Effects of SARS-CoV-2 infection on incidence and treatment strategies of hepatocellular carcinoma in people with chronic liver disease
Lung-Yi Mak,Matthew Shing Hin Chung,Xue Li,Francisco Tsz Tsun Lai,Eric Yuk Fai Wan,Celine Sze Ling Chui,Franco Wing Tak Cheng,Esther Wai Yin Chan,Ching Lung Cheung,Ivan Chi Ho Au,Xi Xiong,Wai-Kay Seto,Man-Fung Yuen,Carlos King Ho Wong,Ian Chi Kei Wong
Abstract BACKGROUND Chronic liver disease (CLD) was associated with adverse clinical outcomes among people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.AIM To determine the effects of SARS-CoV-2 infection on the incidence and treatment strategy of hepatocellular carcinoma (HCC) among patients with CLD.METHODS A retrospective,territory-wide cohort of CLD patients was identified from an electronic health database in Hong Kong.Patients with confirmed SARS-CoV-2 infection [coronavirus disease 2019 (COVID-19)+CLD] between January 1,2020 and October 25,2022 were identified and matched 1:1 by propensity-score with those without(COVID-19-CLD).Each patient was followed up until death,outcome event,or November 15,2022.Primary outcome was incidence of HCC.Secondary outcomes included all-cause mortality,adverse hepatic outcomes,and different treatment strategies to HCC (curative,non-curative treatment,and palliative care).Analyses were further stratified by acute (within 20 d) and post-acute (21 d or beyond) phases of SARS-CoV-2 infection.Incidence rate ratios (IRRs) were estimated by Poisson regression models.RESULTS Of 193589 CLD patients (>95% non-cirrhotic) in the cohort,55163 patients with COVID-19+CLD and 55163 patients with COVID-19-CLD were included after 1:1 propensity-score matching.Upon 249-d median follow-up,COVID-19+CLD was not associated with increased risk of incident HCC (IRR: 1.19,95%CI: 0.99-1.42,P=0.06),but higher risks of receiving palliative care for HCC (IRR: 1.60,95%CI: 1.46-1.75,P <0.001),compared to COVID-19-CLD.In both acute and post-acute phases of infection,COVID-19+CLD were associated with increased risks of allcause mortality (acute: IRR: 7.06,95%CI: 5.78-8.63,P <0.001;post-acute: IRR: 1.24,95%CI: 1.14-1.36,P <0.001) and adverse hepatic outcomes (acute: IRR: 1.98,95%CI: 1.79-2.18,P <0.001;post-acute: IRR: 1.24,95%CI: 1.13-1.35,P <0.001),compared to COVID-19-CLD.CONCLUSION Although CLD patients with SARS-CoV-2 infection were not associated with increased risk of HCC,they were more likely to receive palliative treatment than those without.The detrimental effects of SARS-CoV-2 infection persisted in post-acute phase.
Key Words: SARS-CoV-2 infection;Chronic liver disease;Long COVID;Post-COVID-19 syndrome;Cirrhosis;Hepatocellular carcinoma
INTRODUCTION
In the year 2020,severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection led to coronavirus disease 2019(COVID-19) pandemic across the globe.The disruption to the routines due to social distancing measures has affected all sectors of the society.Healthcare systems were particularly stretched by the enormous influx of SARS-CoV-2 infected patients,inevitably leading to change in clinical practice such as adoption of virtual consultations,suspension of healthcare services including cancellation of planned investigations,procedures and treatments.Colorectal cancer and lung cancer are examples of chronic conditions that were negatively influenced by the pandemic,with significant delays in screening,diagnosis and workup.In subjects with chronic liver disease (CLD),SARS-CoV-2 infection has been associated with an increased risk of short-term mortality,predominantly caused by respiratory failure and observed in cirrhotic patients[1-4].In comparison,it remains controversial whether SARS-CoV-2 infection increases the risk of nonrespiratory causes of death among non-cirrhotic CLD,a condition that affects 1.5 billion persons globally[5,6].There is no data regarding how COVID-19 interplay with the risk of hepatocellular carcinoma (HCC) and subsequent treatment strategy,which not only depends on the general performance status of the subject,the liver reserve,and the tumor status[7],but also the relative resource allocation within the health care system in the event of the pandemic.
Much is unknown regarding precisely how COVID-19 affects prognosis and liver outcomes in CLD.In particular,the detrimental effects of SARS-CoV-2 infection seem to linger beyond the acute phase of infection and are associated with a number of conditions,collectively termed ‘post-acute sequelae of SARS-CoV-2 infection’ (PASC),also known as ‘long COVID’ or ‘post-COVID-19 syndrome’[8,9].Among the numerous conditions associated with PASC (e.g.,pulmonary,neuropsychiatric,gastrointestinal,endocrine,renal,etc.),hepatic effects of recent SARS-CoV-2 infection have not been well-characterized[10].In addition,it was hypothesized that SARS-CoV-2 infection will lead to long-lasting impacts on the quality of cirrhosis care,resulting from the initial intense period of prioritization of healthcare services with delays in routine care,and subsequent return of backlog presentations of illness and protracted period of suboptimal outcomes[11].Therefore,it is important to understand the immediate and long-term consequences of SARS-CoV-2 infection in patients with CLD,and how they affect the incidence and oncological treatment for HCC.
In this study,we determined the risk of incident HCC,all-cause mortality,adverse hepatic outcomes,and the impact on treatment strategies for patients with liver cancer in a large cohort of patients with CLD and laboratory proven SARSCoV-2 infection,in comparison to a contemporaneous cohort of patients with CLD who did not have SARS-CoV-2 infection in Hong Kong.
MATERIALS AND METHODS
Data source and study population
Our data were extracted from territory-wide cohort of patients with anonymized electronic health records provided by the Hong Kong Hospital Authority (HA),and COVID-19 vaccination records were available from the Department of Health (DH),The Government of Hong Kong Special Administrative Region.Electronic medical records of patients with COVID-19 were retrieved from the HA,and included demographics,disease diagnoses,drug prescriptions,laboratory tests,hospital admissions,emergency departments,and inpatient procedures.The HA data were linked to the COVID-19 vaccination records provided by the DH using the unique identification numbers.This linked database has been used extensively for studies on COVID-19 vaccine safety[12-14] and PASC[15].
This study included patients with CLD between January 1,2020 and November 15,2022.CLD was defined as patients having any of the following diagnoses: (1) Viral hepatitis B (HBV) infection;(2) viral hepatitis C;(3) chronic hepatitis;(4)fatty liver disease;(5) alcoholic liver disease (ALD);(6) alcoholic hepatitis;(7) Wilson’s disease,(8) autoimmune hepatitis;and (9) primary biliary cholangitis and primary sclerosing cholangitis.Each of the above diseases was identified by International Classification of Disease,9th Revision,Clinical Modification (ICD-9-CM) diagnosis code,prescription of hepatitis antivirals,or positive hepatitis B surface antigen test (Supplementary Table 1).Patients who were identified by a positive result on the SARS-CoV-2 reverse transcription polymerase chain reaction test or rapid antigen test during the observational period were classified into the COVID-19+CLD group.The index date was set at the first date of SARSCoV-2 infection for patients in the COVID-19 group (i.e.only the first infection was eligible for analysis).Patients who did not have confirmed SARS-CoV-2 infection during the observational period were classified into the control group,i.e.COVID-19-CLD.The pseudo-index date of COVID-19-CLD patients was set at the first date of the respective year (i.e.,January 1,2020,January 1,2021,or January 1,2022) to maintain a similar follow-up period between the COVID-19+CLD and COVID-19-CLD groups.Patients in the COVID-19-CLD group were matched 1:1 by propensity-score with patients in the COVID-19+CLD group of each index year sequentially starting from 2020 until 2022,and without replacement.Unmatched control patients were eligible for matching with COVID-19+CLD patients in the subsequent index year,with the baseline characteristics of COVID-19-CLD groups updated using the new pseudo-index date (i.e.January 1 of the following year).Each patient was observed from the index or pseudo-index date to the occurrence of outcomes,death,or the end of observational period (i.e.,November 15,2022),whichever occurred earlier.

Table 1 Baseline characteristics of chronic liver disease patients with coronavirus disease 2019 and chronic liver disease patients without coronavirus disease 2019 (matched controls) after 1:1 propensity score matching
Patients who died on or before the index date,or had less than 21 d of follow-up (i.e.,patients with COVID-19 diagnosed on or after October 26,2022) were further excluded[16].
Outcomes definition
The primary study outcome was HCC incidence.The secondary outcomes included: (1) All-cause mortality;(2) adverse hepatic outcomes cirrhosis,HCC,liver decompensation (composite outcome including hepatorenal syndrome,liver failure,hepatic coma/encephalopathy,ascites,and variceal bleeding);(3) curative treatment to HCC (hepatic resection,liver transplantation,radiofrequency ablation of liver);(4) non-curative treatment to HCC (transarterial chemoembolization,radiotherapy to liver,systemic chemotherapy or immunotherapy);and (5) palliative care.
Each of the above outcomes was identified by ICD-9-CM diagnosis and procedure code,prescription of antivirals for hepatitis,and fibrosis-4 index (FIB-4)[17] (Supplementary Table 1).
Acute and post-acute phases of SARS-CoV-2 infection
The risks of study outcomes during the acute and post-acute phase of SARS-CoV-2 infection were further stratified and analyzed among those with index date at 2022 amid the Omicron predominance period.The acute phase of infection was defined as the first 20 d after the index date[18] and the post-acute phase of infection was defined from 21 d of the index date onwards.For the analysis of the post-acute phase of infection when the index date was set to 21 d after COVID-19 diagnosis,patients who died within 20 d of the index date,or had less than 21 d of follow-up were excluded.
Definition of covariates
Baseline characteristics were captured based on ICD-9-CM diagnosis,procedure codes,and treatment records as follows:age,sex,pre-existing comorbidities [Charlson Comorbidity Index (CCI),cirrhosis,HCC,liver decompensation],oncological treatment received prior to the index date (curative treatment to HCC,non-curative treatment to HCC,palliative care),and COVID-19 vaccination status (Supplementary Table 1).The FIB-4[17] was also used to enhance case identification for cirrhosis.Fully vaccinated patients were defined as those with at least two doses of BNT162b2(Comirnaty) or three doses of COVID-19 Vaccine (Vero Cell),Inactivated (CoronaVac)[19].
Statistical analysis
Descriptive statistics of baseline characteristics between the COVID-19 groups and matched control groups were presented as mean and standard deviation,or median and interquartile range (IQR) for continuous variables,and count and proportion for categorical variables.
We constructed propensity-score models conditional on age,sex,CCI,and COVID-19 vaccination status in a logistic regression model.We performed 1:1 propensity-score matching using a caliper width of 0.05.Standardized mean differences (SMDs) of each covariate between the groups after propensity-score matching were calculated,which was interpreted as balanced when the SMD was below the threshold of 0.1[20].The incidence rate ratio (IRR) and corresponding 95% confidence intervals (CIs) were estimated using the Poisson regression model.
Subgroup analyses were performed on several patient groups,including age groups (≤ 50vs>50 years),causes of CLD(HBVvsother causes),the presence of cirrhosis,the presence of multiorgan dysfunction,COVID-19 vaccination status(fully vaccinatedvsnot fully vaccinated),respective years of COVID-19 diagnosis (year of 2020vs2021vs2022).Multiorgan dysfunction was defined as patients having any 3 or more organ system malfunctions in the following categories,including: (1) Neurological;(2) psychiatric;(3) respiratory;(4) cardiovascular;(5) hematologic;(6) endocrine;(7) nephrological;(8) hepatic;(9) gastrointestinal;and (10) dermatologic disorder.Each subgroup analysis was reconstructed with a new propensity-score model,and the pairs of patients with COVID-19 and their respective controls were rematched.Furthermore,subgroup analyses among COVID-19+CLD patients were performed on two patient groups,including hospitalization groups (hospitalizedvsnon-hospitalized) and receipt of antiviral medications for COVID-19 infection.COVID-19+CLD patients were identified as antiviral users if they received any of the following antiviral medications,including: (1) Molnupiravir;(2) nirmatrelvir/ritonavir;and (3) remdesivir.Each subgroup analysis among COVID-19+CLD patients was also re-constructed with a new propensity-score model,and rematched between hospitalized and non-hospitalized patients or antiviral users and non-users,respectively.
All statistical analyses were performed using Stata (version 17).The analyses were conducted by Chung MSH and analyzed independently by Xi X and Au ICH for quality assurance.All significance tests were two-tailed,wherePvalues<0.05 were considered statistically significant.
Role of the funding source
This work was supported by a research grant from Collaborative Research Fund,University Grants Committee,HKSAR Government (principal investigator,Wong ICK;reference no.C7154-20GF).Lai FTT,Wong CKH,and Wong ICK are partially supported by the Laboratory of Data Discovery for Health (D24H) funded by AIR@InnoHK administered by Innovation and Technology Commission.The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
RESULTS
Baseline characteristics
A total of 193589 CLD patients of whom 57323 patients had confirmed SARS-CoV-2 infection between January 1,2020 and November 15,2022 in Hong Kong,and 136266 CLD patients without SARS-CoV-2 infection were identified(Figure 1).After applying the exclusion criteria followed by 1:1 propensity-score matching,55163 patients with COVID-19 and CLD (COVID-19+CLD) and 55163 matched controls (COVID-19-CLD) were included in the present study.The baseline characteristics are presented in Table 1.Baseline age (58.8vs58.7),gender (male gender: 51.2%vs51.6%),medical comorbidities (CCI: 3.2vs3.1),and COVID-19 vaccination status (fully vaccinated: 50.1%vs50.0%) were balanced between the two groups.Additionally,underlying cirrhosis (3.4%vs3.1%),decompensated liver disease (2.8%vs2.6%),HCC (2.2%vs2.2%),and previous treatment for HCC (curative: 2.0%vs1.9%;non-curative: 4.3%vs3.9%;palliative care:3.3%vs3.0%) were matched between COVID-19 patients with CLD and controls with CLD (all SMD <0.1).Of note,the majority (95.0%) of included subjects came from year 2022 when the omicron strain of SARS-CoV-2 was ubiquitous.

Figure 1 Study flowchart of eligible chronic liver diseases patients with and without confirmed coronavirus disease 2019 diagnosis for analysis. CLD: Chronic liver disease;COVID-19: Coronavirus disease 2019.
Incidence rates of clinical sequelae
The median follow-up duration was 249 (IQR: 108-259) days in the COVID-19+CLD group and 318 (IQR: 318-318) days in the COVID-19-CLD group.The crude incidence rates of HCC were 64.2 and 54.0 events per 10000 person-years for COVID-19+CLD and COVID-19-CLD,respectively.There was a trend for increased risk of HCC among COVID-19+CLD group compared to COVID-19-CLD group (IRR: 1.19,95%CI: 0.99-1.42,P=0.06) but did not reach statistical significance.There were 2273 and 1600 events of all-cause mortality,for COVID-19+CLD group and COVID-19-CLD group,respectively.The crude incidence rates of all-cause mortality were 676.5 (95%CI: 648.9-704.9) events per 10000 personyears (2273 events/33601 person-years) for COVID-19+CLD group,and 306.2 (95%CI: 291.4-321.6) events per 10000 person-years (1600 events/52258 person-years) for COVID-19-CLD group.The cumulative incidence of HCC,all-cause mortality,adverse hepatic outcomes,and palliative care among COVID-19+CLD patients and COVID-19-CLD are shown in Figure 2.COVID-19+CLD patients were associated with significantly higher risks of all-cause mortality (IRR: 2.21,95%CI: 2.07-2.36,P<0.001),adverse hepatic outcomes (IRR: 1.74,95%CI: 1.64-1.85,P<0.001),which were predominantly contributed by incident cirrhosis (IRR: 1.79,95%CI: 1.68-1.89,P<0.001),followed by liver decompensation (IRR: 1.36,95%CI: 1.17-1.57,P<0.001),compared to the COVID-19-CLD (Table 2).

Figure 2 The cumulative incidence of study outcomes among chronic liver disease patients with and without severe acute respiratory syndrome coronavirus 2 infection. A: Hepatocellular carcinoma;B: All-cause mortality;C: Adverse hepatic outcomes;D: Palliative care.HCC: Hepatocellular carcinoma;COVID-19: Coronavirus disease 2019.

Table 2 Incidence rates of clinical sequelae of chronic liver disease patients with coronavirus disease 2019 diagnosis in 2020-2022 and controls after 1:1 propensity score matching
Among patients with CLD,there were no significant differences in the risks of receiving curative (IRR: 1.16,95%CI:0.93-1.46,P=0.20) or non-curative (IRR: 0.98,95%CI: 0.86-1.11,P=0.70) treatment to HCC.COVID-19+CLD patients were at higher chance of receiving palliative care (IRR: 1.60,95%CI: 1.46-1.75,P<0.001) compared to COVID-19-CLD patients (Table 2).
Incidence rates of clinical sequelae according to the phase of infection
During the acute phase of infection,patients with CLD who had confirmed SARS-CoV-2 infection in 2022 were associated with significantly higher risks of HCC (IRR: 1.89,95%CI: 1.03-3.47,P=0.04) and all-cause mortality (IRR: 7.06,95%CI:5.78-8.63,P<0.001).The risks of adverse hepatic outcomes were increased (IRR: 1.98,95%CI: 1.79-2.18,P<0.001),not only contributed by an increased risk of HCC,but also cirrhosis (IRR: 1.88,95%CI: 1.71-2.06,P<0.001) and liver decompensation (IRR: 2.85,95%CI: 1.77-4.58,P<0.001).There were no significant differences in the incidence of receiving curative treatment (IRR: 0.57,95%CI: 0.25-1.28,P=0.17) or non-curative treatment (IRR: 1.24,95%CI: 0.77-2.01,P=0.38),but a significantly higher chance of receiving palliative care (IRR: 4.46,95%CI: 3.28-6.06,P<0.001) for the COVID-19 patients,compared to the controls (Table 3).
In the post-acute phase of infection,CLD patients with SARS-CoV-2 infection were still associated with significantly higher risks of HCC (IRR: 1.24,95%CI: 1.00-1.53,P=0.05),all-cause mortality (IRR: 1.24,95%CI: 1.14-1.36,P<0.001) and adverse hepatic outcomes (IRR: 1.24,95%CI: 1.13-1.35,P<0.001),but the risk ratios were numerically diminished compared to the acute phase.Risk of incident cirrhosis (IRR: 1.28,95%CI: 1.17-1.39,P<0.001) and liver decompensation(IRR: 1.26,95%CI: 1.05-1.52,P=0.01) in CLD patients with COVID-19 were maintained compared to controls.There were no significant differences in the incidence of receiving curative treatment (IRR: 1.20,95%CI: 0.92-1.57,P=0.18),noncurative treatment (IRR: 1.02,95%CI: 0.88-1.18,P=0.82),and palliative care (IRR: 1.10,95%CI: 0.98-1.24,P=0.11) for HCC(Table 3).Figure 3 shows the cumulative incidence of HCC,all-cause mortality,adverse hepatic outcomes,and palliative care in the acute and post-acute phases of SARS-CoV-2 infection and Figure 4 shows the proportion of treatment modalities of HCC stratified by the presence and phase of SARS-CoV-2 infection.

Figure 3 The cumulative incidence of study outcomes in the acute phase vs post-acute phase of infection. A: Hepatocellular carcinoma;B: Allcause mortality;C: Adverse hepatic outcomes;D: Palliative care.HCC: Hepatocellular carcinoma;COVID-19: Coronavirus disease 2019.
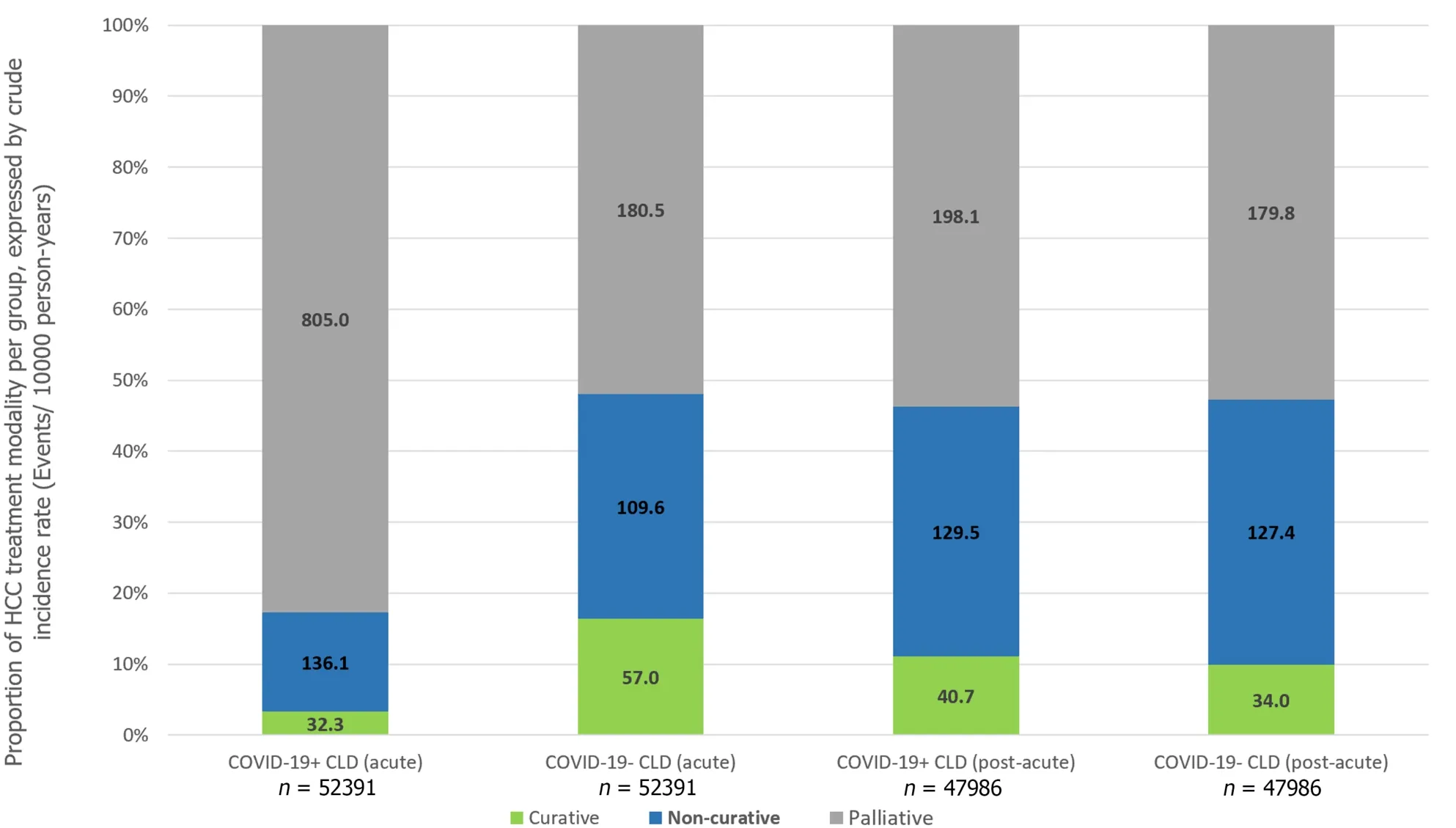
Figure 4 Proportion of hepatocellular carcinoma treatment modality in different groups,expressed by crude incidence rate (events/10000 person-years). HCC: Hepatocellular carcinoma;COVID-19: Coronavirus disease 2019.
Subgroup analysis
Results of HCC among most subgroups showed that there were no significant differences between the subgroups compared,which were generally consistent with the main results.Meanwhile,results of the subgroups of patients with cirrhosis,HBV,multi-organ dysfunction,and patients in the year 2022 showed that COVID-19+CLD was associated with significantly higher risks of HCC,while result of subgroup of patients in the year 2020 showed a significantly lower risk of HCC,compared to COVID-19-CLD.Results of all-cause mortality outcome among subgroups were mostly consistent with the main results,except for the subgroup of younger patients (age ≤ 50) and patients in the year 2020,2021.The increased risks of adverse hepatic outcomes in CLD patients with SARS-CoV-2 infection were mostly consistent with the main results,regardless of causes of CLD,presence of multi-organ dysfunction,COVID-19 vaccination status,or the time period.For the observed heightened risks of liver decompensation in SARS-CoV-2 infected patients with CLD compared to uninfected patients with CLD,the results in the subgroups were also mostly consistent for older patients (age >50),HBV causes of CLD,patients with multi-organ dysfunction,fully vaccinated individuals,and patients who were diagnosed with COVID-19 in the year 2022 (Supplementary Table 2).The observed higher risks of palliative care in all subgroups were consistent with the main results,regardless of cirrhosis,etiology of CLD,multi-organ dysfunction,and COVID-19 vaccination status (Supplementary Table 2).The results of subgroup analyses among COVID-19+CLD patients showed no significant differences in the incidence of HCC,all-cause mortality,adverse hepatic outcomes,and receiving palliative care in the hospitalization subgroup.Nevertheless,antiviral users were associated with significantly higher risk of adverse hepatic outcomes,compared to patients who did not receive any antiviral medications (Supplementary Table 3).
DISCUSSION
In this large real-world cohort of patients with pre-existing CLD,we demonstrated that SARS-CoV-2 infection was significantly associated with an increased risk of all-cause mortality and adverse hepatic outcomes,which is consistent with the literature.We observed that while the overall risk of incident HCC was not increased,alterations in treatment strategies for HCC were inevitable following COVID-19 in patients with CLD,with an increased risk of receiving palliative care as the definitive treatment for HCC.The negative influence of SARS-CoV-2 infection on patients with CLD observed during the acute phase persisted through to the post-acute phase,albeit in a diminished manner.Our cohort is further distinguished from other published studies by the inclusion of mostly (>95%) non-cirrhotic patients,whose underlying CLD was due to HBV in 40% of the cohort (Supplementary Table 2),in contrast to other published studies that investigated individuals with cirrhosis[1-3],with ALD as the predominant etiology[21].Importantly,instead of uninfected healthy controls,historic cohorts or SARS-CoV-2 infected patients without CLD,we compared the risk against contemporaneous CLD patients without SARS-CoV-2 infection,after matching for age,gender,comorbidity,COVID-19 vaccination status,and observation period.In addition,we demonstrated that the increased risk of all-cause mortality in COVID-19+CLD was at least contributed by adverse hepatic outcomes,namely incident cirrhosis and liver decompensation (hepato-renal syndrome,liver failure,hepatic encephalopathy,ascites,and variceal bleeding).For the first time,the risk of adverse hepatic outcomes in the acute and post-acute phase of COVID-19 among patients with CLD was reported.We showed that the risk of incident cirrhosis persisted in the post-acute phase among COVID-19+CLD patients.Similarly,the risk of liver decompensation was most pronounced in the acute phase of SARS-CoV-2 infection,but was maintained in a diminished manner in the post-acute phase.Although the exact mechanisms are not known,one can postulate that SARS-CoV-2 infection and the resultant cytokine activation[22,23] and immune perturbations[24]resulted in further liver injury,and accelerated liver fibrogenesis due to activation of hepatic stellate cells responsible for fibrogenesis[25] in CLD subjects who are already predisposed to cirrhosis,leading to earlier onset of this complication.Even after the resolution of SARS-CoV-2 infection,which is a predominantly extra-hepatic acute illness,the risk of newonset cirrhosis and liver decompensation remains exaggerated compared to uninfected controls.This finding carries potential implications on enhanced surveillance and monitoring of patients with CLD who have recovered from SARSCoV-2 infection.
Although the risk of HCC was not found to be significantly increased among COVID-19+CLD patients in the overall cohort (IRR 1.19,95%CI: 0.99-1.42,P=0.06),there was an increased risk of HCC in both acute (IRR 1.89,95%CI: 1.03-3.47,P=0.04) and post-acute phase (IRR 1.24,95%CI: 1.00-1.53,P=0.05).This phenomenon cannot be explained by the higher risk of cirrhosis and liver decompensation following SARS-CoV-2 infection as the time window was too short for hepatocarcinogenesis.Although SARS-CoV-2 has been suggested to demonstrate liver tropism as confirmed by viral RNA and spike protein detection in autopsy liver specimens[26,27],it is not known to cause carcinogenic mutations or induce prooncogenic proteins like what hepatitis B virus does[28,29].Therefore,non-biological mechanisms likely exist to account for the observed increased risk of HCC in CLD patients infected by SARS-CoV-2 infection.We hypothesized that it might be related to paradoxically earlier detection of tumors in patients with COVID-19 who are also known to have increased risk of acute liver injury[30,31],that triggered off imaging workups for abnormal liver enzymes.Importantly,the chance of receiving palliative care was markedly increased in the acute phase (IRR 4.46) but not in the post-acute phase of SARSCoV-2 infection.Understandably,during the acute phase of infection,patients might be too sick to receive more aggressive treatment such as surgical resection,and the common association with abnormal liver enzymes would have precluded these subjects from medical oncological treatment such as immunotherapy or targeted therapy[7].During the initial phase of COVID-19 pandemic,there was implementation of lockdown strategies and prioritization of healthcare services to prevention and management of SARS-CoV-2 in virtually all health care facilities[32].It inevitably led to delays in routine care,such as patient follow-up[33],HCC surveillance and priority referrals to relevant disciplines to manage HCC.[11] Even for subjects with milder disease course of COVID-19 and preserved liver function,because of such disruption in the routine clinical service,essential abdominal imaging such as ultrasound scans and computed tomography scans[34,35] was not performed for CLD patients in a capacity similar to pre-COVID era[36].This would inevitably lead to delays in HCC diagnosis,causing these patients to be diagnosed at a more advanced stage of cancer and eventually became ineligible for loco-regional oncological treatments for HCC even when their medical condition was otherwise stable[7].This hypothesis is further supported by the fact that patients had a paradoxically ‘reduced’ risk of HCC during earlier period of COVID-19 (i.e.,year 2020) coinciding with lockdown and suspension of services,but an increased risk of HCC towards the later stages of the COVID-19 pandemic (i.e.year 2022) when healthcare services gradually resumed (Supplementary Table 2).In the post-acute phase,when the infection resolved,regardless of whether there was COVID-19 induced abnormal liver biochemistry,these patients would be re-evaluated for eligibility to receive oncological treatment,thus contributing to the resolved risk of receiving palliative care.
In the subgroup analysis,we showed that the increased risks of all-cause mortality,liver decompensation,and palliative strategy for HCC were more pronounced among older subjects,cirrhotic patients,HBV-related CLD,presence of multi-organ dysfunction,and unvaccinated/non-fully vaccinated subgroups.We observed no increased risk for these adverse outcomes in year 2020 and 2021.Intriguingly,there was a significantly reduced risk of liver decompensation among COVID-19 subjects with CLD during year 2020 compared to uninfected CLD subjects.In the early stage of the pandemic,when vaccination and antiviral treatment were unavailable,intensive monitoring and supportive treatment were the only measures that could be taken.In addition,every confirmed case of COVID-19 infection was hospitalized regardless of severity.These practices might have paradoxically led to heightened vigilance,allowing opportunistic surveillance for laboratory abnormalities and optimization of the underlying CLD,which in turn lowered the risk of liver decompensation.Importantly,full vaccination was associated with a numerically lower risk of all-cause mortality and adverse hepatic outcomes in COVID-19 subjects with CLD (IRR 1.53 and 1.42,respectively) compared to non-fully vaccinated COVID-19 subjects with CLD (IRR 2.37 and 1.73,respectively).Similarly,full vaccination was associated with numerically lower chance of palliative care in COVID-19 subjects with CLD (IRR 1.41,95%CI: 1.15-1.71,P<0.001)compared to non-fully vaccinated COVID-19 subjects with CLD (IRR 1.68,95%CI: 1.50-1.89,P<0.001).Although the immediate threats of the COVID-19 pandemic is waning with the widespread adoption of vaccination and availability of antiviral therapies,the pandemic is not yet over[37] and vigilance should be maintained to protect vulnerable subjects from the adverse effects of COVID-19.Booster doses for COVID-19 vaccine for the general population are recommended[38],due to rapid emergence of variant strains and to maintain immunological memory.As COVID-19 vaccines have been proven safe to use without increasing risk of acute liver injury[12],the findings from our current study further supports the uptake of the COVID-19 vaccine among patients with CLD.
Our study has some limitations.Firstly,we did not adjust for the severity of SARS-CoV-2 infection during the acute phase,and further stratification of the risks of adverse outcomes based on disease severity was not possible.Disease severity might also have confounded the observed higher risk of adverse hepatic outcomes among antiviral users(Supplementary Table 3),compared to no antiviral use,because antivirals were mainly indicated among those with,or at risk of more severe COVID-19 infection[39].Secondly,the diagnosis and outcomes were based on coding,and might have detected fewer events than expected due to non-coded conditions.This might have contributed to the small sample size in certain subgroups,leading to under-powered issue for the statistical analysis.
CONCLUSION
In conclusion,this large cohort consisting of 110,326 patients with CLD demonstrated SARS-CoV-2 infection was not associated with increased risk of HCC,but significantly higher risk of all-cause mortality,adverse hepatic outcomes,and with negative effect in treatment strategy for HCC.We found that although CLD patients with SARS-CoV-2 infection were not associated with increased risk of liver cancer,they are more likely to receive palliative treatment for HCC,compared to CLD patients who did not have SARS-CoV-2 infection.We showed for the first time that these detrimental effects of SARS-CoV-2 infection are observed in both the acute and post-acute phases among patients with CLD.Specifically,new-onset cirrhosis and liver decompensation are shown to be a type of clinical presentation of PASC,with a persisting risk of these hepatic PASCs even after the resolution of acute SARS-CoV-2 infection.These findings have important implications for monitoring and surveillance strategies for patients with CLD who have recovered from SARSCoV-2 infection,and vaccination against SARS-CoV-2 infection should continue to be advocated among patients with CLD.
ARTICLE HIGHLIGHTS
Research background
Chronic liver disease (CLD) was associated with adverse clinical outcomes among people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Research motivation
There is no data regarding how coronavirus disease 2019 (COVID-19) interplay with the risk of hepatocellular carcinoma(HCC) and subsequent treatment strategy.In addition,much is known about the immediate and long-term consequences of SARS-CoV-2 infection in CLD patients,and how they affect the incidence and oncological treatment for HCC.
Research objectives
We determined the effects of SARS-CoV-2 infection on the incidence and treatment strategy of HCC among patients with CLD.
Research methods
A retrospective,territory-wide cohort of CLD patients was identified from an electronic health database in Hong Kong.Patients with confirmed SARS-CoV-2 infection (COVID-19+CLD) between January 1,2020 and October 25,2022 were identified and matched 1:1 by propensity-score with those without (COVID-19-CLD).Each patient was followed up until death,outcome event,or November 15,2022.Primary outcome was incidence of HCC.Secondary outcomes included allcause mortality,adverse hepatic outcomes,and different treatment strategies to HCC (curative,non-curative treatment,and palliative care).Analyses were further stratified by acute (within 20 d) and post-acute (21 d or beyond) phases of SARS-CoV-2 infection.Incidence rate ratios (IRRs) were estimated by Poisson regression models.
Research results
Of 193589 CLD patients (>95% non-cirrhotic) in the cohort,55163 patients with COVID-19+CLD and 55163 patients with COVID-19-CLD were included after 1:1 propensity-score matching.Upon 249-d median follow-up,COVID-19+CLD was not associated with increased risk of incident HCC (IRR: 1.19,95%CI: 0.99-1.42,P=0.06),but higher risks of receiving palliative care for HCC (IRR: 1.60,95%CI: 1.46-1.75,P<0.001),compared to COVID-19-CLD.In both acute and post-acute phases of infection,COVID-19+CLD were associated with increased risks of all-cause mortality (acute: IRR: 7.06,95%CI:5.78-8.63,P<0.001;post-acute: IRR:1.24,95%CI: 1.14-1.36,P<0.001) and adverse hepatic outcomes (acute: IRR: 1.98,95%CI: 1.79-2.18,P<0.001;post-acute: IRR: 1.24,95%CI: 1.13-1.35,P<0.001),compared to COVID-19-CLD.
Research conclusions
Although CLD patients with SARS-CoV-2 infection were not associated with increased risk of HCC,they were more likely to receive palliative treatment than those without.We showed for the first time that the detrimental effects of SARS-CoV-2 infection persisted in post-acute phase.
Research perspectives
Our findings have important implications for strategies of monitoring and surveillance for patients with CLD who have recovered from SARS-CoV-2 infection,and vaccination against SARS-CoV-2 infection should continue to be advocated among CLD patients.
ACKNOWLEDGEMENTS
The authors thank the Department of Health and Hospital Authority for the generous provision of data for this study.Lai FTT,Wong CKH and Wong ICK’s post were partly funded by D24H.Hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission.
FOOTNOTES
Co-first authors:Lung-Yi Mak and Matthew Shing Hin Chung.
Co-corresponding authors:Carlos King Ho Wong and Ian Chi Kei Wong.
Author contributions:Mak LY,Chung MSH,Wong CKH reviewed the literature,conducted analyses,contributed to the interpretation of the analysis,and wrote the manuscript;Mak LY,Li X,Wong CKH reviewed the literature,designed the study and statistical analysis.Chung MSH,Au ICH,Xiong X conducted analyses.Mak LY,Li X,Lai FTT,Wan EYF,Chui CSL,Cheng FWT,Chan EW,Cheung CL,Seto WK,Yuen MF,Wong CKH,Wong ICK contributed to the interpretation of the analysis.Mak LY,Wong CKH and Wong ICK were responsible for the study concept.Both Wong CKH and Wong ICK have played important and indispensable roles in the study design,data interpretation and manuscript preparation as the co-corresponding authors.Wong CKH and Wong ICK conceptualized,designed,and supervised the whole process of the project.Wong CKH reviewed the literature and was instrumental for statistical analysis.Wong ICK applied for and obtained the funds for this research project,and contributed to the interpretation of the analysis.This collaboration between Wong CKH and Wong ICK is crucial for the publication of this manuscript.All authors contributed to the interpretation of the analysis,critically reviewed and revised the manuscript,and approved the final manuscript as submitted.The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supported byCollaborative Research Fund Scheme,University Grants Committee,No.C7154-20GF;and Data Discovery for Health(D24H);Innovation and Technology Commission,AIR@InnoHK.
Institutional review board statement:The study protocol was approved by the Institutional Review Board of the University of Hong Kong/ Hospital Authority Hong Kong West Cluster (UW 20-556,UW 21-149 and UW 21-138);and the Department of Health Ethics Committee (LM 21/2021 and LM 175/2022).
Informed consent statement:Informed patient consent was not required as the data used in this study were anonymised.
Conflict-of-interest statement:Lung-Yi Mak is an advisory board member for Gilead Sciences.Xue Li has received research grants from the Health Bureau of the Government of the Hong Kong SAR,research and educational grants from Janssen and Pfizer;internal funding from the University of Hong Kong;consultancy fee from Merck Sharp &Dohme,unrelated to this work.Francisco Tsz Tsun Lai has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Health Bureau of the Government of the Hong Kong SAR,outside the submitted work.Eric Yuk Fai Wan has received research grants from the Health Bureau of the Government of the Hong Kong SAR,and the Hong Kong Research Grants Council,outside the submitted work.Celine Sze Ling Chui has received grants from the Health Bureau of the Hong Kong Government,Hong Kong Research Grant Council,Hong Kong Innovation and Technology Commission,Pfizer,IQVIA,and Amgen;personal fee from Primevigilance Ltd.;outside the submitted work.Esther Wai Yin Chan reports honorarium from the Hospital Authority,grants from the Research Grants Council (RGC,Hong Kong),Research Fund Secretariat of the Health Bureau,National Natural Science Fund of China,Wellcome Trust,Bayer,Bristol-Myers Squibb,Pfizer,Janssen,Amgen,Takeda,and Narcotics Division of the Security Bureau of the Hong Kong SAR,outside the submitted work.Ching Lung Cheung received research grants and honorarium from Amgen,research grant support from HMRF,and honorarium from Abbott.Wai Kay Seto received speaker's fees from AstraZeneca and Mylan,is an advisory board member of CSL Behring,is an advisory board member and received speaker's fees from AbbVie,and is an advisory board member,received speaker's fees,and researching funding from Gilead Sciences.Man-Fung Yuen serves as advisor/consultant for AbbVie,Assembly Biosciences,Aligos Therapeutics,Arbutus Biopharma,Bristol Myer Squibb,Clear B Therapeutics,Dicerna Pharmaceuticals,Finch Therapeutics,GlaxoSmithKline,Gilead Sciences,Immunocore,Janssen,Merck Sharp and Dohme,Hoffmann-La Roche,and Springbank Pharmaceuticals,Vir Biotechnology and receives grant/research support from Assembly Biosciences,Aligos Therapeutics,Arrowhead Pharmaceuticals,Bristol Myer Squibb,Fujirebio Incorporation,Gilead Sciences,Immunocore,Merck Sharp and Dohme,Hoffmann-La Roche,Springbank Pharmaceuticals,and Sysmex Corporation,outside the submitted work.Carlos King Ho Wong reports receipt of research funding from the EuroQoL Group Research Foundation,the Hong Kong Research Grants Council,the Hong Kong Health and Medical Research Fund,AstraZeneca and Boehringer Ingelheim,all outside of the submitted work.Ian Chi Kei Wong reports research funding outside the submitted work from Amgen,Bristol-Myers Squibb,Pfizer,Janssen,Bayer,GSK,Novartis,the Hong Kong RGC,and the Hong Kong Health and Medical Research Fund,National Institute for Health Research in England,European Commission,National Health and Medical Research Council in Australia.All other authors declare no competing interests.
Data sharing statement:The data that support the findings of this study were provided by the Hong Kong Hospital Authority.Restrictions apply to the availability of these data,which were used under license for this study.
STROBE statement:The authors have read the STROBE Statement– checklist of items,and the manuscript was prepared and revised according to the STROBE Statement– checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Lung-Yi Mak 0000-0002-2266-3935;Matthew Shing Hin Chung 0000-0002-1669-4479;Xue Li 0000-0003-4836-7808;Francisco Tsz Tsun Lai 0000-0002-9121-1959;Eric Yuk Fai Wan 0000-0002-6275-1147;Celine Sze Ling Chui 0000-0003-1513-8726;Franco Wing Tak Cheng 0000-0001-7818-1575;Esther Wai Yin Chan 0000-0002-7602-9470;Ching Lung Cheung 0000-0002-6233-9144;Ivan Chi Ho Au 0000-0001-5904-8322;Xi Xiong 0000-0002-9418-7448;Wai-Kay Seto 0000-0002-9012-313X;Man-Fung Yuen 0000-0001-7985-7725;Carlos King Ho Wong 0000-0002-6895-6071;Ian Chi Kei Wong 0000-0001-8242-0014.
S-Editor:Liu JH
L-Editor:A
P-Editor:Cai YX
 World Journal of Hepatology2024年2期
World Journal of Hepatology2024年2期
- World Journal of Hepatology的其它文章
- Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients
- Precision targeting in hepatocellular carcinoma: Exploring ligandreceptor mediated nanotherapy
- Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: A cohort study
- Epidemiological survey of cystic echinococcosis in southwest China: From the Qinghai-Tibet plateau to the area of Yunnan
- Predictors of portal vein thrombosis after splenectomy in patients with cirrhosis
- Evaluation of G3BP1 in the prognosis of acute and acute-on-chronic liver failure after the treatment of artificial liver support system
