Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients
Dmitry Victorovich Garbuzenko
Abstract This editorial describes the contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis (LC) patients according to the current guidelines.Gastroesophageal variceal bleeding is the most dangerous complication of portal hypertension in LC patients.Risk stratification and determination of an individual approach to the choice of therapeutic measures aimed at their prevention and management has emerged as one of the top concerns in modern hepatology.According to the current guidelines,in the absence of clinically significant portal hypertension,etiological and nonetiological therapies of LC is advisable for the primary preventing gastroesophageal variceal bleeding,whereas its presence serves as an indication for the administration of non-selective β-blockers,among which carvedilol is the drug of choice.Non-selective β-blockers,as well as endoscopic variceal ligation and transjugular intrahepatic portosystemic shunt can be used to prevent recurrence of gastroesophageal variceal bleeding.Pharmacotherapy with vasoactive drugs(terlipressin,somatostatin,octreotide),endoscopic variceal ligation,endovascular techniques and transjugular intrahepatic portosystemic shunt are recommended for the treatment of acute gastroesophageal variceal bleeding.Objective and accurate risk stratification of gastroesophageal variceal bleeding will allow developing individual strategies for their prevention and management,avoiding the first and further decompensation in LC,which will improve the prognosis and survival of patients suffering from it.
Key Words: Liver cirrhosis;Portal hypertension;Gastroesophageal variceal bleeding;Prevention;Management
INTRODUCTION
Liver cirrhosis (LC) is the final stage of many chronic liver diseases and has long been considered a static,irreversible pathological process.However,research in recent years has refuted this well-established notion.LC is now considered as a dynamic,potentially reversible disorder,where there are a compensated stage (without or with clinically significant portal hypertension (CSPH),which is characterized by hepatic venous pressure gradient (HVPG) values ≥ 10 mmHg and gastroesophageal varices (GEV) forming) and a decompensated stage,often accompanied by fatal complications associated with portal hypertension (PH) and/or liver failure[1].Hence,LC decompensation is the most important stratification variable of a bad prognosis[2].After the first decompensation,a progressive growth in portal pressure increases a likelihood of further decompensation,a resistance to treatment,a risk of death,and the need for liver transplantation[3].
Based on the modern concept of the natural history of LC,at the Baveno VI consensus workshop,held in 2015,recognized the use of “advanced chronic liver disease” as a term equivalent to LC to refer to cases of chronic liver disease with a risk of complications[4].Since LC is an exclusively histological term,the approval of the new concept expanded the spectrum of the clinical course of the disease,allowing non-invasive methods to be used for its diagnosis and staging.Now LC patients can be stratified by a risk of complications,and therapeutic approaches are individualized.In 2021,at the Baveno VII consensus workshop,the main provisions adopted at the Baveno VI workshop were approved,and given the latest achievements,practical recommendations for personalized care for PH were developed[5].
This editorial describes the contemporary concepts of prevention and management of gastroesophageal variceal bleeding in LC patients according to the current guidelines.
DIAGNOSIS OF CLINICALLY SIGNIFICANT PORTAL HYPERTENSION
The development of CSPH in compensated LC patients is an important prognostic factor,since it leads to an increased risk of first decompensation,one of the clinical manifestations of which is gastroesophageal variceal bleeding[1].The gold standard of its diagnosis is HVPG measurement[6].The normal range of HVPG is 1-5 mmHg,whereas a values of ≥ 10 mmHg indicates the presence of CSPH[7].It should be recognized that until now,HVPG measurement is possible only in specialized centers.In addition,the procedure invasiveness and the need for repeated measurements increases a risk of possible complications and raises costs.
These limitations have contributed to the development of non-invasive methods for assessment of advanced chronic liver disease.One of them is the liver stiffness measurement by transient elastography.This is a fast,simple to perform,and are well tolerated procedure by patients with immediately available results[8].According to the current guidelines,liver stiffness values by transient elastography of less than 10 kPa in the absence of other known clinical/imaging signs excludes LC,a values between 10 and 15 kPa suggest of it,and a values of more than 15 kPa indicates the presence of LC with a high probability[9].At the Baveno VII consensus workshop,criteria were established for the exclusion or identification of CSPH by liver stiffness values that,combined with platelet count.According to them,liver stiffness values by transient elastography of more than 25 kPa indicates the presence of CSPH,whereas with liver stiffness values of less than 15 kPa and a normal platelet count,it is unlikely.LC patients with liver stiffness values between 20 and 25 kPa and platelet count less than 150 × 109/L or with liver stiffness values between 15 and 20 kPa and platelet count less than 110 ×109/L have a risk of developing CSPH with a probability of about 60%.They need additional screening[5].For example,esophagogastroduodenoscopy (EGDS) has traditionally been used to detect GEV,the degree of dilation of which correlates with HVPG and,accordingly,with a risk of bleeding[10].
PREVENTING FIRST LIVER CIRRHOSIS DECOMPENSATION
Primary preventing gastroesophageal variceal bleeding
Therapeutic measures in compensated LC patients with or without CSPH,should be aimed at preventing first decompensation,in particular,primary preventing gastroesophageal variceal bleeding (Figure 1).In the absence of hyperdynamic circulatory state,etiological and non-etiological therapies of LC may be beneficial for preventing GEV forming[11].Indeed,it has been found that alcohol abstinence positively affects the prognosis in alcohol-related LC,including patients with CSPH[12],and a sustained virologic response in LC associated with chronic HBV and HCV infection improves liver morphology and reduces HVPG[13].The use of statins[14],anticoagulants[15],gut microbiota modulation[16] and targeted therapy of liver fibrosis[17] seems promising as a non-etiological treatment.

Figure 1 Algorithm for primary preventing gastroesophageal variceal bleeding in liver cirrhosis patients.
The drugs of choice for the primary preventing gastroesophageal variceal bleeding are non-selective β-blockers (NSBB),which is associated with their positive effect on the hyperdynamic circulatory state in CSPH.They can both reduce heart rate and cardiac output by blocking β1-adrenergic receptors,and decrease portal inflow as a result of splanchnic vasoconstriction caused by an endogenous α-adrenergic effect against the background of the blocking vasodilating β2-adrenergic receptors.In addition,NSBBs suppress the small intestinal bacterial overgrowth and prevent bacterial translocation contributing to systemic inflammation characteristic of LC,by accelerating orocecal transit[18].
Liver stiffness values of more than 25 kPa in compensated LC patients,which indicates the presence of CSPH,may be a criterion for prescribing NSBBs[19].In the PREDESCI trial,the administration of NSBBs (propranolol and carvedilol)reduced HVPG and improved hyperdynamic circulatory state,which contributed to lowering the risk of first decompensation in compensated LC patients with CSPH[20].The results of the PREDESCI trial were confirmed by quantifying the benefits of NSBBs in preventing LC decompensation[21].A meta-analysis of 15 studies has shown that a reduction in portal pressure by NSBBs contribute to lowering the risk of complications,death or liver transplantation in LC patients[22].Thus,the indication for prescribing NSBBs in compensated LC patients is a CSPH in the presence of GEV[5].
The aim of PH pharmacotherapy with NSBBs should be HVPG reduction to less than 12 mmHg or 20% of the baseline,without allowing significant arterial hypotension and other adverse effects.However,since HVPG measurement is not widely available,and a decrease in heart rate does not correlate with HVPG reduction,the dose of NSBBs is adjusted to the maximum tolerated doses[23].According to the current guidelines,NSBBs should be prescribed at a dose that decrease heart rate at rest by 25% or up to 55 beats per minute at the initial bradycardia.Daily doses of propranolol can vary from 20 mg orally (initial) to 320 mg (maximum) and should be individually determined according to clinical response.The dose of carvedilol should be titrated from the initial daily dose of 6.25 mg.The maximum dose is 25 mg/d[24].In some systematic reviews and meta-analyses,it has been shown that correctly determined therapeutic dosages of carvedilol more significantly reduce HVPG compared to propranolol,making it more effective in preventing gastroesophageal variceal bleeding in LC patients[25,26].In the majority of responders to carvedilol therapy,the HVPG-response is maintained over a long period,which improves the clinical outcome[27].As a consequence,at the Baveno VII consensus workshop,carvedilol was recommended as the drug of choice for preventing first decompensation in compensated LC patients with CSPH[5].
In compensated LC patients after the start of NSBBs therapy,there is no need to monitor the presence and dynamics of GEV during follow-up due to the lack of influence of the results of EGDS on therapeutic tactics.An exception may be in the case of a decision to withdrawal of NSBBs with the effectiveness of etiological treatment.In particular,their withdrawal is possible in patients who,1-2 years after the elimination of the etiological factor,had a complete eradication of GEV and,according to transient elastography or HVPG,there are no signs of CSPH[28].
Endoscopic band ligation (EBL) is recommended in compensated LC patients with contraindications or intolerance to NSBBs with high-risk GEV for preventing first bleeding[5].
PREVENTING FURTHER LIVER CIRRHOSIS DECOMPENSATION
Further LC decompensation is an unfavorable prognostic stage associated with an even higher mortality rate than during the first decompensation,therefore,patients with it are candidates for liver transplantation.Further LC decompensation is characterized by recurrent gastroesophageal variceal bleeding,refractory ascites (requires >3 large volume paracentesis within 1 year),recurrent encephalopathy,the development of spontaneous bacterial peritonitis,hepatorenal syndrome/acute kidney injury,as well as jaundice[5].
Preventing recurrent gastroesophageal variceal bleeding (secondary prophylaxis)
The combined use of NSBBs and EBL is the treatment of choice for secondary prophylaxis of gastroesophageal variceal bleeding[5].This approach proved to be more effective than the use of each technique separately,both in preventing recurrent bleeding[29] and in improving survival[30].With regard to the isolated use of NSBBs in secondary prophylaxis of gastroesophageal variceal bleeding,a recent systematic review showed the advantages of carvedilol over propranolol,which was accompanied by lower rates of recurrent bleeding,liver-related death,and further nonbleeding decompensation[31].
In recent years,the issue of the expediency of prescribing NSBBs to decompensated LC patients with ascites has been discussed due to their ability to reduce increased cardiac output,which is a compensatory reaction to hypovolemia to maintain systemic and renal perfusion[32].In this regard,at the Baveno VII consensus workshop,it was recommended that in decompensated LC patients with ascites,in case of persistently low blood pressure (systolic blood pressure <90 mmHg or mean arterial pressure <65 mmHg) and/or the presence of hepatorenal syndrome/acute kidney injury,the dose of NSBBs should be reduced or they should be completely canceled.Once blood pressure returns to baseline and/or after eliminating signs of hepatorenal syndrome/acute kidney injury,NSBBs can be re-initiated or re-titrated initially at a dose lower than when discontinuation.If patients remain intolerant to NSBBs,EBL is recommended to prevent gastroesophageal variceal bleeding[5].
Transjugular intrahepatic portosystemic shunt (TIPS) is the method of choice for preventing recurrent gastroesophageal variceal bleeding with the ineffectiveness of the combined use of NSBBs and EBL taking into account rebleeding severity and other complications of PH,with careful patient selection to minimize hepatic encephalopathy[33].Compared to the combined use of NSBBs and EBL,polytetrafluoroethylene (PTFE)-covered TIPS have a significant benefit of preventing gastroesophageal variceal rebleeding[34],decrease the threat of further decompensation[35],and postoperative reduction of HVPG below 12 mmHg can contribute to recompensation[36].
Managing acute gastroesophageal variceal bleeding
At the Baveno VII consensus workshop,a number of changes and additions were made to the previously adopted recommendations for the management of LC patients with acute gastroesophageal variceal bleeding[5],although the general principles remained the same.If possible,all LC patients with acute gastroesophageal variceal bleeding should be hospitalized in the intensive care unit for resuscitation measures aimed at preserving tissue perfusion.It is important to quickly begin restoration of circulating blood volume to ensure and maintain hemodynamic stability.The threshold for red blood cell transfusion should be a hemoglobin level of 7-8 g/dL,taking into account factors such as cardiovascular diseases,age,hemodynamic status and the presence of ongoing bleeding.If there is a suspicion of acute gastroesophageal variceal bleeding,as early as possible,ideally before the EGDS,vasoactive drugs should be administered: terlipressin(under the control of serum sodium levels),somatostatin,octreotide for at least 5 d[37].
Terlipressin is usually administered 2 mg IV immediately,then 1-2 mg every 4-6 h until hemostasis achieved,or for 3 to 5 d.Somatostatin is administered 250 mg bolus IV initially,followed by 250 mg/h IV infusion for 3 to 5 d.Octreotide is administered 50 mcg bolus IV initially,followed by 50 mcg/h IV infusion until hemostasis achieved or for 3 to 5 d[38].In a systematic review and meta-analysis,vasoactive drugs had similar indicators of mortality risk,control of acute gastroesophageal variceal bleeding,its early and late recurrence,need for transfusion of red blood cells and hospitalization duration.However,the use of terlipressin was accompanied by a higher risk of adverse events[39].At the same time,the administration of proton pump inhibitors started before EGDS,after the diagnosis of acute gastroesophageal variceal bleeding in the absence of strict indications,should be stopped immediately,since their use in LC patients increases the likelihood of spontaneous bacterial peritonitis and other infectious complications[40].
Given the risk of bacterial infection primarily in decompensated LC patients with acute gastroesophageal variceal bleeding,antibiotic prophylaxis is an integral part for therapy.It should be prescribed from the moment of admission by IV administration of ceftriaxone at a dose of 1 g/d.Antibiotic prophylaxis should always be in accordance with local resistance patterns and antimicrobial policies.Antibiotic prophylaxis in LC patients with acute gastroesophageal variceal bleeding significantly reduces the frequency of bacterial infections,all-cause mortality,bacterial infection mortality,rebleeding events and hospitalization duration[41].
Malnutrition in LC patients with acute gastroesophageal variceal bleeding increases the risk of adverse outcomes,therefore,their feeding should be resumed 48-72 h after achieving hemostasis.Because of the lower cost and the lack of complications,enteral nutrition is always preferable to parenteral.If it is carried out through a nasogastric probe,manipulations with it should be performed with extreme caution should be performed with caution due to the risk of pulmonary infection[42].
Correction of hepatic encephalopathy in LC patients with acute gastroesophageal variceal bleeding is carried out by rapid removal of blood from the gastrointestinal tract by lactulose (through a nasogastric probe or in the form of enemas)[43].
Against the background of resuscitation measures in LC patients with acute upper gastrointestinal bleeding,EGDS should be performed within 12 h from the moment of admission.If the patient's condition is unstable,it is carried out as soon as possible,as far as it is safe.The diagnosis of gastroesophageal variceal bleeding is established by the presence of its active manifestations.In the absence of bleeding,indirect signs of the complication are the “white nipple sign” and blood clots on varices,as well as blood in the lumen of the esophagus and/or stomach if other possible causes have been ruled out[44].Tracheal intubation before EGDS is recommended in patients with impaired consciousness and/or active vomiting blood.They are extubated immediately after the procedure is completed.In the absence of contraindications(QT interval prolongation),administration of intravenous erythromycin at 250 mg for 30-120 min before EGDS to improve mucosa visualization by enhancing gastric motility is considered appropriate[45].
In LC patients with acute gastroesophageal variceal bleeding,the method of choice is EBL[5].An additional 5-d course of pharmacotherapy with vasoactive drugs can significantly reduce the risk of their recurrence[46].Endoscopic therapy with tissue adhesiveshistoacryl (N-butyl-2-cyanoacrylate)/thrombin) is recommended for acute bleeding from type 1 GEV (GEV1) and type 2 GEV (GEV2) that extend beyond the cardia,and from type 1 and type 2 isolated gastric varices(IGV1 and IGV2) (Figure 2)[47].In case of refractory gastroesophageal variceal bleeding despite combined pharmacotherapy with vasoactive drugs and EBL,esophageal balloon tamponade with a Sengstaken–Blakemore tube or the installation of a dedicated self-expandable,covered esophageal metal stent should be resorted to.Ideally,this should serve as a bridge to rescue PTFE-covered TIPS[48].PTFE-covered TIPS is recommended in LC patients with uncontrolled acute gastroesophageal variceal bleeding at EGDS or who have successfully undergone EBL but who rebleed at any time during admission (after endoscopy).In addition,select LC patients Child-Turcotte-Pugh (CTP) class B or C with active gastroesophageal variceal bleeding at EGDS are at highest risk for rebleeding and may benet from early or pre-emptive PTFE-covered TIPS within 72 h of admission to improve survival[49].It has been shown that among selected advanced LC patients (CTP class B or C) with acute gastroesophageal variceal bleeding,PTFE-covered TIPS is superior to pharmacotherapy with vasoactive drugs plus EBL in improving transplantation-free survival,reducing failure to control bleeding,without increasing the risk of overt hepatic encephalopathy[50].At the same time,TIPS may be useless in LC patients CTP class C with >14 points,or with a MELD score >30 and a lactate level >12 mmol/L,if liver transplantation is not planned in the short term[5].In patients with bleeding from GEV2 and from IGV1 and IGV2 balloon-occluded retrograde transvenous obliteration (BRTO) is possible as an alternative to endoscopic treatment or TIPS,provided that this is feasible (type and diameter of gastrorenal shunts) and there is experience in its use[51].The combined use of TIPS and BRTO is possible both to control acute gastric variceal bleeding and to reduce the risk of their recurrence,particularly in cases when,despite a reduction in HVPG,portal ow remains diverted to gastrorenal shunts[47].
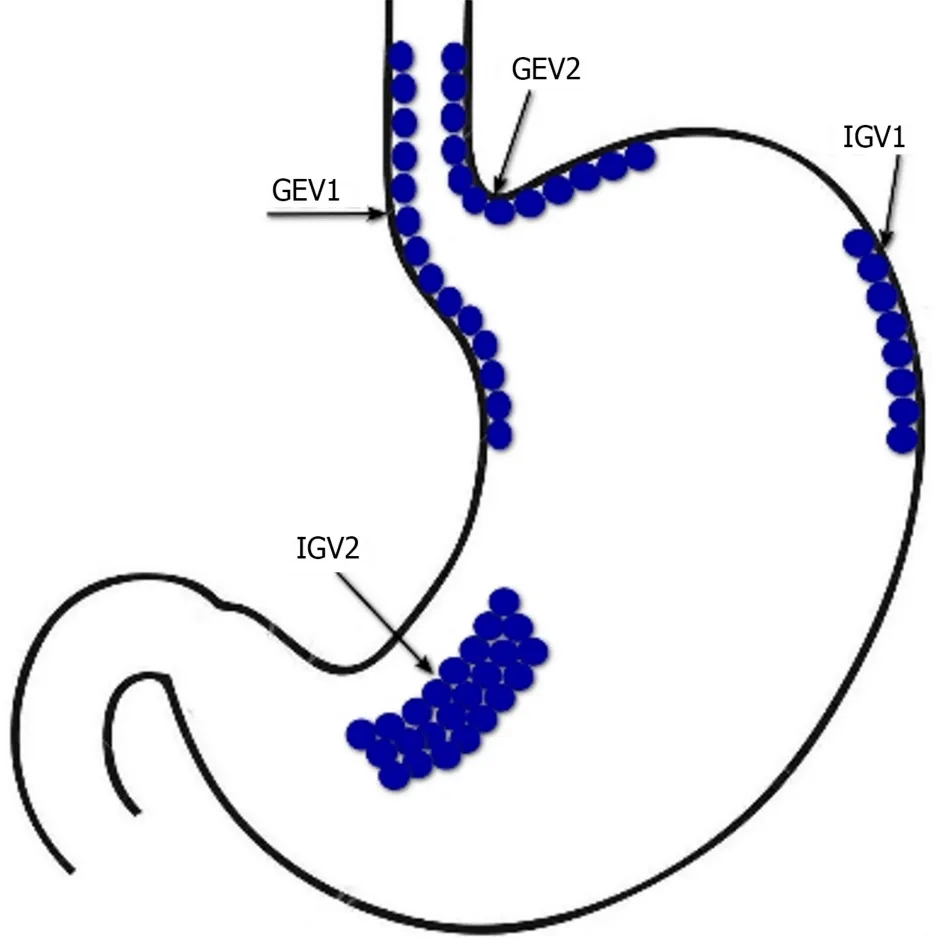
Figure 2 Sarin’s classification of gastric varices. GEV1: Type 1 gastroesophageal varices;GEV2: type 2 gastroesophageal varices;IGV1: Type 1 isolated gastric varices;IGV2: Type 2 isolated gastric varices.
Since the cause of gastroesophageal variceal bleeding is PH,it is obvious that the basis of their treatment should be a reduction in portal pressure,and not correction of blood clotting disorders.Moreover,conventional coagulation screening parameters,for example,prothrombin time/international normalized ratio and activated partial thromboplastin time,reflect the hemostasis state in LC patients is not quite correct[52].Therefore,fresh frozen plasma transfusion is not recommended in gastroesophageal variceal bleeding,since it will not correct coagulopathy and may lead to volume overload and worsening of PH[53].This postulate was,in particular,confirmed in a multicentre cohort study,where fresh frozen plasma transfusion in acute gastroesophageal variceal bleeding was independently associated with poor clinical outcomes[54].There is also no evidence that platelet count and brinogen levels are correlated with the risk of failure to control gastroesophageal variceal bleeding or rebleeding.In addition,the use of recombinant factor VIIa and tranexamic acid are not recommended in gastroesophageal variceal bleeding.At the same time,if pharmacotherapy with vasoactive drugs and/or EBL is ineffective,the decision to eliminate blood clotting disorders should be considered individually[53].
CONCLUSION
PH is the most important event of the natural history of LC,since it can be associated with its first and further decompensation and is responsible for the development of severe,often fatal complications,such as gastroesophageal variceal bleeding.The dynamic character and potential reversibility of LC requires the improvement of invasive and noninvasive methods of its diagnosis,as well as the identification of CSPH.This will allow to objectively and accurately stratify the risk of gastroesophageal variceal bleeding,develop individual strategies for their prevention and management,avoid the first and further decompensation in LC,which will improve the prognosis and survival of patients suffering from it (Figure 3).

Figure 3 Algorithm for preventing and managing gastroesophageal variceal bleeding in liver cirrhosis patients. GEV1: Type 1 gastroesophageal varices;GEV2: Type 2 gastroesophageal varices;IGV1: Type 1 isolated gastric varices;IGV2: Type 2 isolated gastric varices.
FOOTNOTES
Author contributions:Garbuzenko DV contributed to the conception,design,acquisition,analysis,interpretation of data,wrote the manuscript and approved the final version.
Conflict-of-interest statement:Author have no conflict of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Russia
ORCID number:Dmitry Victorovich Garbuzenko 0000-0001-9809-8015.
S-Editor:Liu JH
L-Editor:A
P-Editor:Cai YX
 World Journal of Hepatology2024年2期
World Journal of Hepatology2024年2期
- World Journal of Hepatology的其它文章
- Precision targeting in hepatocellular carcinoma: Exploring ligandreceptor mediated nanotherapy
- Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: A cohort study
- Effects of SARS-CoV-2 infection on incidence and treatment strategies of hepatocellular carcinoma in people with chronic liver disease
- Epidemiological survey of cystic echinococcosis in southwest China: From the Qinghai-Tibet plateau to the area of Yunnan
- Predictors of portal vein thrombosis after splenectomy in patients with cirrhosis
- Evaluation of G3BP1 in the prognosis of acute and acute-on-chronic liver failure after the treatment of artificial liver support system
