Precision targeting in hepatocellular carcinoma: Exploring ligandreceptor mediated nanotherapy
Xia-Qing Zhou,Ya-Ping Li,Shuang-Suo Dang
Abstract Hepatocellular carcinoma (HCC) is the most common primary liver cancer and poses a major challenge to global health due to its high morbidity and mortality.Conventional chemotherapy is usually targeted to patients with intermediate to advanced stages,but it is often ineffective and suffers from problems such as multidrug resistance,rapid drug clearance,nonspecific targeting,high side effects,and low drug accumulation in tumor cells.In response to these limitations,recent advances in nanoparticle-mediated targeted drug delivery technologies have emerged as breakthrough approaches for the treatment of HCC.This review focuses on recent advances in nanoparticle-based targeted drug delivery systems,with special attention to various receptors overexpressed on HCC cells.These receptors are key to enhancing the specificity and efficacy of nanoparticle delivery and represent a new paradigm for actively targeting and combating HCC.We comprehensively summarize the current understanding of these receptors,their role in nanoparticle targeting,and the impact of such targeted therapies on HCC.By gaining a deeper understanding of the receptor-mediated mechanisms of these innovative therapies,more effective and precise treatment of HCC can be achieved.
Key Words: Targeting;Hepatocellular carcinoma;Receptor;Nanomedicine;Chemotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer worldwide and the second leading cause of cancerrelated deaths,claiming approximately 700000 lives each year[1].The 5-year survival rate of HCC in the North American area is 15%-19%,while in China it is only around 12.1%.This high mortality rate is partly due to the aggressive nature of the disease and the fact that most patients are diagnosed at a late stage[2,3].These bring heavy mental pressure and economic burden to the patient's family and society.The incidence of HCC is closely related to chronic viral hepatitis,of which hepatitis B virus (HBV) and hepatitis C virus (HCV) are the main causative factors[4,5].Globally,HBV accounts for 54.4% of liver cancer cases and up to 50% of HCC occurrences.This high rate is attributed to the virus's ability to integrate into the host DNA,causing direct genetic alterations[6].In contrast,HCV-induced HCC usually results from a different mechanism,primarily through chronic inflammation,cirrhosis,and subsequent cellular changes leading to malignancy[7].Patients with HCV-associated cirrhosis are at higher risk of developing HCC compared to HBV[8].Other risk factors for HCC include chronic alcohol abuse,non-alcoholic fatty liver disease (NAFLD),and exposure to aflatoxins[9,10].Aflatoxin B1,produced by Aspergillus fungi and present in contaminated staple foods,is particularly prevalent in certain regions of Africa and Asia,significantly increasing the incidence of HCC in these areas[11].In addition,the rising incidence of obesity and type 2 diabetes has led to an increase in the number of NAFLD-associated HCC cases[12].As a result,innovative therapeutic approaches are urgently needed for the treatment of HCC.
Currently,the therapeutic strategies for HCC are diverse,including options such as liver transplantation,surgical resection,embolization,stereotactic body radiation therapy,ablative procedures,and systemic therapy[13-17].Treatment of HCC is highly dependent on the stage of the disease at diagnosis.Liver transplantation offers the best long-term survival rate for patients with early-stage HCC,with a 5-year survival rate exceeding 70% for suitable patients[18].Surgical resection is another treatment option,and patients with early-stage HCC without cirrhosis have a 5-year survival rate of 50%-70%[19].However,only about 15%-20% of HCC patients are candidates for liver transplantation or surgical resection at the time of diagnosis.Transarterial chemoembolization (TACE) is a widely used treatment for patients with intermediate (stage B) HCC.This approach takes advantage of the unique feature that HCC tumors predominantly receive their blood supply from the hepatic artery[20].By delivering chemotherapeutic agents such as doxorubicin (DOX),or mitomycin C directly to the tumor through the hepatic artery,TACE concentrates the drugs on the tumor while minimizing the impact on surrounding healthy liver tissue[21].Approximately only 10%-15% HCC patients are candidates for TACE,making it an important option for patients with unresectable mid-stage HCC.However,the onset of HCC is insidious and the disease progresses slowly,and most patients are often diagnosed in the late stages,when treatment becomes more challenging and the efficacy of existing therapies is greatly reduced.In the advanced stages of HCC,systemic therapy becomes the primary treatment modality.This includes molecular targeted therapy that specifically targets the molecular pathways which contribute to HCC growth,and immunotherapy that stimulates the body's immune system to attack cancer cells[22].Systemic therapy is preferred for advanced HCC because it is relatively less painful and more cost-effective than other advanced treatments.
In recent years,systemic therapy for HCC has undergone significant evolution with the development of several chemotherapeutic agents.Sorafenib,approved by the United States Food and Drug Administration (FDA) in 2007 as the first systemic treatment for advanced HCC,marked a critical milestone in this journey.As a multikinase inhibitor,Sorafenib disrupts angiogenesis by inhibiting vascular endothelial growth factor receptors (VEGFR) and platelet-derived growth factor receptors (PDGFR) and prevents tumor from obtaining the nutrients and oxygen they need to grow[23].Additionally,Sorafenib blocks the Raf-MEK-ERK signaling pathway by targeting Raf kinases,effectively slowing down cancer cell proliferation.This dual-action mechanism extended the overall survival of HCC patients to about 10.7 months,compared to 7.9 months in the placebo group[24].Following this,Lenvatinib was approved by the FDA in 2018 as a firstline treatment for unresectable HCC,marking another major advancement in HCC treatment.Lenvatinib,inhibiting multiple kinases including VEGFR1-3,fibroblast growth factor receptor1-4,PDGFR,RET,and KIT,demonstrates effective control over tumor proliferation and angiogenesis[25].Clinical trials have shown that Lenvatinib can extend the survival of patients to about 13.6 months.A significant leap was made in 2020 with the FDA approval of the combination therapy of Atezolizumab and Bevacizumab,setting a new standard for first-line treatment in terms of efficacy[26].Atezolizumab,an immunoglobulin G 1 monoclonal antibody,specifically targets and binds to programmed death-ligand 1 (PD-L1),blocking its interaction with the PD-1 receptor and thereby enhancing T-cell activity against cancer cells.Concurrently,Bevacizumab,a humanized monoclonal antibody targeting VEGF,inhibits tumor angiogenesis,further impeding the progression of HCC[27].The progression in second-line treatments for HCC began with the approval of Regorafenib in 2017 and Cabozantinib in 2020.Regorafenib,like Sorafenib but with a broader spectrum of kinase inhibition,notably enhances the antiangiogenic effects through the simultaneous blockade of VEGFR2 and epidermal growth factor homology domain2 pathways[28].Cabozantinib demonstrates significant antitumor activity in HCC,primarily through its dual inhibition of MET and VEGFR2[29].Furthermore,FDA approved Ramucirumab in 2019,an antibody targeted against VEGFR2,providing a specialized option for patients with elevated alpha-fetoprotein levels[30].In addition,nucleic acidbased drugs,including small interfering RNA (siRNA),microRNA,and antisense oligonucleotides,represent another promising area in HCC treatment,particularly in terms of their specificity and p potential to target the molecular basis of the disease[31].For example,In HCC,high levels of Polo-like kinase 1 (PLK1) are associated with aggressive tumor growth and poor prognosis.Researchers have developed siRNA molecules that specifically target and silence thePLK1gene to inhibit the proliferation of HCC cells[32].All of the above advancements provided more targeted and effective options for HCC treatment.
However,despite these advancements,systemic therapy for HCC continues to face significant challenges,including the management of side effects.Sorafenib often causes hand and foot skin reactions in up to 30% of patients.Lenvatinib can lead to hypertension in about 23% of patients,as well as proteinuria and cardiac dysfunction in some patients[33].Immunotherapy also presents unique side effects,including autoimmune reactions such as colitis,hepatitis,dermatitis,and endocrinopathies[34].These side effects result from the nonspecific effects of the chemical drugs that may inadvertently harm healthy cells while killing cancer cells.The uneven drug distribution at the tumor site and the emergence of multi-drug resistance (MDR) further challenge the effectiveness of chemotherapy[35].Consequently,the search for more targeted and effective systemic therapeutical agents remains a key issue that needs to be addressed.
NANOTECHNOLOGY APPLIED IN HCC TREATMENT
Nanomedicine or nanoparticle drug delivery system (NDDS) with particle sizes of 1-1000 nm,offers a revolutionary way to circumvent the side effects associated with traditional systemic therapy.The history of nanomedicine dates back to the late 20thcentury,with the advent of liposomal formulations being one of the earliest applications[36-38].These nanoscale carriers improve the solubility and stability of chemotherapeutic drugs,which often presents a challenge in conventional formulations[39].Moreover,these nanoparticles can deliver therapeutic drugs directly to the tumor site.This targeted approach not only enhances the efficacy by increasing the concentration of drug within the tumor,but also minimizes the impact on surrounding healthy tissues,thereby significantly reducing the adverse side effects typically associated with systemic therapy.In addition,one key mechanism of drug resistance is through the overexpression of efflux pumps,such as P-glycoprotein[40].These pumps are capable of actively transporting chemotherapy drugs out of the cancer cells,significantly reducing the intracellular concentration of these drugs,thereby diminishing their efficacy.However,nanomedicine can bypass these efflux pumps because drugs are encapsulated within nanoparticles that are less likely to be recognized and expelled by these pumps.This property is particularly important for cancers that have become resistant to chemotherapy regimens.Nanomedicine's ability to deliver drugs in a more controlled and precise manner opens new avenues in cancer treatment,offering the potential to significantly improve the efficacy of systemic therapies while simultaneously reducing their side effects[41].This innovative field continues to evolve,with ongoing research and development aimed at further refining and personalizing cancer treatment through advanced nanotechnology.
NDDS has revolutionized the management of HCC at various stages,including surveillance,diagnosis,and treatment[42].Their application in surveillance has notably improved the detection of early-stage HCC,offering higher sensitivity and specificity,which is crucial for timely intervention.On the diagnostic side,contrast-enhanced nanoparticles can improve the clarity and accuracy of imaging modalities such as magnetic resonance imagings and computed tomography scans,resulting in more precise visualization of HCC tumors[43].When it comes to treatment,the unique physiological and biochemical properties of the liver,particularly its dual blood supply from the hepatic artery and the portal vein,are crucial for nanomedicine delivery[44].Specifically,liver tumors typically have abnormal and leaky vasculature,which may enhance the permeability and retention effect,allowing nanoparticles to accumulate more efficiently in tumor tissues than in normal liver tissues[45].In addition,nanomedicines can be designed for active targeting by modifying the surface of nanoparticles with ligands that have a high affinity for receptors overexpressed in liver cancer cells,thereby reducing the impact on healthy liver cells.Another critical aspect of NDDS in HCC treatment is their role in overcoming drug resistance,a common challenge in cancer therapy[46].The liver's complex enzyme system often contributes to this resistance,but nanoparticles can be engineered to circumvent these mechanisms,enhancing the efficacy of drug delivery and reducing the likelihood of resistance development.
The applications of NDDS in HCC systemic treatment are diverse and can be categorized based on their therapeutic function and type.These include targeted therapy,stimuli-responsive therapy,immune-modulating therapy,TACE therapy,nucleic acid-based therapy,and so on.Each category plays a distinct and pivotal role in improving the efficacy of HCC systemic treatment[47,48].Thus,this review paper will primarily focus on targeted therapy,particularly emphasizing ligand-receptor mediated delivery.This approach underscores the crucial role of NDDS in advancing HCC management strategies,highlighting how targeted therapy,through specific ligand-receptor interactions,represents a significant advancement in the precision and effectiveness of HCC treatment.
SURFACE RECEPTOR FOR SPECIFIC TARGETING IN HCC THERAPY
In targeted therapy,the uptake of nanoparticles by HCC cells is facilitated through the interaction between targeting agents on the nanoparticle surface and receptors that are abundantly expressed on the membrane of HCC cells[49].Therefore,a thorough understanding of these surface receptors on HCC cells is essential for the effective design and surface modification of nanoparticles to ensure that they are accurately localized on target cells.Next,we will delve into the key receptors that are characteristically overexpressed on liver cancer cells.We will also discuss their corresponding ligands,which play a key role in targeted therapies for HCC,thus providing a clearer perspective on the strategies employed for receptor-mediated nanotherapies for this complex disease.Figure 1 illustrates a summary of receptors that are overexpressed on hepatoma cells.
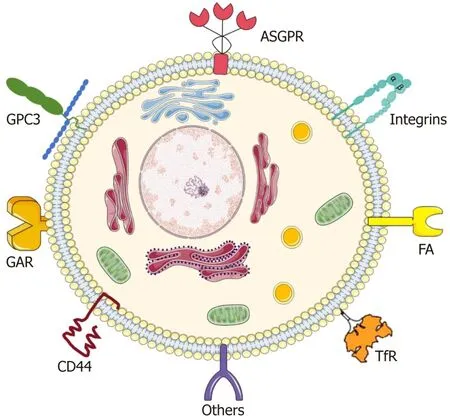
Figure 1 Schematic representation of different types of targeting receptors expressed on hepatocellular carcinoma. ASGPR: Asialoglycoprotein receptor;FA: Folic acid;TfR: Transferrin receptor;GAR: Glycyrrhetinic acid receptors;GPC3: Glypican-3.
Glypican-3
In the area of nano-targeted therapies for HCC,Glypican-3 (GPC3) stands out as a pivotal molecular target.As a heparan sulfate proteoglycan,GPC3 is significantly overexpressed in the cell membrane and cytoplasm of HCC cells,whereas it is conspicuously absent in normal hepatocytes[50].This unique expression pattern makes GPC3 a prime candidate for therapeutic targeting,and a series ofin vitroandin vivostudies have validated this potential.In addition,the presence of GPC3 is strongly associated with advanced HCC stage,higher tumor grade,vascular invasion and poorer patient prognosis[51].Hsuet al[52] discovered that GPC3 mRNA was present in 74.8% of both primary and recurrent HCC cases,in contrast to its mere 3.2% occurrence in normal liver tissues[52].This significant difference highlights GPC3's utility as a biomarker for tumor staging and assessing the aggressiveness of HCC.This is due to the role of GPC3 in promoting HCC growth through the wnt/β-catenin signaling pathway and its potential as a therapeutic target.The pathological process driven by GPC3 in HCC can be demonstrated by the fact that the gene silencing inhibits HCC cell proliferation and induces apoptosis.
Various therapeutic strategies targeting GPC3 have been explored in the treatment of HCC,with a particular focus on anti-GPC3 monoclonal antibodies (mAbs).Among these,GC33 was the first therapeutic mAb developed against GPC3[53].As a humanized mouse antibody,GC33 is known for its high-affinity binding to the C-terminal region of GPC3 and has shown substantial cytotoxic activity against GPC3-positive hepatoma cells.In preclinical studies using xenograft models,GC33 demonstrated a significant ability to reduce tumor size,highlighting its potential as an effective treatment for HCC.In a noteworthy study by Shenet al[54] sorafenib-loaded polymer nanoparticles were modified with the hGC33 antibody[54].These nanoparticles specifically targeted GPC3-positive HepG2 cells,binding to GPC3 on their surface.The treatment was shown to inhibit wnt-induced signal transduction and down-regulate cyclin D1 expression,thereby halting the cell cycle in the G0/1 phase.This led to a reduction in HCC cell migration by inhibiting the epithelial–mesenchymal transition,offering a promising approach to HCC therapy.In addition to GC33,several other mAbs targeting GPC3 are currently being evaluated in various stages of research.These include the human antibodies MDX-1414 and HN3,as well as the humanized mouse antibody YP7.Each of these antibodies offers a unique approach to targeting GPC3,expanding the potential treatment options for HCC.For instance,Hanaokaet al[55] developed YP7-modified albumin-bound paclitaxel nanoparticles[55].This innovative formulation not only induced targeted necrotic cell death,but also enhanced the concentration of paclitaxel within tumors,demonstrating its efficacy in HCC treatment.Table 1 presents various studies that have employed nanotechnology to target GPC-3 in the treatment of HCC[54-58].

Table 1 Summary of nanoformulations utilizing Glypican-3 as a targeting receptor in hepatocellular carcinoma treatment
Asialoglycoprotein receptor
The Asialoglycoprotein receptor (ASGPR),commonly known as the Ashwell-Morell receptor,is predominantly found on the sinusoidal surfaces of hepatocytes and is less common in non-liver cells[59,60].This C-type lectin receptor is chiefly involved in the endocytosis and clearance of glycoproteins from the bloodstream.It binds specifically to glycoproteins that have exposed terminal galactose (GAL) or N-acetylgalactosamine (GalNAc) residues.In HCC,there is an observed increase in ASGPR expression across both early and advanced stages of the disease[61].Utilizing this characteristic,drugs or therapeutic nanoparticles can be effectively conjugated with ligands that precisely target ASGPR.This targeted approach is designed to enhance drug delivery directly to the liver,thereby increasing the concentration of therapeutic agents in the target area while significantly reducing the potential for off-target effects on non-hepatic tissues.
In a recent study,Fariset al[62] developed chitosan nanoparticles,with a size of less than 100 nm,were loaded with simvastatin and modified with Chondroitin sulfate (ChS)[62].ChS,containing GalNAc,has a specific affinity for ASGPR found on hepatocyte membranes.This modification enhanced the cytotoxicity of simvastatin against HepG2 cells,due to its targeted delivery and increased cellular uptake.However,targeting HCC cells presents a unique challenge since both cancerous cells and healthy hepatocytes express ASGPR.Wang’s group tackled this problem by synthesizing nanoparticles conjugated with eight different types of GAL derivatives[63].Their findings revealed that nanoparticles decorated with phenyl β-D-galactoside were particularly effective in delivering drugs to HCC cells,achieving greater specificity compared to normal hepatocytes.To provide a comprehensive overview,Table 2 includes several examples of HCC-targeting ligands that have been modified on nanoparticles for ASGPR-targeted delivery[62,64-69].

Table 2 List of different nanoformulations for Asialoglycoprotein Receptor targeted therapy in hepatocellular carcinoma
Transferrin receptor
The Transferrin receptor (TfR),a membrane glycoprotein,plays a crucial role in cellular iron regulation.When transferrin binds to TfR on the cell surface,the complex is internalized into the cell where the acidic environment of the endosome causes transferrin to release its iron ions[70].There are two primary types of TfR: TfR1 and TfR2,both responsible for mediating cellular iron uptake.TfR1 is ubiquitously expressed and exhibits a significantly higher affinity for transferrin compared to TfR2.In recent years,TfR has gained attention for its notable overexpression in various tumor cells,including HCC.It is particularly pronounced on the surface of several HCC cell lines such as HepG2,J5,Bel-7402,Huh7,and SK-Hep-1.This marked overexpression establishes TfR as a significant target for effective drug delivery strategies in HCC therapy[71].Specifically,research indicates that in human HCC,the mRNA level of TfR1 is upregulated,whereas that of TfR2 is downregulated.This differential expression pattern further highlights the potential of targeting TfR1 in HCC therapy.
Exploiting this trait,Xiaoet al[72] developed innovative transferrin nanovesicles,incorporating Fe3+ions and encapsulating the chemotherapeutic drug sorafenib[72].In bothin vivoandin vitrostudies,SOR@TF-Fe3+NVs demonstrated a preferential accumulation in the liver,specifically targeting HCC cells that overexpress the TfR.This targeted approach not only enhances the therapeutic effectiveness of sorafenib by directing it to the tumor site but also potentially reduces the systemic distribution and associated side effects,highlighting the potential of TfR-targeted therapies in the treatment of HCC.In addition,Malarvizhiet al[73] developed nanoparticles conjugated with human serum transferrin,innovatively incorporating DOX within a poly(vinyl alcohol) nano-core and sorafenib in an albumin nano-shell[73].This design utilized transferrin ligands for targeted delivery,resulting in notably enhanced cellular uptake.The study demonstrated that these transferrin-conjugated nanoparticles achieved synergistic cytotoxicity,effectively inducing cell death in approximately 92% of the targeted cells.This outcome was significantly more efficient compared to the 75% cell death rate observed with nanoparticles that were not modified with transferrin,highlighting the efficacy of transferrinmediated targeting in enhancing the therapeutic impact in HCC treatment.
Folate receptor
In HCC,the rapid proliferation of tumor cells creates an increased demand for essential nutrients and organic compounds,including vital vitamins such as folic acid (FA),biotin,retinoic acid (RA),and dehydroascorbic acid.FA,also known as vitamin B9,vitamin M,and vitamin Bc,is a water-soluble vitamin crucial in eukaryotic cell metabolism[74].It is integral to the biosynthesis of methionine,purine,and pyrimidine,as well as in the interconversion of serine and glycine and histidine catabolism.Animal cells,unable to synthesize FA due to the absence of key enzymes,rely on the uptake of exogenous FA for these vital biosynthetic pathways[75].The FA receptor (FAR),a glycosylphosphatidylinositol-anchored membrane protein,is significantly overexpressed in HCC cells and offers a strategic target for anticancer therapies.FAR mediates the cellular uptake of FA through receptor-mediated endocytosis,a process that efficiently internalizes this essential nutrient.This overexpression of FAR in HCC cells,coupled with the critical role of FA in cellular metabolism,makes FAR a prime target for delivering therapeutic agents.This approach aims to capitalize on the unique metabolic requirements of rapidly proliferating cancer cells,potentially leading to more effective and targeted therapeutic strategies in the treatment of HCC.
3,4-seco-lupane triterpenes show a potent cytotoxic activity against HepG2 cells,however,the poor solubility of the drug has limited its further application.Wanget al[76] formulated FA-conjugated polyethylene glycol albumin nanoparticles which encapsulated lupane triterpenes inside[76].With the help of FA ligand,these nanoparticles showed enhanced toxicity and specific uptake in FAR-positive HepG2 cells,demonstrating their targeted anticancer efficacy.While FA-functionalized drug delivery systems can induce apoptosis in tumor cells,HCC cells often possess various antiapoptotic mechanisms that can hinder the effectiveness of such therapies.To overcome this challenge,down-regulation of anti-apoptotic genes through RNA interference has emerged as a viable strategy to induce cell death in HCC cells.In this context,Xiaet al[77] made a significant contribution by developing selenium nanoparticles loaded with siRNA and linked with FA[77].These nanoparticles,approximately 115 nm in size,demonstrated enhanced cellular uptake and were notably effective in inhibiting the proliferation of HepG2 cells.Furthermore,they were successful in inducing cell cycle arrest at the G0/G1 phase in HepG2 cells.Highlighting the potential of FAR-targeted therapies in the treatment of HCC.Based on the multifunctionality of FA in targeted therapies that can provide dual drug treatment options.Caoet al[78]extended this approach by developing polymeric nanoparticles loaded with both BCL-2 siRNA and DOX and functionalized with FA for targeted delivery[78].This FA-mediated targeting greatly improved the therapeutic efficacy;delivery of BCL-2 siRNAviathese FA-modified nanoparticles produced more pronounced gene silencing,as evidenced by a dramatic reduction in BCL-2 mRNA and protein expression levels.This targeted delivery mechanism not only induces apoptosis of cancer cells more effectively,but also extends the therapeutic effect of co-delivered DOX.The study demonstrates the potential of using FA as a ligand in multidrug nanoparticle systems,providing a more targeted and effective approach to the treatment of cancer,especially in terms of enhanced gene suppression and drug synergy.
Integrins
Integrins,a class of heterodimeric transmembrane glycoproteins,play an important role in regulating various cellular functions,including adhesion,migration,invasion,proliferation,and apoptosis[79].In contrast to normal cells,certain integrins are often overexpressed or aberrantly activated in HCC cells,which is associated with aggressive behavior of cancer cells,including proliferation,invasion,and metastasis.The Arginine-Glycine-Asparagine (RGD) tripeptide is crucial in these cellular interactions,particularly in its specific targeting of integrins[80].RGD peptides have a high affinity for integrin receptors,a feature that is strategically utilized in the design of targeted nanoparticles for HCC treatment.This targeted approach ensures that the nanoparticles,often carrying therapeutic agents,are more likely to adhere to and be absorbed by HCC cells rather than normal cells,thereby enhancing the efficacy and specificity of the treatment directed against these cancer cells.Recently,Wuet al[81] prepared a RGD-modified polydopamine-paclitaxelloaded nanoparticles[81].These nanoparticles are uniquely designed to target HCC cells by specifically recognizing and binding to αvβ3/αvβ5 integrins,which are often overexpressed in HCC cells.Liet al[82] engineered another novel therapeutic approach by developing gold nanoparticles coated with polydopamine and conjugated with RGD peptides[82].The conjugation with RGD peptides enabled the nanoparticles to target integrin αvβ3-overexpressing HepG2 cells specifically.These receptor-mediated targeting led to an enhanced uptake of the nanoparticles by the cancer cells,resulting in increased cytotoxicity compared to non-targeted treatment approaches.
Cancer stem cell biomarker
Recent studies have underscored the critical role of cancer stem cells (CSCs) in HCC,particularly their capacity to initiate tumors and drive recurrence and metastasis[83].These CSCs,a distinct subpopulation within the tumor,are notably resistant to conventional chemotherapies,highlighting the need for targeted therapeutic strategies.Among the notable CSC biomarkers in HCC,Cluster of Differentiation 44 (CD44) has been identified as a key player.This transmembrane glycoprotein,primarily a receptor for hyaluronic acid (HA),also interacts with osteopontin,collagens,and matrix metalloproteinases[84].Nanoparticles can be specifically designed to target CD44,utilizing ligands that bind to this receptor.Cannitoet al’s group has prepared HA and PEGylated liposomes as promising approaches for the treatment of HCC[85].In cell culture experiments,HA-liposomes demonstrated enhanced internalization in Huh7 cells that overexpress CD44 compared to HepG2 cells with lower receptor expression,indicating CD44's potential as a target for nanoparticle-based therapies.
Besides CD44,several other markers have been identified for liver CSC,including CD133,CD90,OV6,and epithelial cell adhesion molecule (EpCAM).CD133,in particular,stands out as one of the most important surface markers for liver CSCs.Jinet al[86] have contributed to this field by developing paclitaxel-loaded PLGA nanoparticles decorated with anti-CD133 antibodies[86].These targeted nanoparticles showed a substantial improvement in therapeutic response by selectively eliminating the CD133 positive subpopulation in bothin vitroandin vivoexperiments.Another breakthrough came from Yamashitaet al[87] who identified EpCAM-positive cancer cell subpopulations in HCC.These cells have the ability to self-renew,initiate tumors,and form distant metastases,etc.EpCAM overexpression in HCC is associated with a poor prognosis,and thus it has been positioned as a potential risk stratification biomarker.Utilizing EpCAM-specific antibodies,researchers have developed modified nanoparticles for effectively treating malignant tumors in HCC patients with EpCAM positive carcinomas.For example,Zhanget al[88] prepared magnetic nanoliposomes targeting EpCAM capable of encapsulating Lenvatinib[88].This nanoparticle showed significant efficacy in inhibiting HCC cell proliferation and promoting apoptosis,as well as specific targeting and magnetic resonance imaging tracking of HCC cells.
Glycyrrhetinic acid receptor
Glycyrrhizic acid (GA),a GA derivative extracted from licorice root,has attracted great interest in the field of HCC therapy.GA has been reported to inhibit cancer cell proliferation,invasion,and metastasis,and induce cell cycle arrest,autophagy,and apoptosis[89,90].Recent advances in nanoparticle technology have witnessed the development of GAmodified drug delivery systems.These nano-delivery systems have shown good hepatocyte and liver targeting efficiency bothin vitroandin vivo.The efficacy of GA in targeting HCC cells is primarily due to its ability to bind to Glycyrrhetinic acid receptors (GAR) present on the surface of these cells.Furthermore,the prevalence of GAR is reportedly higher in tumor tissues compared to normal tissues[91].This differential expression makes GA an optimal ligand for targeted drug delivery in HCC.
For instance,Lvet al’s group developed GA-modified mesoporous silica nanoparticles (MSN) containing Curcumin[92].These nanoparticles not only exhibited satisfactory loading capacity but also increased drug uptake by GA receptorpositive cells.In vitro experiments revealed a significant increase in apoptotic cells treated with MSN/Curcumin/GA,indicating the efficacy of GA-functionalized nanoparticles in inducing apoptosis in HepG2 cells.Similarly,Tianet al[93]prepared liver-targeted DOX delivery using GA-modified chitosan/PEG nanoparticles.These nanoparticles exhibited significant liver-targeting and retention,and the accumulation in the liver was 2.6 times higher than that of non-GAmodified nanoparticles.Furthermore,the DOX-loaded chitosan/PEG–GA nanoparticles effectively inhibited tumor growth in H22 cell-bearing mice,showcasing the potential of GA in enhancing the therapeutic efficacy of nanoparticlebased drug delivery systems in HCC treatment.In conclusion,the incorporation of GA into nanoparticle formulations represents a major advancement in targeted therapy for HCC,which leverages the unique properties of GA to improve drug delivery and therapeutic efficacy.
Other receptors in HCC nanotherapy
In the evolving development of nanotherapies for HCC,several receptors beyond the previously discussed ones are being targeted for more effective treatments.Notably,the epidermal growth factor receptor (EGFR) plays a crucial role in HCC.Often overexpressed in HCC,EGFR is linked to accelerated tumor growth and poor prognosis[94].Targeting EGFR with nanoparticles,such as the adriamycin-loaded polymer-lipid hybrid nanoparticles developed by Gaoet al[95] conjugated with EGFR-specific antibodies,demonstrates enhanced targeting and cytotoxicity against EGFR-expressing HCC cells[95].
Another important target is the low-density lipoprotein receptor (LDLR),which shows increased expression in HCC compared to adjacent liver tissue[96].Utilizing the natural affinity of the major cholesterol transporter,LDL,for the LDLR,nanoparticles can be designed to mimic or conjugate with LDL particles.This approach is employed to deliver therapeutic agents directly to HCC cells,capitalizing on their increased demand for cholesterol.The Wanget al’s group utilized Apolipoprotein B-100,recognized by LDLR,to modify lipid nanoparticles[97].These nanoparticles exhibited higher cellular internalization and tumor targeting in LDLR-overexpressing liver cancers.
c-Met,the receptor for hepatocyte growth factor,is another significant target in HCC.It contributes to cell proliferation,survival,migration,and invasion[98].Nanoparticles carrying c-Met inhibitors,such as crizotinib or cabozantinib,have been developed for targeting HCC cells.These nanoparticles can be functionalized to bind specifically to c-Met,allowing for targeted delivery and disruption of c-Met signaling pathways.
Furthermore,C-X-C chemokine receptor type 4 (CXCR4) plays a multifaceted role in HCC progression,including promoting angiogenesis and tumor cell evasion of immune surveillance.The Chen group developed nanoparticles where the CXCR4 antagonist AMD3100 serves a dual function;it is encapsulated within the nanoparticles and also modifies their surface[99].This innovative design allows AMD3100 to act both as an intracellular delivery agent for siRNA targeting malignant HCC cells and as a CXCR4 blocker,enhancing its anti-cancer efficacy.
In summary,these advancements in targeting EGFR,LDLR,c-Met,and CXCR4 through nanoparticle technology represent significant strides in the personalized treatment of HCC.By exploiting the unique molecular characteristics of HCC cells,these targeted therapies offer the potential for more effective and less toxic treatments.
Multiple receptors: Dual-targeting
Dual-ligand nanoparticle modification is an advanced strategy for the treatment of HCC that enhances targeting and specificity by simultaneously binding to multiple receptors or pathways on cancer cells.This approach enables more precise targeting of HCC cells and ensures better cellular uptake and internalization of the drug,thereby improving drug efficacy and specificity.In addition to this,dual-ligand nanoparticles can provide synergistic therapeutic agents that produce enhanced effects and offer diverse therapeutic strategies by combining different therapeutic modalities.Table 3 lists some successful examples of dual-ligand modified nanoparticles targeting multiple HCC receptors[100-105].

Table 3 Summary of dual-targeted nanoformulations in hepatocellular carcinoma therapy
LIMITATIONS OF NANOTECHNOLOGY IN THE TREATMENT OF HCC
While numerous studies have demonstrated the effectiveness of targeted ligand-modified nanoparticles in enhancing the anticancer properties of drugs for HCC treatment,the advancement of nanotechnology in this field encounters several complex challenges[106-108].Achieving precise targeted drug delivery is a major challenge,which includes not only the precise localization of nanoparticles within a specific body region,but also the control of their release and dosage.This precision is essential to maximize efficacy and minimize adverse effects.Another major challenge is the body's immune response and potential rejection of these nanocarriers.The immune system usually recognizes these nanoparticles as foreign entities,leading to reduced efficacy or adverse immune reactions.Successful application of nanomedicines for the treatment of HCC requires various strategies to evade immune detection and minimize immunogenicity.There are technical difficulties in synthesizing nanoparticles with uniform and predictable properties in a controlled,rapid and reproducible manner.This challenge also includes ensuring precise manufacturing processes for the systematic screening and characterization of nanoparticles,which is critical for maintaining consistency of efficacy.Scaling up production for mass market availability while ensuring quality,performance and biocompatibility is another hurdle.Ensuring costeffectiveness is key to making these advanced treatments economically viable and widely available for clinical use.Regulatory and ethical considerations are also central to the development and application of nanomedicines.These include stringent regulation of safety and efficacy,ethical considerations such as patient privacy,and understanding the long-term impact of nanomaterials on human health and the environment.Addressing these multifaceted challenges requires a multidisciplinary approach that encompasses the fields of materials science,medicine,pharmacology,engineering,and ethics.Collaboration between these disciplines is critical to refining nanoparticle design,improving their therapeutic applications in HCC,and transitioning these advanced technologies from the laboratory to the clinical setting.
CONCLUSION
HCC is one of the most challenging malignant tumors,characterized by its complex nature and increasing morbidity and mortality.Utilizing the unique advantages of nanotechnology to improve the efficacy of HCC heralds a new era of precision medicine.In this review,we delved into the application of nanomedicine in HCC,with special emphasis on the role of ligand-receptor interactions in improving treatment specificity and efficacy.We investigated a series of receptors that are critical to the pathophysiology of HCC,including GPC3,ASGPR,FAR,TfR,Integrins,GAR and several CSC receptors.Targeted therapies developed to interact with these receptors demonstrate how nanomedicines can be tailored to address the various complexities of HCC.These therapies are expected to not only improve efficacy but also reduce side effects compared to conventional therapies.Of particular note are dual-ligand modified nanoparticles.By targeting multiple receptors or pathways simultaneously,these nanoparticles provide a multifaceted approach to combating HCC,a strategy that is critical to addressing challenges such as MDR and enhanced targeting.However,the process of movingfrom laboratory research to clinical application remains fraught with challenges,including ensuring the precision of targeted delivery in the human body,mitigating immune responses,enabling controlled and reproducible nanoparticle synthesis,scaling up production,and addressing cost-effectiveness issues.In addition,regulatory pathways and addressing ethical issues are critical steps in bringing these innovations to patients.As we make progress in developing and refining these targeted therapeutic strategies,the future looks bright for dramatically improving HCC treatment outcomes.However,this will require continued collaboration across multiple scientific and medical disciplines to realize the full potential of nanotechnology in the fight against HCC.
FOOTNOTES
Author contributions:Zhou XQ and Li YP collected and analyzed the information and wrote the manuscript;Dang SS reviewed and edited the manuscript;All authors have given approval to the final version of the manuscript.
Supported byXi'an Jiaotong University Medical "Basic-Clinical" Integration Innovation Project,No.YXJLRH2 022067;and Shaanxi Postdoctoral Research Program “Orlistat-loaded Nanoparticles as A Targeted Therapeutical Strategy for The Enhanced Treatment of Liver Cancer”,No.2023BSHYDZZ09.
Conflict-of-interest statement:The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Xia-Qing Zhou 0009-0009-7562-8832;Ya-Ping Li 0000-0002-0900-5559;Shuang-Suo Dang 0000-0002-8451-1072.
S-Editor:Fan JR
L-Editor:A
P-Editor:Zhang YL
 World Journal of Hepatology2024年2期
World Journal of Hepatology2024年2期
- World Journal of Hepatology的其它文章
- Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients
- Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: A cohort study
- Effects of SARS-CoV-2 infection on incidence and treatment strategies of hepatocellular carcinoma in people with chronic liver disease
- Epidemiological survey of cystic echinococcosis in southwest China: From the Qinghai-Tibet plateau to the area of Yunnan
- Predictors of portal vein thrombosis after splenectomy in patients with cirrhosis
- Evaluation of G3BP1 in the prognosis of acute and acute-on-chronic liver failure after the treatment of artificial liver support system
