Dynamic changes and clinical value of lipocalin 2 in liver diseases caused by microbial infections
Feng Chen,Shan-Shan Wu,Chao Chen,Cheng Zhou
Abstract Lipocalin 2 (LCN2) plays a pivotal role in iron metabolism,particularly in the context of microbial infection resistance (e.g.,viruses,bacteria,parasites,etc.).LCN2 combats microbial infection by directly assisting the body in competing with microorganisms for iron,inducing immune cells to secrete various cytokines to enhance systemic immune responses,or recruiting neutrophils to infectious sites.The liver serves as the primary organ for LCN2 secretion during microbial infections.This review encapsulates recent advances in dynamic changes,clinical values,and the effects of LCN2 in infectious liver diseases caused by various microbial microorganisms.
Key Words: Lipocalin 2;Microbial infection;Immunity;Liver diseases
INTRODUCTION
Lipocalin 2 (LCN2) is a secreted protein from the Lipocalin family with a molecular weight of 25 kDa,consisting of 178 amino acid residues[1].It is widely expressed across various cell types,including neutrophils,macrophages,activated leukocytes,adipocytes,hepatocytes,tumor cells,stromal cells,and osteoblasts.The LCN2 mRNA was initially isolated from a kidney cell cDNA library infected with SV40 in 1989 by Hraba-Reneveyet al[2],and it was named 24p3.Subsequently,in 1993,Kjeldsenet al[3] successfully purified LCN2 from human neutrophils.LCN2’s diverse functions were discovered in different cell types,resulting in several names inspired by its various roles.In addition to the original designation as 24p3[2],it was referred to as 25 kDa protein for its molecular weight[3],neutrophil gelatinase-associated lipocalin (NGAL or HNL) for its aggravating inflammatory responseviarecruitment of neutrophils[4],oncogenic lipocalin for its promotion of tumor growth[5],superinducible protein (SIP24)[6] for its heightened expression in response to fibroblast growth factor in BALB/c 3T3 cells,uterocalin for its elevated level around parturition in the uterus[7],and siderocalin for its function in sequestering iron[8].In 2004,Flo TH published a paper in Nature officially named it LCN2,following the nomenclature of the lipocalin family,a gene group evolutionarily conserved and found in all kingdoms of life.In the Flo TH paper,LCN2 was demonstrated to play an important role in innate immunity against iron-dependent bacterial infections.Over time,the name LCN2 gained widespread acceptance and standardization[9,10].
Similar to other members of the Lipocalins family,LCN2 has a highly conserved core structure,characterized by an eight-stranded,antiparallel β-barrel with the calyx,or ligand-binding site.Compared with other members,LCN2 exhibits a calyx that is unusually shallower and broader,featuring a lining of positively charged residues.Consequently,LCN2 does not directly bind positively charged ions but rather sequesters irons by binding the negatively charged ferric siderophore with a sub-nanomolar dissociation constant[11].The ligands housed in the calyx suggests that LCN2’s primary function is intricately linked to iron metabolism.
LCN2 has been reported to be expressed in many organs,including the liver[12-15],kidney[16-18],lungs[19,20],brain[21,22],heart[23,24],bone marrow[25],and spleen[26],bronchus[27],stomach[28],small intestine[29],pancreas[30],prostate gland[31],thymus[32].Despite its presence in numerous organs,the liver emerges as the major site of LCN2 generation.During the body’s response to harmful microorganisms,90% of the LCN2 upregulation originates from the liver.It has been reported that in mice,upon bacterial infection,the mRNA level of LCN2 increased by 30-40-fold in the liver,in contrast to an approximately 1.5-fold elevation in the spleen and lungs[33].Physiologically,hepatocytes take up iron majorly by transferrin-mediated pathway,and LCN2 was demonstrated to be not essential in hereditary hemochromatosis (HH)[34].However,when the body with HH was infected by microorganisms,such as Salmonella Typhimurium,iron-capturing LCN2 was inducted to confer the host resistance to systemic infection with Salmonella and improve control of bacterial replication[35].Therefore,LCN2 is proposed to play an important role in microbial infection-induced hepatitis.
IN VIRAL INFECTIOUS LIVER DISEASES
As a key organ of detoxification,the liver assumes a crucial role in the body’s defense mechanisms.Viral infections,including hepatitis B virus (HBV) and hepatitis C virus (HCV),can induce a series of inflammatory pathological changes in the liver,leading to varying degrees of hepatitis.This progression may result in fibrosis,cirrhosis,liver failure,and ultimately,liver cancer.Individuals at different stages of HBV-related liver diseases manifest varying degrees of iron metabolic disorders[36].Following viral infection,LCN2 levels begin to rise and exhibit variation during different phases of liver disease,underscoring the potential of LCN2 in the diagnosis and treatment of viral hepatitis.Due to the lack of suitable mouse models for viral hepatitis,studies on LCN2 in viral liver diseases mainly focus on disease diagnosis.
LCN2 is mainly related to the degree of inflammation in the body during viral liver disease and can serve as an indicator of various complications,such as cirrhosis,ascites,peritonitis,hepatorenal syndrome,nephritis,etc.Our previous study showed that the serum LCN2 levels in patients with chronic hepatitis B were significantly higher than those in normal controls[37].Luet al[38] examined the serum of patients with HBV-associated acute-on-chronic liver failure (ACLF) and found that LCN2 levels were significantly higher than that of patients without ACLF.Moreover,the serum LCN2 levels significantly correlated with the total bilirubin,international normalized ratio,and model for endstage liver disease model (MELD).The MELD score was independently associated with the overall survival in patients with HBV-ACLF,and serum LCN2 is also an independent risk factor for hepatorenal syndrome.Thus,the above data showed significant value in predicting the prognosis of HBV-ACLF.
In patients with liver cirrhosis,Gungoret al[39] reported that in patients with stable cirrhosis,serum LCN2 levels were not significantly different when compared with controls,while LCN2 levels in plasma and urine were significantly higher in cirrhosis patients with hepatorenal syndrome.Cox regression analysis revealed that plasma LCN2 and MELD-Na scores independent predicted of mortality.Nevertheless,conflicting perspectives exist,Borkham-Kamphorstet al[40]found that there was no difference in LCN2 between cirrhosis and non-cirrhosis patients,with compensatory cirrhosis patients exhibiting similar LCN2 levels to end-stage liver disease patients.
Concerning the relationship between LCN2 and viral load,studies in the patients infected with HCV showed no correlation between LCN2 levels and HCV viral load[41].The diagnostic value of LCN2 in HCV infection-associated renal glomerular injury remains unclear.Strazzullaet al[42] observed significant increase in urine LCN2 levels in HCV-infected patients after one year of treatment with direct antiviral drugs,whereas Nadaet al[43] reported a decrease in urine LCN2.Both studies,however,showed unchanging glomerular filtration rate.The disparate results may stem from individual differences in patient samples,systemic inflammation,and drug toxicity,necessitating further investigation (Table 1).
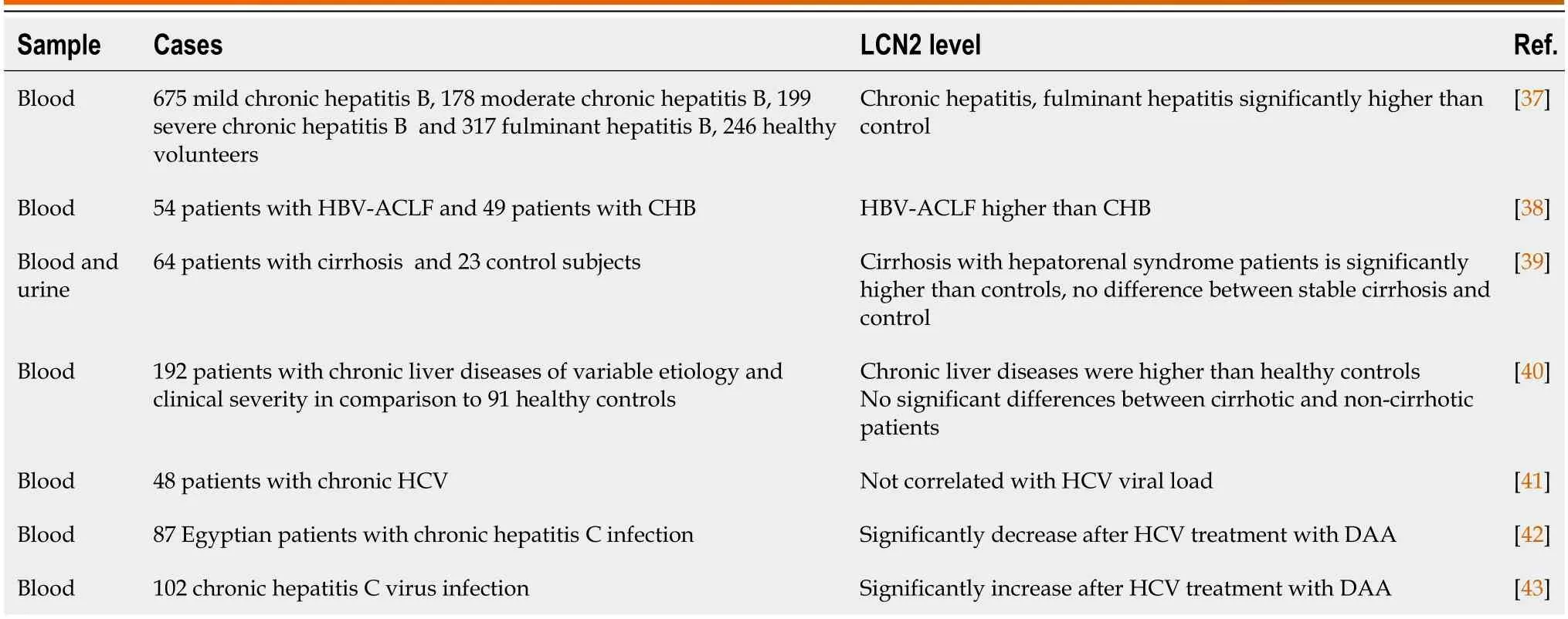
Table 1 Expression of lipocalin 2 in different viral hepatitis
Because of its small molecular weight,LCN2 may directly leak from the kidney into the urine,allowing it to be clinically detected through noninvasive methods.Urinary LCN2 levels can be used to assess kidney injury in chronic liver disease complications or renal toxicity after antiviral drug administration[16,17,44].To evaluate the efficacy of urinary LCN2 as a diagnostic biomarker for different etiology of acute kidney injury (AKI) in cirrhosis and its role as a prognostic marker,Hamdyet al[44] studied 83 patients with liver cirrhotic AKI due to HCV or combined with HBV infections,and they revealed that different urine LCN2 levels matched different types of kidney dysfunction in cirrhotic patients,thus providing suggestions for management decisions in the diagnostic process.It needs to be pointed out that,in addition to a systemic inflammatory reaction,the kidneys themselves are also damaged in the virus-induced hepatorenal syndrome[45],resulting in a significant increase of LCN2.
IN BACTERIAL INFECTIOUS LIVER DISEASES
Bacterial infections pose an escalating global health challenge,and the LCN2 has been repetitively found to play an important role in the body's defense against bacterial infections (Figure 1).

Figure 1 Schematic outline of lipocalin 2 antibacterial effects. During bacterial infections,most lipocalin 2 (LCN2) is secreted by liver cells,while some are derived from immune cells such as macrophages and neutrophils.LCN2 exerts an antibacterial effect mainly in two ways.One,it chelates LCN2-sensitive siderophores to form the LCN2-siderophores-Fe complex,which prevents the bacteria from absorbing iron and inhibits bacterial growth.Two,it stimulates the antibacterial immune response through stimulating immunocytes,which secrete various inflammatory cytokines and chemokines,promoting the migration and phagocytosis of macrophages.The cytokines and chemokines from immunocytes then further stimulate immunocytes and hepatocytes to produce more LCN2,forming a positive feedback loop to enhance the antibacterial immune response.LCN2 derived from hepatocytes and macrophages enters the systemic circulation to fight bacteria,and LCN2 is a component of NETs from neutrophils,which plays an important role in local inflammation.LCN2: Lipocalin 2;LPS: Lipopolysaccharide;IL: Interleukin;CXCL: Chemokines;IFN: Interferon;TNF: Tumor necrosis factor.
LCN2 inhibits bacterial growth primarily by sequestering iron,an essential nutrient for life.Bacterial invasion,growth,and reproduction rely on iron,creating a natural competition with the body’s iron-dependent activities.Mechanistically,bacteria acquire iron by synthesizing siderophores and forming siderophore-Fe complexes.The host employs LCN2 to directly bind the siderophores-Fe complex,thereby controlling bacterial growth.Therefore,LCN2 is an important component of the innate immune system in defense against bacterial infection[46].In LCN2 deficient mice,a challenge with a sublethal dose of Escherichia coli intraperitoneally resulted in a substantially higher amount of bacteremia and bacterial burden in the liver compared to the control mice.However,no significant differences in other components of the immune response,including leukocyte numbers,neutrophil infiltration,and various cytokines,such as toll-like receptor(TLR)-induced tumor-necrosis factor,interleukin (IL)-12,IL-6 and macrophage inflammatory protein (MIP)-2 were found between wild-type and lipocalin-2-deficient mice[9].
In vivo,LCN2 may also act as an antioxidant by regulating iron homeostasis to combat bacteria.Studies in LCN2 deficient mice revealed decreased levels of tissue redox state indicators cysteine and glutathione in the liver and plasma,elevated indices of liver damage such as transaminasemia,lactate dehydrogenase,and increased mortality.Moreover,the application of an iron chelator,Desferoxamine,was able to protect LCN2-deficient mice from LPS-induced toxicity and reduce mortality[47].
As a constituent of innate immunity,the antibacterial effects of LCN2 are intricately regulated by the immune system.During the early phase of infection,the innate immunocytes,such as neutrophils and macrophages,are activated,leading to the secretion of substantial quantities of LCN2.LCN2 then stimulates these immunocytes to produce a variety of cytokines and chemokines,such as IL-6,IL-10,tumor necrosis factor (TNF)-α,monocyte chemoattractant protein(MCP)-1,etc.,thereby enhancing the migration and phagocytosis of macrophages to bolster antibacterial function.Deletion of the LCN2 gene results in impaired functions of immune cells,including compromised homeostasis and morphological development of neutrophils,decreased migration ability and exudation,as well as reduced secretions of cytokines and chemokines,such as TNF-α,IL-6,IL-1β,MCP-1,and MIP-2[48].LCN2 knockout mice exhibit higher expression of Th17 cell polarization markers,with transcription factor RORγt and cytokines IL-17A and IL-21 significantly up-regulated[49].
LCN2 itself also interacts with other immune factors in various types of cells,such as TLR ligands (e.g.,TLR4 ligands),cytokines (e.g.,IL-6,IL-1,IL-22,TNF-α,interferon (IFN)-γ),and growth factors (e.g.,insulin-like growth factor)[33,50,51].It was reported that IL-6 treatment stimulated hepatocytes to produce more LCN2in vitroand in vivo,and the elevation of LCN2 was abrogated in the IL-6R Hep−/−,IL-6−/−mice,and signal transducers and activators of the transcriptions 3(STAT3) Hep−/−mice.Hepatocyte-specific LCN2 knockout mice showed increased susceptibility to infection with Klebsiella pneumoniae or Escherichia coli,leading to increased bacterial translocation from the gut to mesenteric lymph nodes and reduced liver regeneration after partial hepatectomy.These findings suggest that the production of hepatocyte-specific LCN2 depends on IL-6 activation of the STAT3 signaling pathway.Hepatocyte-derived LCN2 protects against bacterial infection and promotes liver regeneration[33].Another study demonstrated that rIL-22-induced antimicrobial activity mediated by IL-22 receptor alpha 1 (IL-22Rα1) and STAT3 signaling is partially dependent on LCN2[51].
During bacterial infection,elevated levels of LCN2 expression are detected in both hepatocytes and neutrophils.The LCN2 from these distinct sources coordinates to combat bacteria,albeit with differing roles.While extracellular LCN2,secreted by liver cells,serves to restrict systemic bacterial infection,neutrophils transported LCN2 are carried in specific granules of neutrophils to local sites,contributing to the resistance against local bacterial infection through a network of neutrophil extracellular traps (NETs).The recently discovered mechanism,NETs,describes the process by which neutrophils kill bacteria through both cell-death-dependent and cell-death-independent pathways.Among the more than 80 proteins identified as components of NETs,LCN2 emerges as one of the crucial proteins.Notably,LCN2 in NETs is exclusively derived from neutrophils and not from hepatocytes or other cells.Complete or specific genetic deletion of the LCN2 gene in neutrophils does not impact NETs formation but does reduce the bactericidal effect of NETsin vitro[52].
LCN2 also resists bacterial invasion by maintaining intestinal microecological stability.It plays an important role in maintaining the species and abundance of intestinal flora and contributes to the gastrointestinal antibacterial barrier.Studies have shown that in the gut of LCN2 knockout mice,there is a significant and dramatic alteration in the species and abundance of intestinal flora change.Notably,iron-dependent strains expand significantly,leading to dysbiosis and continuous colonization by segmented filamentous bacteria (SFB).SFB is a special symbiotic intestinal bacterium within the firmicutes,fundamental for the production of innate and acquired immunity in the gastrointestinal and respiratory tracts and necessary for the maturation of the host intestinal immune barrier[53].Deletion of LCN2 may provide a favorable environment for SFB colonization by inhibiting the antibacterial response of epithelial cells,altering the mucous composition,or establishing the antibacterial barrier.The increased SFB,in turn,can upregulate the level of LCN2.A study reported a significant increase in LCN2 levels in the liver and serum of SFB-colonized mice,accompanied by upregulation of the pro-inflammatory TH17 and TH1 cells in the liver-draining lymph nodes[54].
The dynamic changes in LCN2 levels during bacterial infection present potential applications for the early diagnosis of complications in liver diseases.Behairyet al[55] demonstrated that the level of LCN2 was significantly elevated in the patients with chronic liver diseases complicated with bacterial infection compared with those without bacterial infection.This finding suggests that LCN2 is an early diagnostic indicator for chronic liver disease with bacterial infection.Similarly,Liuet al[56] found that the level of LCN2 in ascites from decompensated liver cirrhosis with spontaneous bacterial peritonitis (SBP) was significantly higher than that in the non-SBP group,and the LCN2 level was positively correlated with ascitic polymorphonuclear leukocyte and negatively correlated with ascitic albumin.Furthermore,the dynamic changes of ascitic LCN2 were able to predict the clinical prognosis of SBP patients.Another study by Cullaroet al[57] showed a close relationship between ascitic LCN2 level and patient mortality,indicating that LCN2 serves as a molecular diagnostic biomarker of peritonitis in liver cirrhotic patients and an independent predictor of short-term hospitalization mortality.In addition,a study in the sepsis animal model of cecal ligation and puncture showed the LCN2 Levels in mouse liver and lung increased significantly during the early hyper-inflammatory phase,despite the dysfunction of innate immunity characterized by a severely decreased expression of most inflammatory mediators.This highlights the potential importance of LCN2 in the diagnosis of sepsis[58].Thus,LCN2 is suggested to be an important clinical biomarker for early diagnosis of sepsis.
As a siderophore-binding protein,LCN2 provides wide application in the treatment of bacterial infections.Due to its multidrug resistance and the lack of effective antimicrobial drugs,carbapenem-resistant Acinetobacter baumannii (A.baumannii) has been designated by the World Health Organization as a priority critical pathogen for the development of novel therapeutics.Sheldonet al[59] observed the transcriptional profile in the A.baumannii-infected mice and revealed that the expression of LCN2 gene was the most highly upregulated during A.baumannii bacteremia.In vitrostudies have also shown that LCN2 inhibits iron-dependent growth of A.baumannii and induces iron-regulated gene expression.In an LCN2 knockout mouse model,although LCN2 gene deletion did not alter the number of microflora in A.baumanniiinfected tissues,it significantly aggravated the severity of infection and increased mortality.
Similarly,in a sepsis animal model induced by the infection of A.baumannii,the injection of recombinant mouse LCN2 prolonged the survival time of mice by decreasing the number of bacteria in macrophages and multiple organs,including the liver,spleen,and lungs[60].LCN2 has been regarded as novel therapeutics to combat A.baumannii infection.
IN PARASITIC INFECTIOUS LIVER DISEASES
Infection caused by a variety of parasites,such as Schistosoma,Leishmania,and Plasmodium,can lead to liver damage.Iron,being an essential trace element for parasite survival,is acquired by intracellular pathogens from multiple sources within host cells,such as heme,ferlactoferrin or ferrictransferin.This ability may contribute to parasites' survival in different environmental conditions within the host[61].As a metabolic enzyme and a cofactor in oxidative transport,iron plays a crucial role in immune surveillance.Therefore,controlling iron homeostasis is one of the central battlegrounds in combating pathogen infection.
LCN2's robust iron-binding ability assumes a crucial role in the body's resistance to parasitic infections[62].Dighalet al[63] studied the intramonocytic labile iron pool (LIP) in Indian post Kala-azar dermal Leishmaniasis and found enhanced gene expressions of the iron influx gateways,including LCN2,possibly contributing to the heightened LIP.Therefore,restricting the availability of iron for parasites is regarded as a potential therapeutic strategy against parasitic infections.
Emerging evidence has shown that macrophage polarization plays a critical role in the initiation and progression of liver diseases.The underlying molecular mechanisms are intricate and involve various signaling pathways,including TLR4/nuclear factor kappa-B (NF-κB),janus kinase/STATs,transforming growth factor-β/smads,peroxisome proliferators-activated receptor,Notch,and miRNA signaling pathways[64].Various factors,such as microorganisms,hypoxia,metabolism,etc.,can influence the macrophage polarization.As an iron-related protein involved in innate immune response,LCN2 is modulated by the host’s immune system and interacts with macrophages.Studies have reported that LCN2 promotes M1 polarization of macrophages underS.japonicumsoluble worm antigens (SWA) treatment.In addition,during the early infection stage in mice treated withschistoma japonicum,the expression of LCN2 significantly increased in the liver,mainly located in macrophagesviathe upregulation of NF-κB signaling.This study highlighted the importance of NF-κB/LCN2 in migration and phagocytosis of M1 macrophages stimulated by SWA,emphasizing the essential role of NF-κB/LCN2 in early innate immune responses to infection[65].Recently,it has been reported that GATA3 is a master regulator for macrophage polarization and infiltration[66,67].Therefore,investigating the relationship between LCN2 and GATA3 in macrophage polarization of various liver diseases may provide valuable insights.
Besides,oxidative stress can also lead to an increase in LCN2.Inin vitroexperiments,the up-regulation of LCN2 expression after H2O2treatment was found to be offset by the addition of antioxidants,dimethyl sulfoxide or cysteamine[68].Al-Shaebiet al[69] found that in the mouse model of malaria induced by Plasmodium chabaudi infection,the expression of the LCN2 gene reduced significantly after treatment with a plant antioxidant,Indigofera oblongifolia leaf extracts (ILE).ILE demonstrated a protective effect on mouse liver injury infected with Plasmodium chabaudi by enhancing the antioxidant capacity of the liver and significantly reducing the red blood cell count and hemoglobin content in mice caused by infection.
CONCLUSION
In summary,LCN2 emerges as a sensitive marker for infections,as its changes can be detected at the very early stage of various pathogenic microorganism infections,even preceding the detection of the commonly used clinical acute phase protein α2 macroglobulin.Infection with a variety of pathogens can lead to liver damage.Monitoring the level of LCN2 allows doctors to evaluate disease progression and treatment efficacy.LCN2 also serves as a predictive marker in certain end-stage liver diseases,holding promise as a novel diagnostic marker.Additionally,due to its robust iron-binding capacity,targeting LCN2 presents great potential in the treatment of infectious diseases.
Indeed,the application of LCN2 in clinical setting is not without challenges.First,given that LCN2 is expressed at different levels in different organs,plasma,and urine,it is crucial to determine the appropriate situations to test specific samples and develop standardized sample-handling protocols.Second,the variability in LCN2 data across studies is attributed to different methods of detection.Addressing this issue would entail standardizing the detection methods and establishing specific thresholds for diagnosis,necessitating future work with large sample verification.Third,LCN2,being a very sensitive indicator of inflammation,is also sensitive to various other factors.Therefore,evaluating and mitigating the impact of confounding factors on LCN2 reading is essential.Finally,despite promising results in drug studies targeting LCN2,extensive experiments are required to confirm whether regulating iron metabolismviaLCN2 may lead to unexpected side effects,as iron involves many aspects of the body’s functions.
FOOTNOTES
Co-first authors:Feng Chen and Shan-Shan Wu.
Author contributions:Chen F and Zhou C contributed to the concept and design of the whole study;Chen F prepared the draft;Chen F and Zhou C wrote and revised the manuscript;Chen C contributed to drawing the figure;All authors contributed to preparing,reading,and approving the nal manuscript;Chen F and Wu SS have been working together on the research of LCN2;Wu SS participated in the conception of the paper and was instrumental and responsible for the comprehensive literature search,figure plotting,preparation and submission of the current version of the manuscript;Chen F and Wu SS have made crucial and indispensable contributions to the publication of this manuscripts as the co-first authors.
Conflict-of-interest statement:All authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Cheng Zhou 0000-0001-6502-5442.
S-Editor:Liu JH
L-Editor:A
P-Editor:Cai YX
 World Journal of Hepatology2024年2期
World Journal of Hepatology2024年2期
- World Journal of Hepatology的其它文章
- Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients
- Precision targeting in hepatocellular carcinoma: Exploring ligandreceptor mediated nanotherapy
- Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: A cohort study
- Effects of SARS-CoV-2 infection on incidence and treatment strategies of hepatocellular carcinoma in people with chronic liver disease
- Epidemiological survey of cystic echinococcosis in southwest China: From the Qinghai-Tibet plateau to the area of Yunnan
- Predictors of portal vein thrombosis after splenectomy in patients with cirrhosis
