-15].In crystal state,a few reports show that there is molecule-ion interaction between CD molecules and simpleinorganicions[16-17],suchasI-3,I-5,and I-7[18-21].Recent studies indicate that the existence of CD molecules can seriously affect the crystal packing modes of inorganic salts.For example,in terms of XRD data,CaCO3nanoparticles formed in water present a calcite structure[22].However,if the isolated nano-particles are grown in the aqueous solution of β-CD,they have an aragonitestructure[23].Thisworkprovidesagoodparadigmforrevealing the significance of the molecule-ion interaction between CD molecules and inorganic ions.Nevertheless,it only refers to the influenceofmolecule-ioninteractionbetween β-CDandCa2+onthe growth and packing behavior of CaCO3and does not mention the effect of this interaction on those of β-CD.
As shown in Fig.1,the molecule-ion interaction between β-CD molecules and inorganic ions is unlike the intermolecular complexation between β-CD and water molecules as well as organic guests.As most of inorganic ions have a small size and a high polarity,the van der Waals interaction between inorganic ions and the cavities of CDs was found to be extremely weak in aqueous solution[24],possibly because inorganic ions are preferred to be outside the hydrophobic cavities of CDs.
For example,like hydrates of β-CD,the single crystal structure of molecule-ion adduct formed by CaCl2with β-CD is just in the form of a cage packing mode[11],in which neither calcium ion nor chloride ion is embedded in the cavity of β-CD.This is why the products formed by molecule-ion interactions are regarded as adducts instead of inclusion complexes.
Previous studies have concentrated on the confirmation of molecule-ion interaction either in aqueous solution or in single crystal state.To the best of our knowledge,there are very few reports on the spectral behavior of molecule-ion adducts in a crystal state so far though numerous papers are associated with the characterization of inclusion complexes formed through intermolecular interaction between CDs and guest molecules in solution as well as in solid state[5-10].

Fig.1 Proposed molecule-ion interaction mode between β-CD and inorganic ions
The absence of such studies is the more striking,since with the development of molecular biology and medical chemistry,it is suggested that many simple inorganic drugs,such as Li2CO3, NaAsO2and so on,play an important role in treating all aspects of health care[25-28].Assuredly,how to reduce their acute oral toxicity in vivo is a significant and particularly useful subject for studies concerning the link between bioinorganic chemistry and supramolecular chemistry.Possibly,this question can be solved by means of the formation of supramolecular adducts between common inorganic drugs and biomacromolecules such as CDs. Further,what is the spectral difference of similar adducts in solid state?In the present work,we attempt to prepare a series of adducts of β-CD with inorganic salts and try to analyze the spectral difference in powder X-ray diffraction(PXRD)patterns among inorganic salts,β-CD and their adducts in order to evaluatetheperformanceofmolecule-ioninteractionsinacrystalstate. We believe that the research will be very useful for pharmaceutical and biomedical analysis as well as preparation of inorganic nanoparticles[27].
1 Experimental
1.1 Materials
β-CD(≥98.0%)was purchased from Shanghai Chemical Reagent Company and recrystallized twice from deionized water. Lithium chloride(LiCl,≥99.5%),ammonium chloride(NH4Cl,≥99.5%),potassium chloride(KCl,≥99.5%),sodium chloride (NaCl,≥99.5%),potassium nitrate(KNO3,≥99.5%)and lanthanum chloride(LaCl3,≥99.0%)were purchased from Shanghai Chemical Reagent and used without further purification.Calcium chloride(CaCl2,≥96%)was purchased from Sinopharm Chemical Reagent Company and used without further purification. NaAsO2(≥98.0%)is purchased from Sigma and used without further purification.All other reagents are of analytical-reagent grade,unless stated otherwise.
1.2 Preparation of molecule-ion adducts
The adducts of β-CD with the selected inorganic salts were prepared under a hydrothermal condition,by mixing β-CD (0.1135 g,0.1 mmol)with an inorganic salt(1∶1,molar ratio)in aqueous solution(50 mL)into an autoclave.Subsequently,the autoclave was heated at 393 K for 4 h.Then the solution was transferred to a temperature controlled water bath.After solvent was removed below 313 K under reduced pressure,the residue was dried thoroughly at 383 K in vacuo,and used without further purification.
1.3 Preparation of physical mixtures
Unground and ground physical mixtures between β-CD and inorganic salts were obtained by mixing them in 1∶1(molar ratio) with a grinding time of 0 and 20 min,respectively.
1.4 Measurements of samples
PXRD spectra of solid samples were performed on a Philips X′Pert Pro X-ray diffractometer.The solid samples were irradi-ated with monochromatized Cu Kαand analyzed with 5°≤2θ≤40°.The voltage and current were 40 kV and 40 mA,respectively.The sample mass was about 5.5 mg for each measurement. In order to diminish the effect of water molecules on PXRD patterns,all samples to be analyzed were kept under the same conditions,i.e.,383 K for 4 h before use.2θ angles(low angle range) of solid samples:β-CD,9.1°(moderate,m),10.8°(weak,w),12.6° (strong,s),15.6°(w),17.3°(w),19.3°(m)19.7°(w),and 22.7° (w);LiCl,30.0°(s),34.9°(s),and 50.1°(m);LiCl-β-CD,9.3° (w),10.9°(w),12.7°(s),15.8°(m),16.3°(m),17.1°(m),19.5° (m),21.3°(m),and 22.9°(m);NH4Cl,22.9°(m),32.6°(s),and 40.2°(w);NH4Cl-β-CD,9.2°(m),10.9°(w),12.8°(s),15.8°(m), 17.3°(m),19.7°(m),21.2°(m),22.8°(s),and 32.0°(m);KCl, 24.9°(w)and 35.4°(s);KCl-β-CD,9.2°(m),12.7°(s),14.8° (w),15.7°(m),17.3°(m),19.3°(m),22.8°(m),and 32.0°(m); NaCl,26.5°(w)and 32.8°(s);NaCl-β-CD,9.1°(w),10.8°(w), 12.7°(s),15.6°(m),17.9°(m),19.6°(m),21.1°(w),24.4°(m), 25.3°(w),28.8°(w),and 32°(w);LaCl3,13.7°(s),24.5°(s), 27.5°(m),31.4°(m),and 34.4°(s);LaCl3-β-CD,8.3°(w),9.1° (w),12.6°(s),12.9°(s),13.5°(s),17.0°(m),18.3°(m),19.6° (m),and 21.0°(m);CaCl2,19.8°(s),25.5°(m),29.1°(s),31.3° (m),and 38.5°(m);CaCl2-β-CD,9.0°(w),10.5°(m),12.5°(s), 15.6°(m),19.5°(m),22.9°(m),and 29.5°(w);KNO3,27.2°(s), 33.0°(m),and 39.6°(m);KNO3-β-CD,9.0°(w),10.5°(m), 12.5°(s),16.1°(m),19.2°(m),and 22.9°(m);NaAsO2,12.6° (w),18.2°(m),25.1°(m),28.4°(s)and 33.6°(m);NaAsO2-β-CD, 8.9°(w),9.7°(w),10.6°(m),12.4°(s),15.4°(m),17.8°(m), 19.5°(s),22.8°(m),28.0°(w),and 34.1°(w).The field emission scanning electron microscope(FESEM)images of solid samples wererecordedonaJEOL-JSM-6700Ffield-emittingmicroscope.
2 Results and discussion
2.1 Comparisons of PXRD spectra among free components,physical mixtures and adducts of β-CD with inorganic salts
First of all,we should examine what is the difference in spectral property among free components,their ground mixture,and prepared adduct in a system.As an example,four PXRD spectra of the adduct system between β-CD and NaCl,including free NaCl,free β-CD,the ground mixture of them(1∶1,molar ratio), and the prepared adduct NaCl-β-CD under the same drying condition,are presented in Fig.2.
In Fig.2A,the strongest characteristic peak of free NaCl occurs at 2θ of 32.9°(200).Although the peak is still observed clearly in both the mixture(Fig.2C)and the adduct(Fig.2D),its position shifts to a low 2θ angle upon mixing(2θ,32.2°)with β-CD especially after adduct(2θ,32.0°)with β-CD,and its intensity is significantly weakened from the mixture to the adduct.The left shift of this peak means that the interlayer distance(d(200))of complexed NaCl in the adduct is increased due to the molecule-ion interaction between NaCl and β-CD,indicating that the ion packing of the complexed NaCl becomes looser than that of free NaCl.

Fig.2 Linear PXRD patterns of free NaCl(A),free β-CD(B), ground mixture(C),and prepared adduct(D)Those signals shown in bold lines come from NaCl.
On the contrary,all major characteristic peaks in the range from 5°to 15°due to β-CD shift toward higher 2θ angles slightly from the ground mixture to the adduct.For example,the first strongest peak(P1)form 12.6°in the free or the mixed sample shifts to 12.7°in the adduct,which represents the(410)plane of crystal.This phenomenon implies that the molecular packing of complexed β-CD becomes closer than that of free β-CD.At the same time,several sharp signals belonging to β-CD,such as the second strongest peak(P2,19.3°)and the third strongest peak(P3, 9.1°),are weakened markedly after adduct with NaCl.
These observations described above reveal that molecule-ion interactions may also appear during the process of mixing especially grinding(Supporting Information),and they have different spectral performances in different molecule-ion systems,suggesting significant effects of inorganic ions.Additionally,the PXRD spectra of either mixtures or adducts are mainly dominated by β-CD,possibly because the mass percentage of inorganic salts is much lower than that of β-CD.
2.2 Effects of inorganic anions on the PXRD spectra of adducts
It is found that there are considerable differences in the PXRD spectra between oxysalt and chloride adducts of β-CD(Supporting Information).
First,several main peaks belonging to β-CD shift to a lower angle slightly from chloride adducts to oxysalt adducts.For example,the peak at 2θ of about 12.7°(P1)in the spectra of the two chloride adducts occurs in a lower angle of 12.4°for NaAsO2-β-CD and 12.5°for KNO3-β-CD.
The shift of the peaks in a low 2θ angle range toward a lower angle reflects that there is a relative lower interlayer force between β-CD molecules in an oxysalt adduct than in a chloride adduct.We hypothesize that this is associated with the size of anions because the presence of a big anion in interlayer between β-CD molecules will lead to the increase of interlayer distances.
Next,the peak at 19.3°(P2)of free β-CD shifts to 19.5°in the two oxysalt adducts(P2),and is strengthened in the presence of NaAsO2.However,the positions of P2in the two chloride adducts appear at 23.0°for NaCl and 22.8°for KCl.
These observations reveal the complexity of molecule-ion in-teractions between β-CD and inorganic salts.As a result,different anions have different influences on the arrangement of β-CD molecules.
The phenomenon that the spectral difference in PXRD patterns between the adducts of β-CD with the same anion but different cations is obviously smaller than that between the adducts of β-CD with the same cation but different anions seems to imply that inorganic anions play a more important role than cations in changing the stacking behavior of β-CD molecules.For instance,in the spectra of NaAsO2-β-CD[29]and NaCl-β-CD,several main characteristic peaks corresponding to β-CD shift towards different directions relative to free β-CD.This obvious spectral difference in 2θ angles between the two adducts indicates that the presence of NaAsO2and NaCl has increased and decreased the interlayer distances(d)in the direction of corresponding crystal faces,respectively.This fact that the size of Na+, AsO-2,and Cl-is insignificant relative to that of the cavity of β-CD provides important evidence that these inorganic ions exist outside the cavities of β-CD molecules.Because if the small ions are embedded within the large cavities,then there will not exist such a difference in d values between the molecule-ion adducts. In addition,there are some new peaks after adduct,such as those at 28.0°and 34.1°in NaAsO2-β-CD and those at 21.1°,25.3°, and 28.8°in NaCl-β-CD.The occurrence of the new peaks efficiently demonstrates the presence of molecule-ion interactions in the adducts.In addition to the effect of inorganic anions,we wish to further investigate how the properties of inorganic cations affect the spectral behavior of β-CD.
2.3 Effects of inorganic cations on the PXRD spectra of adducts
Meanwhile,we find that upon adduct the shifts of main characteristic peaks of free components vary with different cations. The PXRD spectra of the adducts of β-CD with several monochlorides(NH4Cl,KCl,NaCl,and LiCl)are depicted in Fig.3.
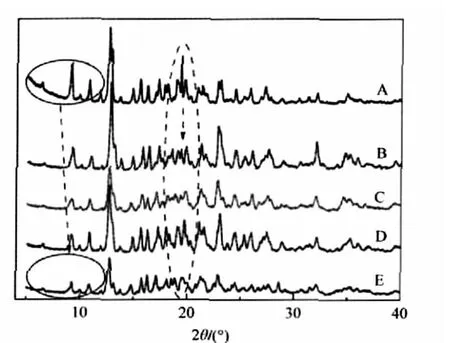
Fig.3 PXRD patterns of β-CD(A),NH4Cl-β-CD(B), KCl-β-CD(C),NaCl-β-CD(D),and LiCl-β-CD(E)
As shown in Fig.3,all those peaks belonging to β-CD on the left side of P1,shift to a higher 2θ angle in the case of four adducts,and the extent of the shift has an increasing order of NH4Cl-β-CD<KCl-β-CD<NaCl-β-CD<LiCl-β-CD.This result indicates that there are different interlayer forces between corresponding crystal faces of the polycrystalline bodies mainly resulted from β-CD molecules.Importantly,such an order can be explained by the fact that the order of the radii of these ions is Li+<Na+<K+<NH+4.In the case of the same anion,the presence of cations brings the arrangement of β-CD molecules closer.Li+ions,due to the highly positive charge density,produce a larger effect on the molecular arrangement of β-CD.It may be because some crystal lattice water molecules are replaced by the cations, which causes the occurrence of stronger electrostatic interaction between these ions and some of the end hydroxyl groups of β-CD molecules,leading to the possibility that the smaller the cation,the stronger the hydrogen bonding interaction between β-CD molecules.Interestingly,the observation is just contrary to the situation of those analyzed anions above.These results indicate that the existence of inorganic ions located at interstitial sites of β-CD molecules can modulate the stacking behavior of β-CD to a different extent dependent on the nature of inorganic salts.This should be an important reason why β-CD as well as many organic additives is being widely applied in controlling the size and shape of inorganic nanoparticles.
In order to estimate the possible influence of ion charges on electrostatic interaction,the PXRD data of KCl-β-CD and two polychlorides(CaCl2-β-CD and LaCl3-β-CD)are compared(Supporting Information).The strongest characteristic peak(P1,12.7°) in KCl-β-CD has shifted to a lower 2θ angle in CaCl2-β-CD(P1, 12.5°),but it shifts to a higher 2θ angle(P1,13.5°)in LaCl3-β-CD. This result is different from the situation in monochloride adducts described before because the order of the radii of the three metal ions is Ca2+<La3+<K+,implying that the shift orientation of the characteristic peak not only is affected by the radii of metal ions,but also depends on charges of metal ions in the case of the same anions.

Fig.4 SEM images of β-CD(A),NaCl-β-CD(B),KCl-β-CD (C),and NH4Cl-β-CD(D)
Interestingly,the SEM images display that the surface structure of β-CD was seriously affected by the existence of different cations(Supporting Information).As shown in Fig.4,β-CD shows the convexity of hexagonal prism with sizes from 2 to 10 μm. However,in the presence of NaCl,KCl,and NH4Cl,the surface morphology of β-CD changes to square,tile,and bar shapes,respectively.The order of particle sizes from small to big is NH4Cl-β-CD<KCl-β-CD<NaCl-β-CD<β-CD.The changes in SEM images prove that different molecule-ion interactions induce the difference of the crystallization behavior of β-CD, which supports the results obtained from PXRD.
3 Conclusions
In summary,although inorganic salts cannot form stable inclusion complexes with β-CD in aqueous solution,they may influence the arrangement behavior of β-CD molecules in the process of crystallization to a different extent,resulting in structural difference of molecule-ion adducts in solid state.Also,there is a close relationship between the performance of PXRD patterns and their impact factors such as formation conditions of adducts, natures of anions or cations,ionic charges and so on.The comparison from SEM images supports the results from PXRD.The present work offers a framework that may be especially useful for the study of those systems that involve the molecule-ion interaction between sugars and salts in biophysical chemistry, molecular biology,and preparation of inorganic nanoparticles.
Supporting Information available free of charge via the internet at http://www.whxb.pku.edu.cn.
1 Liu,Y.;You,C.C.;Zhang,H.Y.Supramolecular chemistry. Tianjin:Nankai University Press,2001 [刘 育,尤长城,张衡益.超分子化学.天津:南开大学出版社,2001]
2 Song,L.X.;Bai,L.;Xu,X.M.;He,J.;Pan,S.Z.Coord.Chem. Rev.,2009,253:1276
3 Tong,L.H.Chemistry of cyclodextrins.Beijing:Science Press, 2001:192 [童林荟.环糊精化学.北京:科学出版社,2001:192]
4 Cai,W.S.;Sun,T.T.;Liu,P.;Chipot,C.;Shao,X.G.J.Phys. Chem.B,2009,113:7836
5 Yu,Y.M.;Cai,W.S.;Chipot,C.;Sun,T.T.;Shao,X.G.J.Phys. Chem.B,2008,112:5268
6 Yu,Y.M.;Cai,W.S.;Shao,X.G.J.Incl.Phenom.Macro.,2006, 56:225
7 Kamigauchi,M.;Kawanishi,K.;Onishi,H.;Ishida,T.Chem. Pharm.Bull.,2007,55:729
8 Liu,Y.;Ke,C.F.;Zhang,H.Y.;Wu,W.J.;Shi,J.J.Org.Chem., 2007,72:280
9 Wang,E.J.;Lian,Z.X.;Cai,J.W.Carbohyd.Res.,2007,342: 767
10 Rodriquez-Llamazares,S.;Yutronic,N.;Jara,P.;Englert,U.; Noyong,M.;Simon,U.Eur.J.Org.Chem.,2007:4298
11 Marques,J.;Anjo,L.;Marques,M.P.M.;Santos,T.M.;Paz,F.A. A.;Braga,S.S.J.Organomet.Chem.,2008,693:3021
12 Nicolis,I.;Coleman,A.W.;Charpin,P.;deRango,C.Acta Crystallogr.Sect.B,1996,52:122
13 Kurokawa,G.;Sekii,M.;Ishida,T.;Nogami,T.Supramol.Chem., 2004,16:381
14 Spencer,J.N.;He,Q.;Ke,X.M.;Wu,Z.Q.;Fetter,E.J.Solution Chem.,1998,27:1009
15 Buvari,A.;Barcza,L.J.Incl.Phenom.Macro.,1989,7:379
16 Matsui,Y.;Ono,M.;Tokunaga,S.Bull.Chem.Soc.Jpn.,1997, 70:535
17 Masar,M.;Bodor,R.;Kaniansky,D.J.Chromatogr.A,1999,834: 179
18 Nimz,O.;Gessler,K.;Uson,I.;Laettig,S.;Welfle,H.;Sheldrick, G.M.;Saenger,W.Carbohydr.Res.,2003,338:977
19 Charalampopoulos,V.G.;Papaioannou,J.C.;Karayianni,H.S. Solid State Sci.,2006,8:97
20 Papaioannou,J.C.;Charalampopoulos,V.G.;Xynogalas,P.; Viras,K.J.Phys.Chem.Solids,2006,67:1379
21 Charalampopoulos,V.G.;Papaioannou,J.C.Carbohydr.Res., 2007,342:2075
22 Sonawane,S.H.;Shirsath,S.R.;Khanna,P.K.;Pawar,S.; Mahajan,C.M.;Paithankar,V.;Shinde,V.;Kapadnis,C.V.Chem. Eng.J.,2008,143:308
23 Zhang,X.Y.;Liao,Z.J.;Yang,L.;Hu,Z.G.;Jiang,K.;Guo,Y. M.Acta Chim.Sin.,2003,61:69 [张秀英,廖照江,杨 林,胡志国,蒋 凯,郭玉明.化学学报,2003,61:69]
24 Yamashoji,Y.;Fujiwara,M.;Matsushita,T.;Tanaka,M.Chem. Lett.,1993:1029
25 Wolf,R.;D′avino,M.;De Angelis,F.;Ruocco,E.;Lombardi,M. L.J.Eur.Acad.Dermatol.Vener.,2000,14:97
26 Manna,P.;Sinha,M.;Sil,P.C.Redox.Rep.,2008,13:67
27 Song,L.X.;Dang,Z.J.Phys.Chem.B,2009,113:4998
28 Song,L.X.;Bai,L.J.Phys.Chem.B,2009,113:9035
29 Dang,Z.;Song,L.X.;Pan,S.Z.;Wang,M.Acta Phys.-Chim.Sin., 2009,25:1059 [党 政,宋乐新,潘淑臻,王 莽.物理化学学报,2009,25:1059]

