PICC与TIVAP在脑胶质瘤患者中的应用比较
赵爽爽 戴慧

[摘要] 目的 比较经外周静脉穿刺的中心静脉导管(peripherally inserted central venous catheter,PICC)与完全植入式静脉输液港(totally implantable venous access port,TIVAP)在脑胶质瘤患者中的应用差异。方法 回顾性分析2016年1月至2022年10月在杭州市肿瘤医院行PICC或TIVAP置管的130例脑胶质瘤患者的临床资料,根据置管方式将其分为PICC组(55例)和TIVAP组(75例),比较两组患者置管前后1周血浆D-二聚体(D-dimer,D-D)水平变化、导管相关总并发症、一次性置管成功率、因导管并发症拔管率和导管留置时间。结果 置管后1周,PICC组患者的D-D水平显著高于本组置管前1周和TIVAP组(P<0.05);TIVAP组患者置管前后的D-D水平比较差异无统计学意义(P>0.05)。TIVAP组患者的导管相关并发症发生率、因导管并发症拔管率均显著低于PICC组,一次性置管成功率显著高于PICC组,导管留置时间显著长于PICC组(P<0.05)。结论 脑胶质瘤患者PICC置管后1周D-D水平升高,在该时段应加强深静脉血栓防治管理。与PICC相比,TIVAP对D-D水平影响较小,临床应用效果更好,值得广泛推广。
[关键词] 经外周静脉穿刺的中心静脉导管;完全植入式静脉输液港;胶质瘤
[中图分类号] R739.4;R473.73 [文献标识码] A [DOI] 10.3969/j.issn.1673-9701.2024.16.014
Comparison of PICC and TIVAP in patients with brain glioma
ZHAO Shuangshuang, DAI Hui
Department of Radiation Oncology, Hangzhou Cancer Hospital, Hangzhou 310002, Zhejiang, China
[Abstract] Objective To compare the application difference between peripherally inserted central venous catheter (PICC) and totally implantable venous access port (TIVAP) in brain glioma patients. Methods The clinical data of 130 patients with brain glioma who received PICC or TIVAP catheterization in Hangzhou Cancer Hospital from January 2016 to October 2022 were retrospectively analyzed, and they were divided into PICC group (55 cases) and TIVAP group (75 cases) according to the catheterization method. The changes of plasma D-dimer (D-D) level one week before and after catheterization, total catheter-related complications, the success rate of one-time catheterization, extubation rate due to catheter complications and catheter indwelling time were compared between two groups. Results One week after catheterization, D-D level in PICC group was significantly higher than that before catheterization and TIVAP group (P<0.05). There was no significant difference in D-D level in TIVAP group before and after catheterization (P>0.05). The rate of catheter-related complications and extubation rate due to catheter complications in TIVAP group were significantly lower than those in PICC group, the success rate of one-time catheterization was significantly higher than that in PICC group, and the catheter indwelling time was significantly longer than that in PICC group (P<0.05). Conclusion D-D level in brain glioma patients increased one week after PICC catheterization, and the prevention and treatment of deep vein thrombosis should be strengthened during this period. Compared with PICC, TIVAP has less effect on D-D level and better clinical application effect, which is worthy of widespread promotion.
[Key words] Peripherally inserted central venous catheter; Totally implantable venous access port; Glioma
脑胶质瘤是指起源于脑神经胶质细胞的肿瘤,是最常见的原发性颅内肿瘤,约占所有恶性脑肿瘤的80%[1]。中国脑胶质瘤年发病率为(5~8)/10万,5年病死率在全身肿瘤中仅次于胰腺癌和肺癌。脑胶质瘤的治疗包括手术、放疗、化疗、靶向治疗、电场治疗及对症支持治疗[2]。在治疗过程中往往需要长期反复输液,经外周静脉穿刺的中心静脉导管(peripherally inserted central venous catheter,PICC)和完全植入式静脉输液港(totally implantable venous access port,TIVAP)具有使用时间长、无需反复穿刺等特点,为患者提供一条无痛性治疗途径,是最常用的中心静脉置管方式。临床实践发现PICC与TIVAP的应用效果存在一定差异,多项研究表明在乳腺癌、泛癌种实体恶性肿瘤中,与PICC相比,TIVAP的并发症发生率更低,医疗花费更少[3-6]。
恶性肿瘤患者本身存在高凝状态,中心静脉导管患者静脉血栓栓塞(venous thromboembolism,VTE)的发病风险是无中心静脉导管者的8.5倍[7]。但目前各国指南均不推荐常规预防性抗凝治疗[8]。D-二聚体(D-dimer,D-D)是交联纤维蛋白特异性降解产物,D-D水平的升高反映凝血和纤溶系统增强,可作为体内高凝状态较敏感的指标[9]。研究表明D-D高水平人群发生VTE的风险是D-D低水平者的1.7~3.4倍[10-11]。本研究对PICC或TIVAP深静脉置管脑胶质瘤患者的临床资料进行回顾性分析,旨在比较两组患者置管前后血浆D-D水平变化及临床应用效果。
1 资料与方法
1.1 一般资料
回顾性分析2016年1月至2022年10月在杭州市肿瘤医院行PICC或TIVAP置管的130例脑胶质瘤患者的临床资料。根据置管方式将其分为PICC组(55例)和TIVAP组(75例)。本研究经杭州市肿瘤医院伦理委员会审查通过[伦理审批号:(HZCH-2023)快审第(007)号]。因大多数患者已去世,豁免签署受试者知情同意书。纳入标准:①年龄18~80岁:②病理诊断为脑胶质瘤;③符合PICC或TIVAP置管条件。排除标准:①病历资料不完整;②导管留置时间≤7d;③置管前后4周应用影响凝血功能及血小板功能的药物;④置管前4周有血栓、感染、创伤、心肌梗死、严重肝肾功能不全的患者。
1.2 置管方法和维护
PICC组:由具有PICC置管合格证的护士对患者静脉进行评估并操作。将贵要静脉作为首选穿刺静脉,其次为肱静脉或头静脉,超声引导下成功穿刺后经穿刺点将管鞘沿导丝送入,导管尖端放置在上腔静脉中下部,100U/ml肝素生理盐水封管;TIVAP组:由外科医生对患者静脉进行评估并操作。实施局部麻醉,选择右侧颈内静脉穿刺,超声引导下穿刺针自体表穿刺点进入颈内静脉,在导丝引导下将导管置入血管,导管尖端放置在上腔静脉和右心房交界处,100U/ml肝素生理盐水封管。建立皮下隧道和囊袋,放置并固定注射座于囊袋中,囊袋均位于同侧锁骨下胸壁,以可吸收线缝合皮肤。两组患者术后均立即行胸部X线检查导管形态及尖端位置。导管日常维护均由经培训合格的护士进行。
1.3 观察指标
比较两组患者置管前后1周的D-D水平、导管相关总并发症[包括导管相关性静脉血栓(catheter related thrombosis,CRT)、导管相关性血流感染(catheter-related bloodstream infection,CRBSI)、导管堵塞、导管移位、过敏性皮炎]发生率、一次性置管成功率、因导管并发症拔管率和导管留置时间。
1.4 统计学方法
采用SPSS 20.0统计软件对数据进行分析。D-D水平不符合正态分布,以中位数(四分位数间距)[M(Q1,Q3)]表示,组间比较采用两独立样本Mann-Whiteny U检验或两相关样本Wilcoxon符号秩检验。导管留置时间符合正态分布,以均数±标准差(![]() )表示,组间比较采用两独立样本t检验。计数资料以例数(百分率)[n(%)]表示,组间比较采用χ2检验。P<0.05为差异有统计学意义。
)表示,组间比较采用两独立样本t检验。计数资料以例数(百分率)[n(%)]表示,组间比较采用χ2检验。P<0.05为差异有统计学意义。
2 结果
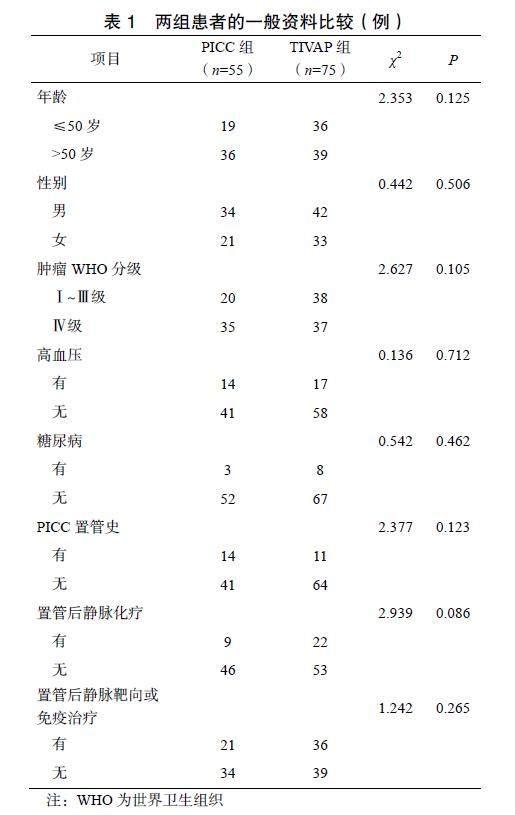
2.1 两组患者的一般资料比较
两组患者的一般资料比较差异均无统计学意义(P>0.05),见表1。TIVAP置管均为首次。
2.2 两组患者置管前后的D-D水平比较
置管后1周,PICC组患者的D-D水平显著高于置管前1周(P<0.05),TIVAP组患者置管前后的D-D水平比较差异无统计学意义(P>0.05),置管后PICC组患者的D-D水平显著高于TIVAP组(P<0.05),见表2。
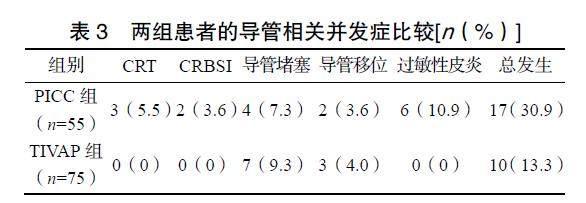
2.3 两组患者的导管相关并发症比较
中位随访5个月,TIVAP组患者的导管相关并发症发生率显著低于PICC组(χ2=5.957,P=0.015),见表3。其中,PICC组发生CRT 3例,1例腋静脉段血栓形成,伴患侧手臂肿胀及疼痛,为症状性静脉血栓,另外2例为附壁血栓,为无症状性静脉血栓,分别发生于置管后第57、41、35天;PICC组发生CRBSI 2例,患者均有畏寒、发热,导管血培养示革兰阳性球菌,分别发生于置管后第21、56天,拔除PICC导管及抗炎治疗后感染得以控制。
2.4 两组患者的导管相关指标比较
TIVAP组患者的一次性置管成功率显著高于PICC组(P=0.012),因导管并发症拔管率显著低于PICC组(P=0.028),导管留置时间显著长于PICC组(P<0.001),见表4。
3 讨论
脑胶质瘤患者在治疗过程中需要化疗、脱水降颅内压、肠外营养、输血、抗生素等治疗。PICC或TIVAP可避免反复静脉穿刺,减轻药物对周围静脉的损伤,从而提高患者的生活质量、延长生存时间[12-13]。当患者需要行深静脉置管时,选择何种深静脉置管方式是医生和患者均需要考虑的问题。本研究结果显示,在脑胶质瘤患者中TIVAP较PICC更具优势,TIVAP对D-D水平影响更小、一次性置管成功率更高、导管相关总并发症发生率及因导管并发症拔管率更低、导管留置时间更长。
高凝状态、血流淤滞和内皮损伤是静脉血栓形成的三大要素[4,14]。PICC或TIVAP不仅减少重复静脉穿刺,还可减轻药物对血管壁的刺激而保护外周静脉。但PICC或TIVAP置管过程及保留导管期间仍可对血管内皮造成机械性损伤,影响血管腔内血流状态,引起凝血和纤溶系统改变促进VTE的发生,VTE是PICC或TIVAP最常见的并发症[6,14-15]。D-D是反映凝血和纤溶系统的理想指标。研究显示置管前D-D水平>5mg/L是PICC相关性VTE形成的高危因素(OR=36.6,P<0.001),而纤维蛋白原及纤维蛋白原降解产物无影响[16]。本研究发现PICC置管后1周D-D水平较置管前1周升高,而TIVAP置管后1周对D-D水平无影响。分析其原因:①TIVAP穿刺点选取的是颈内静脉(直径1~2cm),PICC选取的是外周血管如贵要静脉、肱静脉或头静脉(直径0.4~0.5cm),外周静脉较颈内静脉血管管腔细,血流速度相对更慢[5-6];②TIVAP导管置入血管内的长度10~15cm,PICC导管置入血管内的长度35~40cm,PICC导管置入血管内的长度更长,导管与血管壁的接触面积更大[6,13];③TIVAP注射座固定在胸壁皮下、活动性小,而PICC固定在手臂上,受手臂活动的影响PICC活动度大[13]。PICC较TIVAP更容易造成血流淤滞及血管内皮损伤,因此,对D-D水平的影响更大。
置管后1周是血管对中心静脉导管的急性应激期,VTE高发,此阶段D-D应激性升高,随着时间的延长血管对中心静脉导管逐渐适应,VTE发生率明显下降,D-D水平逐渐降低。Luo等[17]对PICC置管后的恶性肿瘤患者每隔1周行血管超声检查,发现CRT形成中位时间是置管后3d,85.48%的患者CRT发生在置管后1周内,11.29%发生在2~3周内,3.23%发生在4~6周内。D-D高水平人群发生VTE的风险是D-D低水平者的1.7~3.4倍[10-11]。虽然目前各国指南均不推荐对行深静脉置管的肿瘤患者进行常规预防性抗凝治疗,但PICC置管后1周D-D急剧升高,对此类患者应加强凝血功能监测、VTE筛查,必要时预防性抗凝治疗。
本研究中TIVAP较PICC的一次性置管成功率更高、导管留置时间更长。有研究显示TIVAP与PICC的一次性置管成功率无显著差异[4,18],可能是本研究样本量较少所致。多项研究表明,肿瘤患者TIVAP导管平均留置时间220~393d,较PICC导管平均留置时间(94~122d)长[4-5,19],原因可能是TIVAP组患者因并发症导致的导管拔除率较低,其次是治疗间歇期TIVAP更多的被保留。
本研究显示TIVAP组导管相关总并发症发生率及因导管并发症拔管率低于PICC组。多项研究均表明TIVAP的总并发症发生率(4.5%~32.0%)较PICC(11.4%~47.0%)更低[3-6,18-21]。PICC或TIVAP相关的并发症包括CRT、CRBSI、导管堵塞、导管移位、静脉炎、过敏性皮炎、疼痛和导管意外拔除等[4-6,18],其中CRT关注度最高[3-4,6]。本研究中PICC组患者CRT的发生率高于TIVAP组,这与其他研究结果一致[4-6,19-21]。两项Meta分析结果显示TIVAP可降低60%~80%的CRT发生风险[12-13]。
CRBSI是PICC或TIVAP最严重的并发症,未控制的血流感染可导致患者死亡。本研究中PICC组CRBSI发生率为3.6%,而TIVAP组未发生。Chopra等[22]报道肿瘤患者PICC的CRBSI死亡风险高达31%~36%。随着TIVAP及PICC置管技术的逐步成熟、导管维护更加规范、抗菌药物导管的应用,CRBSI的整体发生率逐渐下降[4,23]。导管堵塞、导管移位、过敏性皮炎均属于导管留置期间常见的轻微并发症。本研究中这些轻微并发症的发生率波动在0~10.9%,这与其他研究中报道的结果类似[6,19,21]。
综上所述,脑胶质瘤患者PICC置管后1周D-D水平升高,在该时段应加强VTE防治管理。整体上,TIVAP对D-D水平的影响更小,临床应用效果更好,值得更广泛推广。
利益冲突:所有作者均声明不存在利益冲突。
[参考文献]
[1] BARNHOLTZ-SLOAN J S, OSTROM Q T, COTE D. Epidemiology of brain tumors[J]. Neurol Clin, 2018, 36(3): 395–419.
[2] JIANG T, NAM D H, RAM Z, et al. Clinical practice guidelines for the management of adult diffuse gliomas[J]. Cancer Lett, 2021, 499: 60–72.
[3] CLATOT F, FONTANILLES M, LEFEBVRE L, et al. Randomised phase Ⅱ trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer[J]. Eur J Cancer, 2020, 126: 116–124.
[4] TAXBRO K, HAMMARSKJOLD F, THELIN B, et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: An open-label, randomised, two-centre trial[J]. Br J Anaesth, 2019, 122 (6): 734–741.
[5] MOSS J G, WU O, BODENHAM A R, et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): A randomised controlled trial[J]. Lancet, 2021, 398(10298): 403–415.
[6] PATEL G S, JAIN K, KUMAR R, et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies[J]. Support Care Cancer, 2014, 22(1): 121–128.
[7] ASHRANI A A, GULLERUD R E, PETTERSON T M, et al. Risk factors for incident venous thromboembolism in active cancer patients: A population based case-control study[J]. Thromb Res, 2016, 139: 29–37.
[8] STREIFF M B, ABUTALIB S A, FARGE D, et al. Update on guidelines for the management of cancer- associated thrombosis[J]. Oncologist, 2021, 26(1): e24–e40.
[9] OLSON J D. D-dimer: An overview of hemostasis and fibrinolysis, assays, and clinical applications[J]. Adv Clin Chem, 2015, 69: 1–46.
[10] VAN HYLCKAMA VLIEG A, BAGLIN C A, LUDDINGTON R, et al. The risk of a first and a recurrent venous thrombosis associated with an elevated D-dimer level and an elevated thrombin potential: Results of the THE-VTE study[J]. J Thromb Haemost, 2015, 13(9): 1642–1652.
[11] HANSEN E S, RINDE F B, EDVARDSEN M S, et al. Elevated plasma D-dimer levels are associated with risk of future incident venous thromboembolism[J]. Thromb Res, 2021, 208: 121–126.
[12] JIANG M, LI C L, PAN C Q, et al. Risk of venous thromboembolism associated with totally implantable venous access ports in cancer patients: A systematic review and Meta-analysis[J]. J Thromb Haemost, 2020, 18(9): 2253–2273.
[13] WANG P, SOH K L, YING Y, et al. Risk of VTE associated with PORTs and PICCs in cancer patients: A systematic review and Meta-analysis[J]. Thromb Res, 2022, 213: 34–42.

