乳腺癌细胞条件培养基对骨髓间充质干细胞生物学行为的影响
刘丹阳 李永涛 张海燕 李林 刘洋 沈雷
基金項目:黑龙江省教育厅科学技术研究项目(2020-KYYWF-0010)
作者单位:1齐齐哈尔医学院组织学与胚胎学教研室(邮编161006),2基础医学科研中心;3齐齐哈尔市第一医院普外科
作者简介:刘丹阳(1982),女,讲师,主要从事肿瘤微环境与肿瘤生物学方面研究。E-mail:519110362@qq.com
摘要:目的 探讨MCF-7乳腺癌细胞条件培养基对骨髓间充质干细胞(BMSC)增殖、凋亡和迁移的影响及分子机制。方法 正常环境下培养的BMSC为对照组,以MCF-7细胞条件培养基培养的BMSC为MCF-7条件培养基组,向MCF-7条件培养基组添加10 nmol/L GSK690693(Akt抑制剂)为Akt抑制剂组,向MCF-7条件培养基组添加10 μmol/L Reparixin(CXCR1/2抑制剂)为CXCR1/2抑制剂组。MTT实验检测各组BMSC增殖情况,Annexin V-FITC/PI双标记流式细胞凋亡实验检测各组BMSC凋亡率,Transwell细胞迁移实验检测各组BMSC的迁移能力,酶联免疫吸附试验检测两种细胞培养上清液和MCF-7细胞条件培养基中白细胞介素(IL)-8蛋白含量,Western blot检测各组BMSC的蛋白激酶B(Akt)/磷酸化Akt(p-Akt)和哺乳动物雷帕霉素靶蛋白(mTOR)/磷酸化mTOR(p-mTOR)蛋白表达。结果 与对照组相比,MCF-7条件培养基组BMSC的细胞增殖水平、迁移数目以及p-Akt和p-mTOR蛋白相对表达量均增高,细胞凋亡率降低(P<0.05);与MCF-7条件培养基组相比,CXCR1/2抑制剂组和Akt抑制剂组BMSC的细胞增殖水平、迁移数目以及p-Akt和p-mTOR蛋白相对表达量均降低,细胞凋亡率增加(P<0.05);MCF-7细胞条件培养基和MCF-7培养上清液中IL-8蛋白含量均较BMSC培养上清液中IL-8蛋白含量高(P<0.05)。结论 MCF-7细胞条件培养基通过激活Akt-mTOR信号通路促进BMSC增殖和迁移,抑制BMSC凋亡,其中IL-8-CXCR1/2轴发挥关键作用。
关键词:乳腺肿瘤;肿瘤微环境;细胞增殖;细胞凋亡;细胞运动;骨髓间充质干细胞
中图分类号:R737.9 文献标志码:A DOI:10.11958/20231487
Effect of breast cancer cell conditioned medium on biological behavior of bone marrow mesenchymal stem cells
LIU Danyang1, LI Yongtao2, ZHANG Haiyan1, LI Lin1, LIU Yang3, SHEN Lei2
1 Department of Histology and Embryology, 2 Medical Research Center, Qiqihar Medical College, Qiqihar 161006, China;
3 Department of General Surgery, Qiqihar City First Hospital
Abstract: Objective To explore effects of MCF-7 breast cancer cell conditioned medium on proliferation, apoptosis and migration of bone marrow mesenchymal stem cells (BMSC) and its molecular mechanism. Methods BMSC cultured under normal environment was used as the control group. BMSC cultured with MCF-7 cell conditioned medium was selected as the MCF-7 conditioned medium group. The MCF-7 conditioned medium group supplemented with 10 nmol/L GSK690693 (Akt inhibitor) was used as the Akt inhibitor group. The MCF-7 conditioned medium group added with 10 μmol/L Reparixin (CXCR1/2 inhibitor) was used as the CXCR1/2 inhibitor group. BMSC proliferation was detected by MTT assay. Annexin V-FITC/PI double labeled flow cytometry was used to detect BMSC apoptosis rate in each group. Transwell cell migration assay was used to detect the migration ability of BMSC in each group. The contents of interleukin (IL)-8 protein in two kinds of cell culture supernatant and MCF-7 cell conditioned medium were detected by enzyme-linked immunosorbent assay. The protein expression levels of protein kinase B (Akt)/phosphorylated Akt (p-Akt) and mammalian target of rapamycin (mTOR)/phosphorylated mTOR (p-mTOR) in BMSC of each group were detected by Western blot assay. Results Compared with the control group, cell proliferation level, migration number and the relative expression levels of p-Akt, p-mTOR protein of BMSC were increased in the MCF-7 conditioned medium group, and the apoptosis rate was decreased (P<0.05). Compared with the MCF-7 conditioned medium group, cell proliferation level, migration number and the relative expression levels of p-Akt, p-mTOR protein of BMSC were decreased in the CXCR1/2 inhibitor group and the Akt inhibitor group, and the apoptosis rate was increased (P<0.05). The contents of IL-8 protein in MCF-7 cell conditioned medium and MCF-7 culture supernatant were higher than that in the BMSC culture supernatant (P<0.05). Conclusion MCF-7 cell conditioned medium promotes BMSC proliferation and migration and inhibits BMSC apoptosis by activating Akt-mTOR signaling pathway, in which IL-8-CXCR1/2 axis plays a key role.
Key words: breast neoplasms; tumor microenvironment; cell proliferation; apoptosis; cell movement; bone marrow mesenchymal stem cell
乳腺癌是困扰女性健康的恶性肿瘤。虽然临床上治疗管腔(luminal)A型乳腺癌的疗效较好[1],但luminal A型乳腺癌发生、侵袭、扩散的病理机制尚不清楚。研究发现,间充质干细胞(mesenchymal stem cell,MSC)分泌的外泌体能促进MCF-7乳腺癌细胞生长[2]。肿瘤微环境含有的MSC及其分化的肿瘤相关成纤维细胞(cancer associated fibroblast,CAF)通过旁分泌机制调节癌症干细胞(CSC)生长[3]。MSC具有促进肿瘤发生和转移的能力[4]。研究还发现,肿瘤相关巨噬细胞表达白细胞介素(interleukin,IL)-8[即趋化因子(C-X-C motif chemokine ligand,CXCL)-8]增强了乳腺癌和卵巢癌的转移能力[5-6]。口腔鳞状细胞癌表达的IL-8通过趋化因子受体(C-X-C motif chemokine receptors,CXCR)2-转化生长因子-β(transforming growth factor beta,TGF-β)通路募集骨髓间充质干细胞(bone marrow mesenchymal stem cell, BMSC)迁移到肿瘤微环境,促进了癌细胞的生长和转移能力[7]。由此可见,IL-8和MSC在构成肿瘤微环境、调控肿瘤生长或转移中均发挥关键作用。本实验以MCF-7乳腺癌细胞条件培养基刺激BMSC,观察CXCR1/2-蛋白激酶B(Akt)-哺乳动物雷帕霉素靶蛋白(mTOR)信号轴对BMSC增殖、凋亡及迁移的影响及其分子机制,为抑制luminal A型乳腺癌发生发展提供参考。
1 材料与方法
1.1 主要材料 BMSC和MCF-7细胞购自武汉普诺赛生命科技有限公司。RPMI-1640和DMEM高糖培养基购自美国Hyclone公司。胎牛血清购自浙江天杭生物科技有限公司。兔抗人Akt和磷酸化Akt(p-Akt)抗体、兔抗人mTOR和磷酸化mTOR(p-mTOR)抗体、兔抗人β-肌动蛋白(β-actin)抗体、辣根过氧化物酶(HRP)标记的山羊抗兔IgG二抗均购自英国Abcam公司。Reparixin(CXCR1/2抑制剂)购自美国Med Chem Express公司,GSK690693(Akt抑制剂)购自美国Selleck公司。IL-8酶联免疫吸附试验(ELISA)试剂盒购自武汉博士德生物工程有限公司。Annexin V-FITC/PI双标记流式细胞凋亡试剂盒、MTT、BCA蛋白检测试剂盒和聚偏二氟乙烯膜(PVDF)均购自上海碧云天生物技术有限公司。IX53倒置荧光显微镜购自日本Olympus公司,FACSAriaⅡ流式细胞仪购自美国BD公司,Tanon 6200型发光成像仪购自上海天能公司,SpectraMax iD3酶标仪购自美国Molecular Devices公司。
1.2 实验方法
1.2.1 细胞培养 BMSC用含10%胎牛血清的RPMI-1640培养基培养。MCF-7细胞用含10%胎牛血清的DMEM高糖培养基培养。两种细胞均采用3代对数生长期状态良好的细胞进行实验。
1.2.2 MCF-7细胞条件培养基制备 将MCF-7细胞在5%CO2、37 ℃培养12 h后,吸出培养上清液,1 000 r/min离心3 min后,弃掉沉淀提取上清液。按照体积比4∶1将无胎牛血清的RPMI-1640培养基和MCF-7细胞培养上清液混匀,则为MCF-7细胞条件培养基。
1.2.3 BMSC分组 正常环境下培养的BMSC为对照组。以MCF-7细胞条件培养基培养的BMSC为MCF-7條件培养基组。向MCF-7条件培养基组添加10 nmol/L GSK690693为Akt抑制剂组,向MCF-7条件培养基组添加10 μmol/L Reparixin为CXCR1/2抑制剂组。
1.2.4 MTT实验检测各组BMSC增殖情况 将BMSC以4×103个/孔接种至96孔细胞培养板,孵育48 h后每孔加入20 μL MTT溶液,孵育4 h后弃掉上清液,每孔加150 μL二甲基亚砜,摇动样品,于酶标仪490 nm波长处检测各组BMSC光密度(OD)值。OD值代表细胞增殖水平,实验重复3次。
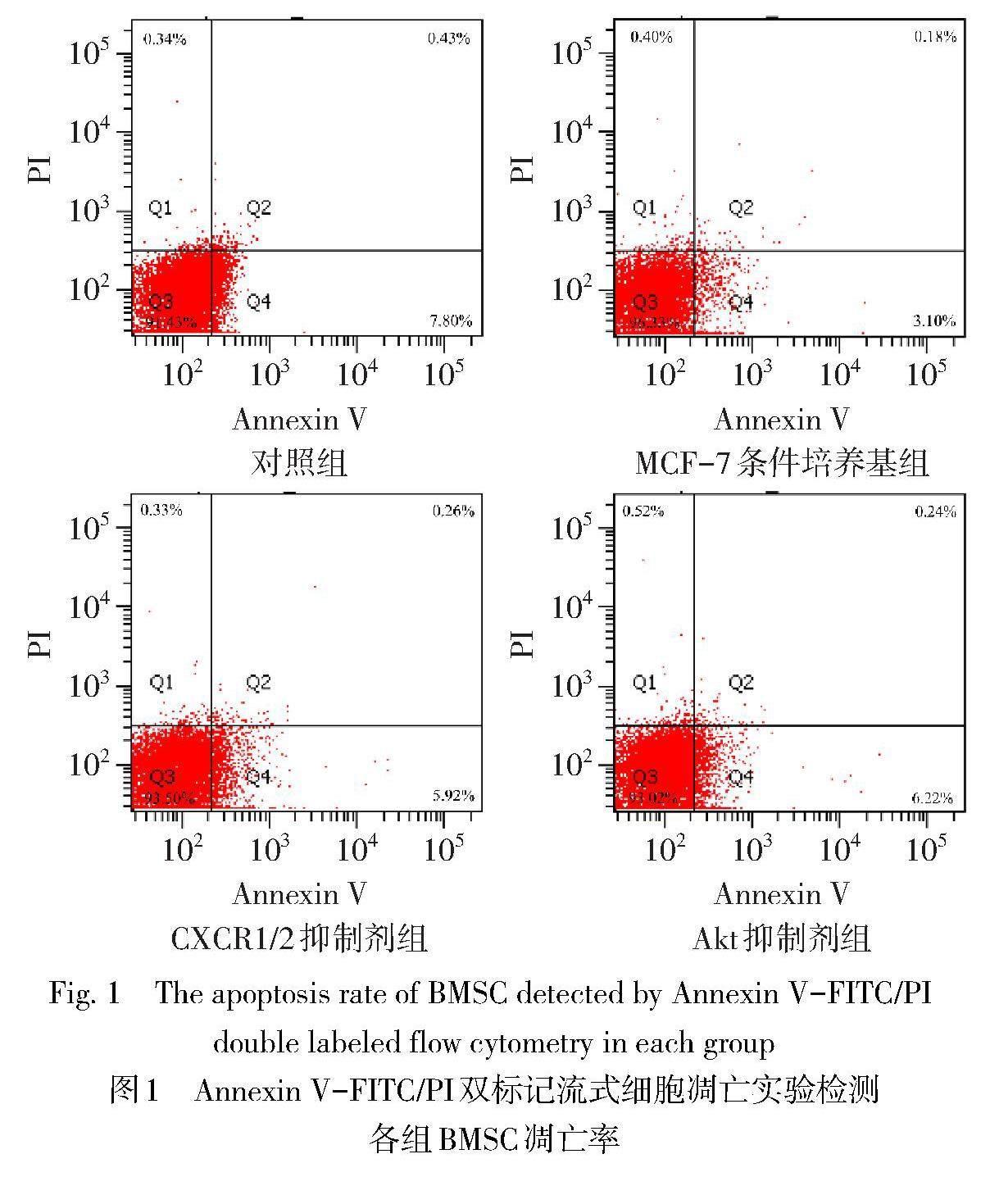
1.2.5 Annexin V-FITC/PI双标记细胞凋亡实验检测各组BMSC凋亡率 各组取1×105个细胞,1 000 r/min离心3 min,弃上清液后,用195 μL Annexin V-FITC结合液重悬各组BMSC,加入5 μL Annexin V-FITC和10 μL碘化丙啶(PI),避光室温孵育25 min,流式细胞仪检测各组BMSC凋亡率(早期凋亡率Q4+晚期凋亡率Q2),实验重复3次。
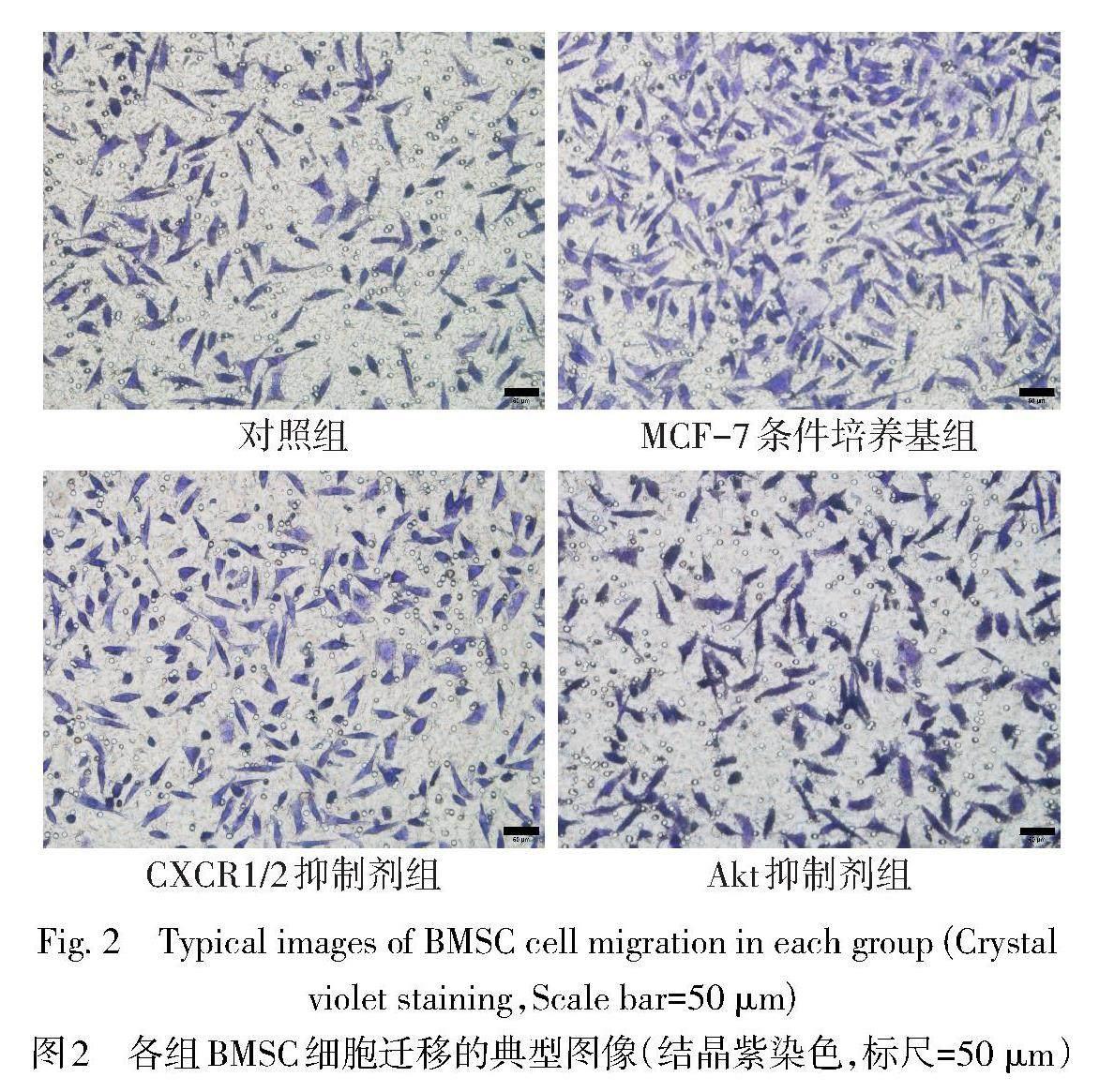
1.2.6 Transwell细胞迁移实验检测各组BMSC的迁移能力 将200 μL BMSC悬液以1×105个/室接种至Transwell细胞小室。根据BMSC实验分组,Transwell细胞小室下方腔室加入相应试剂或培养基。孵育14 h后,4%多聚甲醛溶液室温固定1 h,1.0%结晶紫染色40 min[8]。置于200倍倒置显微镜下拍照,Image J 1.8.0图像软件计算各组BMSC迁移数目,实验重复3次。
1.2.7 ELISA检测两种细胞培养上清液和MCF-7细胞条件培养基中IL-8蛋白含量 正常培养BMSC和MCF-7细胞12 h后,分别提取两种细胞的培养上清液,并制备MCF-7细胞条件培养基,方法同1.2.2。按照IL-8 ELISA试剂盒说明书进行操作,酶标仪450 nm波长处检测3种液体的OD值,计算IL-8蛋白含量,实验重复3次。
1.2.8 Western blot检测各组BMSC的Akt/p-Akt和mTOR/p-mTOR蛋白表达 将各組BMSC接种到25 cm2透气斜口培养瓶中(2×106个/瓶),37 ℃、5%CO2孵育48 h后,RIPA裂解液裂解各组BMSC,BCA蛋白浓度试剂盒检测各组BMSC蛋白浓度。80 V电泳30 min;120 V电泳45 min。75 V 180 min转膜到PVDF膜。5%脱脂奶粉封闭PVDF膜90 min。分别添加兔抗人p-Akt抗体(1∶600)、Akt抗体(1∶550)、p-mTOR抗体(1∶500)、mTOR抗体(1∶450)和β-actin抗体(1∶1 000),4 ℃孵育过夜。HRP标记山羊抗兔IgG(1∶800)室温孵育4 h。发光成像仪显影曝光,Image J 1.8.0图像分析软件计算各蛋白条带灰度值,实验重复3次。
1.3 统计学方法 采用GraphPad Prism 8.0.2软件进行统计学分析。符合正态分布的计量资料以均数±标准差[([x] ±s)
]表示,多组间均数比较采用单因素方差分析,组间多重比较行Tukey-q检验。P<0.05为差异有统计学意义。
2 结果
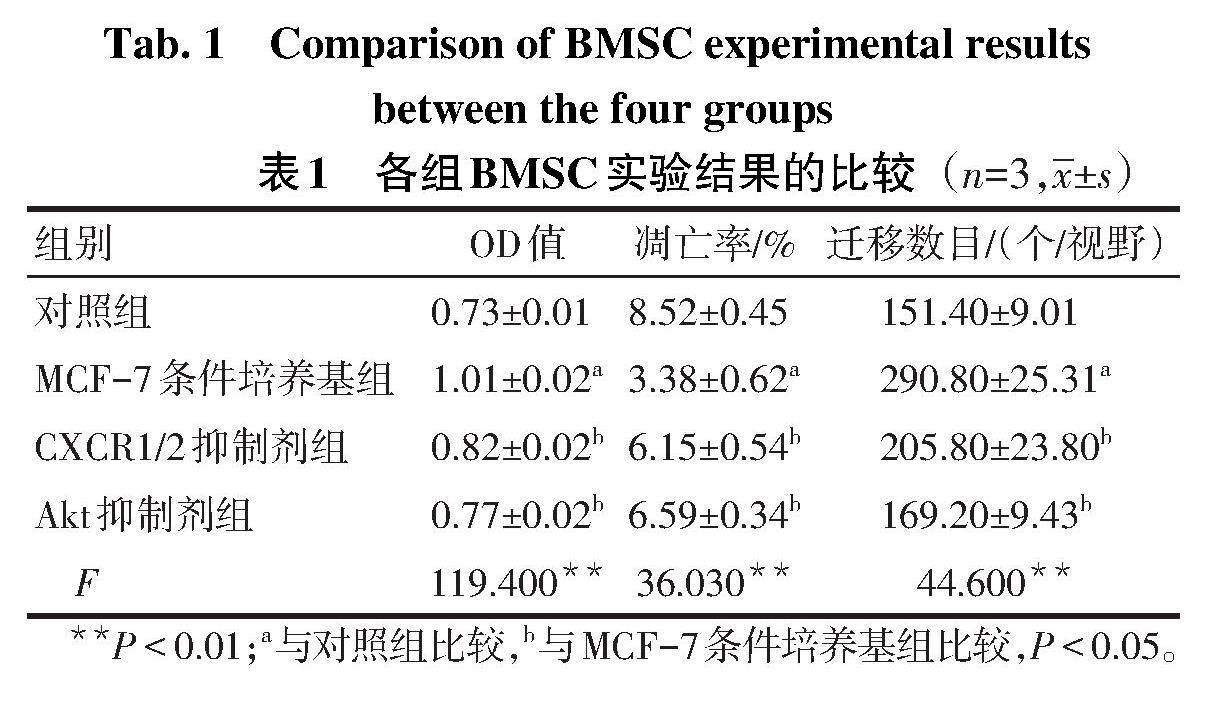
2.1 各组BMSC增殖情况比较 与对照组比较,MCF-7条件培养基组BMSC的增殖水平升高(P<0.05);与MCF-7条件培养基组比较,CXCR1/2抑制剂组和Akt抑制剂组BMSC的增殖水平均降低(P<0.05),见表1。
2.2 各组BMSC凋亡率比较 与对照组比较,MCF-7条件培养基组BMSC凋亡率降低(P<0.05);与MCF-7条件培养基组比较,CXCR1/2抑制剂组和Akt抑制剂组BMSC凋亡率均增高(P<0.05),见表1、图1。
2.3 各组BMSC迁移能力比较 与对照组比较,MCF-7条件培养基组BMSC迁移数目增加(P<0.05);与MCF-7条件培养基组比较,CXCR1/2抑制剂组和Akt抑制剂组BMSC迁移数目均减少(P<0.05),见表1、图2。
2.4 两种细胞培养上清液和MCF-7细胞条件培养基中IL-8蛋白含量比较 MCF-7细胞条件培养基和MCF-7培养上清液中IL-8蛋白含量均高于BMSC培养上清液(P<0.05),见图3。
2.5 各组Akt、p-Akt、mTOR、p-mTOR蛋白表达比较 与对照组比较,MCF-7条件培养基组p-Akt和p-mTOR蛋白相对表达量均增高(P<0.05);与MCF-7条件培养基组比较,CXCR1/2抑制剂组和Akt抑制剂组p-Akt和p-mTOR蛋白相对表达量均降低(P<0.05),见图4、表2。
3 讨论
趋化因子参与构成乳腺癌微环境,影响肿瘤微环境MSC、血管内皮细胞、成纤维细胞和免疫细胞的生物特性[9]。IL-8可调节血管生成、肿瘤干细胞存活以及免疫抑制等促肿瘤功能[10]。IL-8还可招募MSC迁移至肿瘤组织,进入肿瘤微环境的MSC分化为CAF,进而促进肿瘤发展及转移[9]。因此,趋化因子可能是介导MSC、CAF及肿瘤进展的关键调控因子,干扰MSC迁移至肿瘤组织可抑制乳腺癌发生发展。Morein等[11]认为,乳腺癌细胞高表达IL-8和单核细胞趋化蛋白-1(monocyte chemoattractant protein-1,MCP-1)等趋化因子,能够促进乳腺肿瘤细胞及肿瘤干细胞增殖和存活,控制肿瘤细胞衰老,并诱导上皮-间质转化和基质金属蛋白酶的表达;促进对化疗和内分泌治疗的抵抗,并介导肿瘤-基质相互作用。Zhang等[12]发现,乳腺癌细胞诱导巨噬细胞表达高水平的IL-15和粒细胞-巨噬细胞集落刺激因子(granulocyte colony-stimulating factor,G-CSF),通过抑制CD8+T细胞的募集来调节肿瘤微环境,进而促进乳腺癌细胞生长及耐药。Wu等[13]发现,乳腺癌干细胞分泌IL-8,增强了雌激素受体的活性,促进肿瘤细胞抵抗雌激素和细胞周期蛋白依赖性激酶4/6(cyclin-dependent kinase 4/6,CDK4/6)抑制剂的治疗而产生耐药性,同时促进肿瘤细胞转移。本研究结果亦显示,MCF-7细胞培养上清液中含有IL-8蛋白;与对照组比较,MCF-7条件培养基组BMSC的增殖水平增高,细胞凋亡率降低;CXCR1/2抑制剂组或Akt抑制剂组上述指标均较MCF-7条件培养基组呈相反变化。提示MCF-7细胞条件培养基含有的IL-8可激活Akt信号通路,促进BMSC增殖,抑制其凋亡,阻断CXCR1/2或抑制IL-8表达可成为预防luminal A型乳腺癌进展的潜在治疗策略。考虑其原因可能是MCF-7细胞条件培养基中高表达的IL-8等促癌趋化因子可缩短BMSC细胞周期,促进细胞增殖,抑制细胞凋亡[11]。
迁移和侵袭是恶性肿瘤发展的关键环节,肿瘤-基质-炎症网络构成luminal A型乳腺癌转移的关键因素[14]。趋化因子可促进肿瘤细胞和内皮细胞迁移及血管生成,并募集MSC迁移到肿瘤组织而成为热点分子[11,15]。Park等[16]发现,乳腺癌细胞表达的IL-8通过细胞外调节蛋白激酶(extracellular regulated protein kinases,ERK)通路促进间充质标志物的表达、肿瘤发生和肺转移。本研究结果亦显示,MCF-7条件培养基组BMSC迁移数目较对照组增加,而CXCR1/2抑制剂组或Akt抑制剂组BMSC迁移数目较MCF-7条件培养基组减少,提示MCF-7条件培养基可激活BMSC的Akt-mTOR信号通路,促进BMSC迁移,进而归巢到乳腺癌微环境。考虑其原因可能为MCF-7细胞高表达的IL-8结合于BMSC细胞膜表面CXCR1/2,进而激活BMSC的Akt-mTOR信号通路,从而促进细胞微丝或微管收缩形成伪足,引起细胞定向迁移[17]。也有研究发现,人卵巢癌细胞分泌的IL-6和IL-8通过转录激活子3(signal transducer and activator of transcription 3,STAT3)、Akt等信号通路促进肿瘤细胞生长、迁移和侵袭[18]。罗后宙等[19]认为,人前列腺癌组织高表达IL-8、淋巴细胞抗原6G(lymphocyte antigen 6G,Ly-6G)和瓜氨酸化组蛋白H3(citrullinated histone H3,H3CIT),可通过激活磷脂酰肌醇3-激酶(phosphoinositide 3 kinase,PI3K)/Akt信号通路促进肿瘤细胞的增殖、迁移和侵袭。Deng等[20]研究发现,三阴性乳腺癌细胞过表达IL-8,通过PI3K/Akt和丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路促进肿瘤细胞的迁移和生长。本研究结果亦显示,MCF-7条件培养基组BMSC的p-Akt和p-mTOR蛋白表达量均较对照组升高,而CXCR1/2抑制剂组和Akt抑制剂组上述指标均较MCF-7条件培养基组呈相反变化,提示MCF-7细胞条件培养基中的IL-8结合于BMSC细胞膜表面CXCR1/2,可激活BMSC的Akt及其下游的mTOR信号通路。
綜上所述,MCF-7细胞条件培养基中的IL-8结合于BMSC细胞膜表面CXCR1/2,可激活Akt-mTOR信号通路,促进BMSC的增殖和归巢到肿瘤微环境,进而促进luminal A型乳腺癌细胞的生长和转移。
参考文献
[1] GAO J J,SWAIN S M. Luminal a breast cancer and molecular assays:a review[J]. Oncologist,2018,23(5):556-565. doi:10.1634/theoncologist.2017-0535.
[2] WANG S,SU X,XU M,et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway[J]. Stem Cell Res Ther,2019,10(1):117. doi:10.1186/s13287-019-1220-2.
[3] KARNOUB A E,DASH A B,VO A P,et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis[J]. Nature,2007,449(7162):557-563. doi:10.1038/nature06188.
[4] ARAVINDHAN S,EJAM S S,LAFTA M H,et al. Mesenchymal stem cells and cancer therapy: insights into targeting the tumour vasculature[J]. Cancer Cell Int,2021,21(1):158. doi:10.1186/s12935-021-01836-9.
[5] ZHANG H,WANG Z,WANG F,et al. IL-6 and IL-8 are involved in JMJD2A-regulated malignancy of ovarian cancer cells[J]. Arch Biochem Biophys,2020,684:108334. doi:10.1016/j.abb.2020.108334.
[6] NIE G,CAO X,MAO Y,et al. Tumor-associated macrophages-mediated CXCL8 infiltration enhances breast cancer metastasis: suppression by Danirixin[J]. Int Immunopharmacol,2021,95:107153. doi:10.1016/j.intimp.2020.107153.
[7] MENG L,ZHAO Y,BU W,et al. Bone mesenchymal stem cells are recruited via CXCL8-CXCR2 and promote EMT through TGF-β signal pathways in oral squamous carcinoma[J]. Cell Prolif,2020,53(8):e12859. doi:10.1111/cpr.12859.
[8] 刘娜,张晓东,李永涛,等. 高糖环境下P物质活化Akt通路促进间充质干细胞增殖和成血管内皮细胞分化的研究[J]. 天津医药,2022,50(5):461-465. LIU N,ZHANG X D,LI Y T,et al. An experimental study of substance P promotes the proliferation of bone marrow mesenchymal stem cells and the differentiation of vascular endothelial cells by activating Akt pathway in high glucose environment[J]. Tianjin Med J,2022,50(5):461-465. doi:10.11958/20212234.
[9] HOUTHUIJZEN J M,JONKERS J. Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment[J]. Cancer Metastasis Rev,2018,37(4):577-597. doi:10.1007/s10555-018-9768-3.
[10] MATSUSHIMA K,YANG D,OPPENHEIM J J. Interleukin-8:an evolving chemokine[J]. Cytokine,2022,153:155828. doi:10.1016/j.cyto.2022.155828.
[11] MOREIN D,ERLICHMAN N,BEN-BARUCH A. Beyond cell motility:the expanding roles of chemokines and their receptors in malignancy[J]. Front Immunol,2020,11:952. doi:10.3389/fimmu.2020.00952.
[12] ZHANG W,ZHANG Q,YANG N,et al. Crosstalk between IL-15Rα+ tumor-associated macrophages and breast cancer cells reduces CD8+ T cell recruitment[J]. Cancer Commun (Lond),2022,42(6):536-557. doi:10.1002/cac2.12311.
[13] WU M,ZHANG X,ZHANG W,et al. Paracrine secretion of IL8 by breast cancer stem cells promotes therapeutic resistance and metastasis of the bulk tumor cells[J]. Cell Commun Signal,2023,21(1):59. doi:10.1186/s12964-023-01068-6.
[14] LIUBOMIRSKI Y,LERRER S,MESHEL T,et al. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer[J]. Front Immunol,2019,10:757. doi:10.3389/fimmu.2019.00757.
[15] TEIJEIRA A,GARASA S,OCHOA M C,et al. IL8, neutrophils,and NETs in a collusion against cancer immunity and immunotherapy[J]. Clin Cancer Res,2021,27(9):2383-2393. doi:10.1158/1078-0432.CCR-20-1319.
[16] PARK J,TACAM M J,CHAUHAN G,et al. Nonphosphorylatable PEA15 mutant inhibits epithelial-mesenchymal transition in triple-negative breast cancer partly through the regulation of IL-8 expression[J]. Breast Cancer Res Treat,2021,189(2):333-345. doi:10.1007/s10549-021-06316-2.
[17] LIANG N,CHANG W,PENG A,et al. Dermal mesenchymal stem cells from psoriatic lesions stimulate hacat cell proliferation,differentiation,and migration via activating the PI3K/AKT signaling pathway[J]. Dermatology,2022,238(2):283-291. doi:10.1159/000515767.
[18] ZHANG R,ROQUE D M,READER J,et al. Combined inhibition of IL-6 and IL-8 pathways suppresses ovarian cancer cell viability and migration and tumor growth[J]. Int J Oncol,2022,60(5):50. doi:10.3892/ijo.2022.5340.
[19] 羅后宙,陈国强. 中性粒细胞胞外诱捕网通过上调DU145人前列腺癌细胞IL-8表达促进前列腺癌细胞增殖、侵袭及迁移[J]. 细胞与分子免疫学杂志,2023,39(3):261-267. LUO H Z,CHEN G Q. Neutrophil extracellular traps promote the proliferation, invasion and migration of prostate cancer cells by upregulating IL-8 expression in DU145 human prostate cancer cells[J]. Chin J Cell Mol Immunol,2023,39(3):261-267. doi:10.13423/j.cnki.cjcmi.009563.
[20] DENG F,WENG Y,LI X,et al. Overexpression of IL-8 promotes cell migration via PI3K-Akt signaling pathway and EMT in triple-negative breast cancer[J]. Pathol Res Pract,2021,223:152824. doi:10.1016/j.prp.2020.152824.
(2023-10-11收稿 2024-01-04修回)
(本文编辑 陈丽洁)

