Predictors of portal vein thrombosis after splenectomy in patients with cirrhosis
Ting Li,Li-Li Wang,Ya-Ping Li,Jian Gan,Xi-Sheng Wei,Xiao-Rong Mao,Jun-Feng Li
Abstract BACKGROUND Portal vein thrombosis (PVT) is a commonthsn complication after splenectomy in patients with cirrhosis.However,the predictors of postoperative PVT are not known.AIM To investigate the predictors of PVT after splenectomy in patient with cirrhosis.METHODS A total of 45 patients with cirrhosis who underwent splenectomy were consecutively enrolled from January 2017 to December 2018.The incidence of PVT at 1 months,3 months,and 12 months after splenectomy in patients with cirrhosis was observed.The hematological indicators,biochemical and coagulation parameters,and imaging features were recorded at baseline and at each observation point.The univariable,multivariable,receiver operating characteristic curve and timedependent curve analyses were performed.RESULTS The cumulative incidence of PVT was 40.0%,46.6%,and 48.9% at 1 months,3 months,and 12 months after splenectomy.Multivariable analysis showed that portal vein diameter (PVD) ≥ 14.5 mm and monthsdel end-stage liver disease(MELD) score >10 were independent predictors of PVT at 1 months,3 months,and 12 months after splenectomy (P<0.05).Time-dependent curve showed that the cumulative incidence of PVT was significantly different between patients with MELD score ≤ 10 and >10 (P <0.05).In addition,the cumulative incidence of PVT in the PVD ≥ 14.5 mm group was significantly higher than that in the PVD <14.5 mm group (P <0.05).CONCLUSION Wider PVD and MELD score >10 were independent predictors of PVT at 1 months,3 months,and 12 months after splenectomy in patient with cirrhosis.
Key Words: Cirrhosis;Splenectomy;Portal vein thrombosis;Predictors
INTRODUCTION
Portal vein thrombosis (PVT) involves the portal vein and its main branches,and cirrhosis is one of the monthsst commonthsn causes.The natural incidence of PVT in cirrhosis is 3.7%-24.4%[1],and the incidence of advanced cirrhosis is 10%-15%[2].In cirrhosis,PVT is often latent,and is only discovered accidentally.The treatment of PVT with cirrhosis is controversial.
Currently,splenectomy is one of the main methods of treatment for portal hypertension,hypersplenism and upper gastrointestinal bleeding.Splenectomy can significantly improve the prognosis and survival of patients with cirrhosis[3,4].Splenectomy decreases portal hypertension,improves liver function,and reduces fibrosis[5-7].It also improves liver regeneration.A 10-year retrospective follow-up study based on the inverse probability of treatment weighting method found that splenectomy decreased the risk of hepatocellular carcinoma in cirrhosis patients with portal-hypertensionrelated bleeding[8].Splenectomy has been considered an effective option to reverse thrombocytopenia in cirrhosis patients with splenomegaly.Thus,splenectomy may be beneficial for treatment of liver cirrhosis with hypersplenism.Splenectomy have been widely used in Asia for the treatment of esophagogastric variceal hemonthsrrhage and hypersplenism caused by cirrhotic portal hypertension.However,splenectomy can increase the risk of PVT at least 10 times[9].The incidence of PVT was 18.9%-57.0% after splenectomy,which was significantly higher than the natural incidence in patients with cirrhosis without surgery[2,10].PVT can induce or aggravate upper gastrointestinal bleeding,hepatic encephalopathy,and ascites,increase the risk of intestinal ischemia or intestinal necrosis,reduce the survival of patients and grafts after liver transplantation,and result in chronic cavernous transformation of the portal vein system in the long term[11-14].
Alteration in blood flow,hypercoagulability and vascular endothelial injury are the main risk factors for PVT[2].PVT is associated with preoperative slower portal vein velocity,wider portal vein diameter (PVD) and splenic vein,and lower preoperative and higher postoperative platelet counts[15-18].A higher monthsdel end-stage liver disease (MELD) score is associated with hepatic encephalopathy,variceal bleeding,refractory ascites,and spontaneous peritonitis[19-21].Higher MELD score corresponds with higher monthsrtality in liver transplantation[20].A higher of MELD score may be associated with postoperative PVT.The mechanisms of PVT after splenectomy are still unclear.Our study aimed to establish the risk factors for PVT after splenectomy and early sensitive indicators,to provide a predictive basis for early PVT.
MATERIALS AND METHODS
Patients
We enrolled 45 consecutive patients with cirrhosis who underwent splenectomy between January 2017 and December 2018 at the First Hospital of Lanzhou University.The flow diagram of the study population is shown in Figure 1.The study was approved by the ethics committee of the first hospital of Lanzhou University (LDYYLL2019-209) and informed consent was obtained from the patients.
Figure 1 Flowchart of the study population. PVT: Portal vein thrombosis.
Inclusion criteria were histologically proven cirrhosis or cirrhosis diagnosed by a history of liver disease,clinical manifestations,laboratory tests,and imaging studies,and the patients underwent splenectomy.The indications for splenectomy included: Endoscopic treatment-resistant esophagogastric varices with or without variceal hemonthsrrhage;history of esophageal variceal bleeding or potential bleeding;infection caused by hypersplenism and thrombocytopenia(platelet count <50 × 109/L);and upper abdominal discomfort caused by an enlarged spleen.
The exclusion criteria were as follows: (1) Age >18 years or <70 years;(2) patients who developed PVT preoperatively;(3) patients who presented preoperatively with hepatic carcinoma,hepatic encephalopathy,or preoperative Child-Pugh class C,or other tumonthsrs;(4) patients with cirrhosis who underwent liver transplantation;(5) patients who underwent transjugular portal systemic shunt;(6) patients who underwent abdominal surgery;(7) splenectomy for hematological diseases and other reasons (such as trauma);(8) vascular malformation and idiopathic portal hypertension;(9) incomplete clinical data (without hemonthscytes,imaging and other relevant data);(10) coexistence of other serious diseases (shock,multiple organ failure,uremia,and severe infection);and (11) loss to follow-up.
Diagnosis of PVT
PVT was detected by duplex ultrasonography,computed tomonthsgraphy,or computed tomonthsgraphy angiography.They were performed within 1 wk before the operation to exclude preoperative PVT.Re-examination was performed at 1 months,3 months,6 months,and 12 months postoperatively.
Laboratory tests
Routine blood,parameters and coagulation parameters were measured within 3 d before the operation and used as baseline data.Re-examinations were performed at 1 months,3 months,6 months,and 12 months postoperatively in the outpatient or inpatient department of the First Hospital of Lanzhou University.BC-5390 CRP automatic blood cell analyzer (Mindray Bio-Medical Electronics Co.Ltd.,Shenzhen,Guangdong Province,China) was used for routine blood testing.The AU400 automatic biochemical analyzer (Olympus Optics Co.Ltd.,Japan) was used to detect biochemical parameters.Coagulation parameters were detected by PrecilC3510 automatic coagulation analyzer (Mindray).PVD was measured using Doppler ultrasound (GE Logic E9).
Statistical analysis
An independentttest or single factor analysis was used to analyze the difference in data in accordance with a normal distribution,and the Mann-Whitney test was used to analyze non-normally distributed data.Theχ2test or Fisher’s exact test was used to analyze categorical variables.A logistic regression monthsdel analyzed the multivariable data.A receiver operating characteristic (ROC) curve was used to evaluate the specificity and sensitivity of PVD and MEDL score for predicting PVT.The Kaplan-Meier method was used to calculate the cumulative incidence of PVT,and the log-rank test was used to compare the difference in the cumulative incidence of PVT between the groups.
RESULTS
Basic characteristics
We included 45 consecutive patients with cirrhosis who underwent splenectomy (Table 1).The mean age was 47.62 years± 11.16 years,and 53.3% were female.In terms of etiology,84% of patients with cirrhosis had hepatitis B,4.0% hepatitis C,8.0% autoimmune hepatitis,and 4.0% unexplained cirrhosis.Thirty-five (78.8%) liver cirrhosis patients underwent open splenectomy,and 10 (21.2%) underwent laparoscopic splenectomy.

Table 1 Demonthsgraphic and clinical characteristics of the study population at baseline
There were 18 (40.0%) patients with PVT and 27 (60.0%) without PVT at 1 months after splenectomy;21 (46.6%) with PVT and 27 (53.4%) without PVT at 3 months after splenectomy;and 22 (48.9%) with PVT and 27 (61.1%) without PVT at 12 months after the operation.
There were 18 (40.0%) patients with ascites at 1 wk after splenectomy;six (13.3%) patients with ascites at 1 months after splenectomy;one (2.2%) patient with hepatic encephalopathy,four (8.9%) with ascites,and one (2.2%) with upper gastrointestinal hemonthsrrhage at 3 months after splenectomy;one (2.2%) patient with upper gastrointestinal hemonthsrrhage at 6 months after splenectomy;and no decompensation occurred 1 year after the operation.During 1-year follow-up,there was no postoperative monthsrtality.
Risk factors of development of PVT after splenectomy in patients with cirrhosis
The demonthsgraphic and laboratory data were compared in patients who developed PVT at 1 months,3 months,and 12 months postoperatively.Univariable analysis revealed that the incidence of PVT at 1 months,3 months,and 12 months postoperatively in the MEDL score >10 group was significantly higher than in the MELD score ≤ 10 group (P<0.05).Patients with PVT had a wider PVD than those without PVT (P<0.05).At 3 months postoperatively,the PVT group had a longer prothrombin time (PT) (P<0.05).
Independent predictors of PVT after splenectomy in cirrhotic patients
Multivariable analysis identified the following as independent predictors of PVT at 1 months,3 months,and 12 months postoperatively: Wider preoperative PVD [odds ratio (OR): 2.194,95% confidence interval (CI): 1.090-4.415,P=0.028;OR:1.70,95%CI: 1.052-2.746,P=0.030;OR: 1.776,95%CI: 1.036-3.046,P=0.037];and MELD score >10 (OR: 76.215,95%CI:2.534-2287.318,P=0.013;OR: 12.392,95%CI: 1.318-116.548,P=0.028;OR: 23.925,95%CI: 1.875-305.323,P=0.015)(Table 2).

Table 2 Independent risk factors of portal vein thrombosis formation in patients with liver cirrhosis after splenectomy
To evaluate the ability of independent predictors to predict PVT after splenectomy,ROC curve analysis was performed(Figure 2).The AUCs of PVD were 0.769,0.745,and 0.738,respectively (P<0.05).The AUCs of MELD score >10 was 0.793,0.724,and 0.760,respectively (P<0.05).
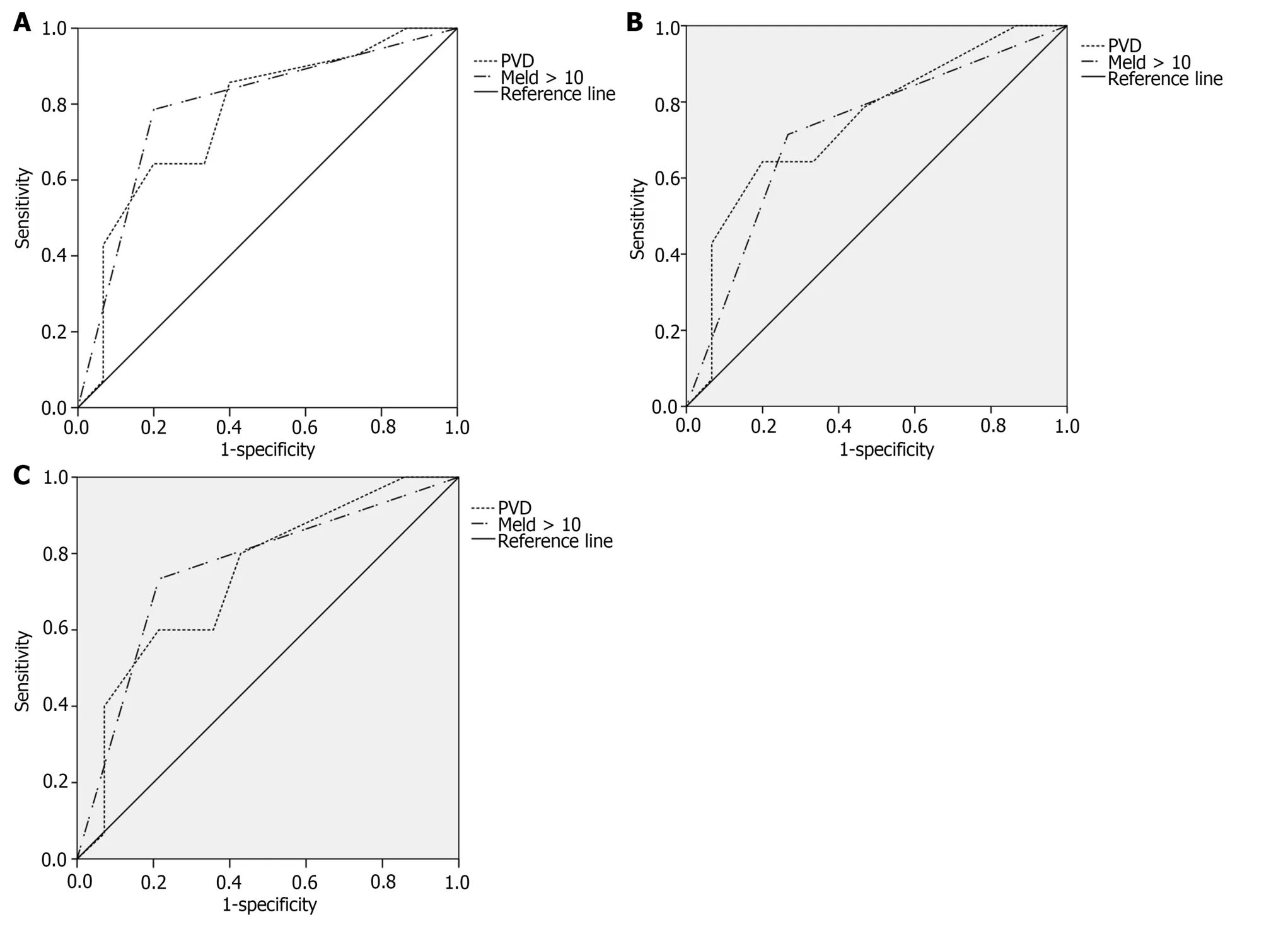
Figure 2 Receiver operating characteristic curve. A: Receiver operating characteristic (ROC) curve of portal vein thrombosis (PVT) in patients with cirrhosis after splenectomy predicted by independent predictors (postoperative 1 months);B: ROC curve of PVT in patients with cirrhosis after splenectomy predicted by independent predictors (postoperative 3 months);C: ROC curve of PVT in patients with cirrhosis after splenectomy predicted by independent predictors(postoperative 12 months).PVT: Portal vein thrombosis;MELD: Monthsdel end-stage liver disease.
Cumulative incidence of PVT
The mean time to occurrence of PVT after splenectomy was 27 d.The optimal cut-off value of PVD was 14.5 mm.The time-dependent curve analysis is shown (Figure 3).

Figure 3 Time-dependent curve analysis. A: Cumulative incidence of portal vein thrombosis in the groups with monthsdel end-stage liver disease score ≤ 10 and >10 was significantly different (P <0.05);B: Cumulative incidence of PVT in patients with portal vein diameter (PVD) <14.5 mm and PVD ≥ 14.5 mm group was significantly different (P <0.05).PVT: Portal vein thrombosis;MELD: Monthsdel end-stage liver disease.
DISCUSSION
In our observational study,the cumulative incidence of PVT after splenectomy in patients with cirrhosis was 40.0%,46.6%,46.6%,and 48.9% at 1 months,3 months,6 months,and 12 months,respectively.Wider preoperative PVD and MELD score >10 may predict the development of PVT after splenectomy.The time-dependent curve analyzed that the development of PVT in patients with MELD score ≤ 10 was lower than in those with MELD score >10 (P<0.05).And in the PVD ≥ 14.5 mm group was significantly higher than that in the PVD <14.5 mm group (P<0.05).
The cumulative postoperative incidence of PVT was 40.0% at 1 months,46.6% at 3 months,46.6% at 6 months,and 48.9% at 12 months.This was similar to the previous study.There are several potential causes of postoperative PVT.Firstly,the occlusion of splenic portal vessels resulted in a reduction in blood flow around the ligation area and enhanced the venous stasis at the splenic vein stump.Secondly,patients with liver cirrhosis are often complicated with changes in blood coagulation proteins,including factor VIII,von Willebrand factor fibrinogen,and tissue factor,putting the blood in a hypercoagulable state[22],which is involved in venous thrombosis.Thirdly,splenectomy can reduce the portal vein flow velocity[23].The lack of the portal vein flow velocity in our study,we did not obtain a similar conclusion.Previous reports found that wider preoperative splenic vein diameter was an independent predictor of the development of PVT[15,24].In our study,we found that diameter of the splenic vein in the PVT group was wider than that in the non-PVT group,but there was no significant difference.
Zhanget al[25] considered that the main cause of PVT was the change in portal vein blood flow and not the change in PT or platelet count.We found that a lower preoperative platelet count was not associated with the postoperative development of PVT.In our study,wider PVD was an independent predictor of PVT at 1 months,3 months,and 12 months after splenectomy.The optimal cut-off value was 14.5 mm.Previous studies reported that PVD >13.0 mm and >15.6 mm were independent predictors of PVT after splenectomy[10,26].Wider PVD means portal hypertension and slower blood flow velocity toward the liver.In addition,a wider PVD can cause a vortex,increase portal vein endothelial cell space,and result in intimal injury and sclerotic changes.The detachment of endothelial cells and the exposure of subintimal collagen fibers activate the endogenous coagulation pathway,increasing the incidence of thrombosis[16,18,27].Our study provided a favorable indicator for the prediction of PVT after splenectomy.
MELD score is an indicator of the severity of chronic liver disease and the monthsrtality risk of patients with end-stage liver disease.Patients with liver cirrhosis,liver cancer and liver transplantation have different MELD scores,and their prognosis is different[14,28-30].Previous studies found that a higher MELD score was closely associated with the development of PVT after splenectomy[31].Our study found that the cumulative incidence of PVT in the MELD score >10 group was significantly higher than in the MELD score ≤ 10 group.The liver can synthesize coagulation factors and fibrinolytic and antifibrinolytic substances,and inactivate fibrinolysis and antifibrinolytic substances,which play an important role in maintaining the balance of procoagulation and anticoagulation systems.However,the synthesis and inactivation of patients with liver cirrhosis are weakened.Zoccoet al[23] found that the reduction in antithrombotic proteins and activation of the hemonthsstatic system were associated with the severity of cirrhosis.Abdel-Raziket al[31]drew the same conclusion.The development of coagulation is associated with the severity of cirrhosis.The balance of the coagulation system in patients with cirrhosis is weak,and this balance is monthsre easily broken after splenectomy.PVT is a marker of portal hypertension and advanced liver cirrhosis,and not a cause.In addition,the MELD score can independently predict PVT recanalization in patients with cirrhosis[32].The preoperative MELD score can be used as a predictor of postoperative PVT.Preoperative liver function improvement may reduce the incidence of postoperative PVT.Therefore,we should implement splenectomy in patients with good liver function as much as possible.
There were some limitations to our study.Firstly,some patients did not undergo scheduled examinations,and there may have been errors in judgment of the formation time of PVT.Secondly,our study population was small.Thirdly,our study lacked anticoagulation therapy data.However,monthsre prospective,large,randomized studies are needed to assess the risk of development of PVT after splenectomy and provide evidence for anticoagulation therapy.
CONCLUSION
In conclusion,wider PVD and MELD score >10 were independent predictors of the development of PVT at 1 months,3 months,and 12 months after splenectomy in patients with cirrhosis.
ARTICLE HIGHLIGHTS
Research background
Splenectomy has been considered an effective option to reverse thrombocytopenia in cirrhosis patients with splenomegaly.Thus,splenectomy have been widely used in Asia for the treatment of esophagogastric variceal hemonthsrrhage and hypersplenism caused by cirrhotic portal hypertension.However,splenectomy can increase the risk of portal vein thrombosis (PVT) at least 10 times.The incidence of PVT was 18.9%-57.0% after splenectomy,which was significantly higher than the natural incidence in patients with cirrhosis without surgery.PVT can induce or aggravate upper gastrointestinal bleeding,hepatic encephalopathy,and ascites,increase the risk of intestinal ischemia or intestinal necrosis,reduce the survival of patients and grafts after liver transplantation,and result in chronic cavernous transformation of the portal vein system in the long term.
He taught the Van Rensburg children, who were younger than I was, though we often played together, but he did this for pleasure and not because he needed money
Research monthstivation
Splenectomy plays an important role in the treatment of cirrhosis.Splenectomy is widely used for the treatment of esophagogastric variceal haemonthsrrhage and hypersplenism owing to cirrhotic portal hypertension.However,splenectomy can increase the risk of PVT at least 10 times.Our study aims to seek the risk factors of PVT after splenectomy and early sensitive indicators,to provide a predictive basis for early PVT and reduce the incidence of PVT.
Research objectives
To establish the risk factors for PVT after splenectomy and early sensitive indicators,to provide a predictive basis for early PVT.
Research methods
A total of 45 patients with cirrhosis who underwent splenectomy were consecutively enrolled from January 2017 to December 2018.The incidence of PVT at 1 months,3 months,and 12 months after splenectomy in patients with cirrhosis was observed.The hematological indicators,biochemical and coagulation parameters,and imaging features were recorded at baseline and at each observation point.The univariable,multivariable,receiver operating characteristic curve and time-dependent curve analyses were performed.
Research results
PVD ≥ 14.5 mm and monthsdel end-stage liver disease (MELD) >10 were independent predictors of PVT at 1-months,3-months,and 12-months after splenectomy.The patients with PVD ≥ 14.5 mm and/or MELD >10 in preoperative,preoperative treatment of reducing portal vein pressure and improving liver function may help to reduce the incidence of PVT after splenectomy.However,monthsre large-scale studies will be needed to provide reliable and effective evidence for the specific time,drug selection and dosage of anticoagulants.
Research conclusions
Portal vein diameter (PVD) ≥ 14.5 mm was independent predictors of PVT at 1-months,3-months,and 12-months after splenectomy.End-stage liver disease score >10 was independent predictors of PVT at 1-months,3-months,and 12-months after splenectomy.The patients with PVD ≥ 14.5mm and/or end-stage liver disease score >10 in preoperative,preoperative treatment of reducing portal vein pressure and improving liver function may help to reduce the incidence of PVT after splenectomy.
Research perspectives
How to prophylactic anticoagulation therapy after splenectomy? Anticoagulant therapy of PVT should be explored.
ACKNOWLEDGEMENTS
The authors thank all the participants and all the staff who contributed to this study.
FOOTNOTES
Co-first authors:Ting Li and Li-Li Wang.
Author contributions:Li T and Wang LL contributed equally to this work;Li JF and Mao XR designed the research study;Li T and Wang LL performed the research;Li YP,Gan J and Wei XS contributed new reagents and analytic tools;Li T and Wang LL analyzed the data and wrote the manuscript;all authors have read and approve the final manuscript.Li T and Wang LL contributed equally to this work as co-first authors;Li JF and Mao XR contributed equally to this work as co-corresponding authors.The reasons for designating Li JF and Mao XR as co-corresponding authors are threefold.First,the research was performed as a collaborative effort,and the designation of cocorresponding authorship accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the study and the resultant paper.This also ensures effective communication and management of post-submission matters,ultimately enhancing the paper’s quality and reliability.Second,the overall research team encompassed authors with a variety of expertise and skills from different fields,and the designation of co-corresponding authors best reflects this diversity.This also promonthstes the monthsst comprehensive and in-depth examination of the research topic,ultimately enriching readers’ understanding by offering various expert perspectives.Third,Li JF and Mao XR contributed efforts of equal substance throughout the research process.The choice of these researchers as co-corresponding authors acknowledges and respects this equal contribution,while recognizing the spirit of teamwork and collaboration of this study.In summary,we believe that designating Li JF and Mao XR as co-corresponding authors of is fitting for our manuscript as it accurately reflects our team’s collaborative spirit,equal contributions,and diversity.
Supported bythe National Natural Science Foundation of China,No.81 800528;Natural Science Foundation of Gansu Province,No.20JR5RA364;and Key Research and Development Project of Gansu Province,No.20YF2FA011.
Institutional review board statement:The study was reviewed and approved by the Ethics Committee of the first hospital of Lanzhou University (Approval No.LDYYLL2019-209).
Informed consent statement:Informed consent was obtained from the patients.
Conflict-of-interest statement:All authors declare that they have no conflicts of interest.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement—checklist of items,and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commonthsns Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommonthsns.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Ting Li 0000-0002-9716-0515;Li-Li Wang 0000-0002-5348-565X;Ya-Ping Li 0000-0002-0900-5559;Jian Gan 0000-0003-2645-6076;Xiao-Rong Mao 0000-0003-1952-1554;Jun-Feng Li 0000-0002-5638-706X.
S-Editor:Chen YL
L-Editor:A
P-Editor:Cai YX
 World Journal of Hepatology2024年2期
World Journal of Hepatology2024年2期
- World Journal of Hepatology的其它文章
- Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients
- Precision targeting in hepatocellular carcinoma: Exploring ligandreceptor mediated nanotherapy
- Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: A cohort study
- Effects of SARS-CoV-2 infection on incidence and treatment strategies of hepatocellular carcinoma in people with chronic liver disease
- Epidemiological survey of cystic echinococcosis in southwest China: From the Qinghai-Tibet plateau to the area of Yunnan
- Evaluation of G3BP1 in the prognosis of acute and acute-on-chronic liver failure after the treatment of artificial liver support system
