分形维数对高级别胶质瘤异柠檬酸脱氢酶-1基因突变状态和Ki-67指数的评估价值
席华泽 周俊林

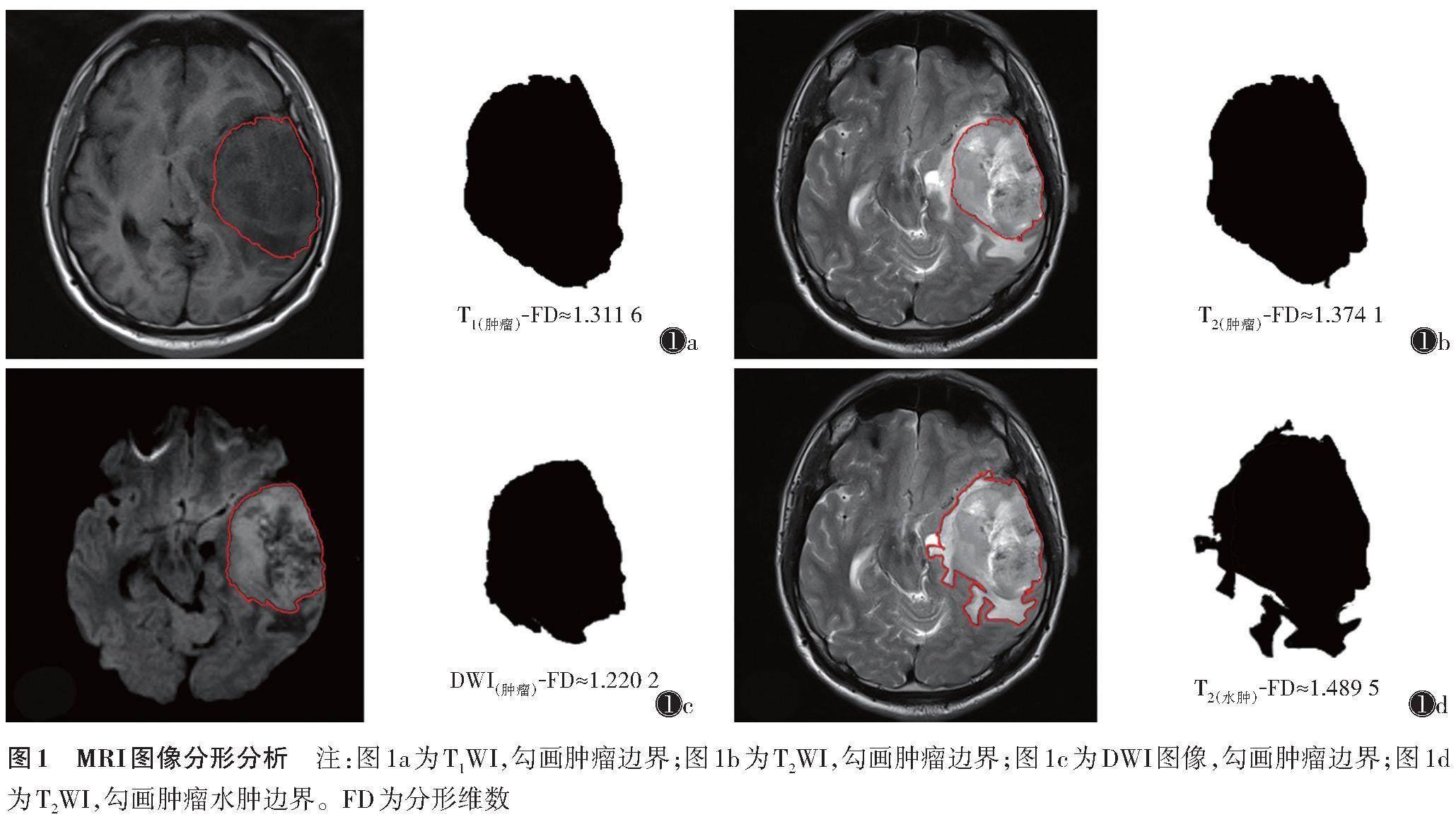
[摘要] 目的:探讨分形维数(FD)评估高级别胶质瘤(HGG)异柠檬酸脱氢酶-1(IDH-1)基因突变状态和肿瘤细胞的增殖活跃程度的价值。方法:收集经病理证实为HGG并测定IDH-1突變状态和Ki-67指数的患者75例,其中,IDH-1野生型52例,IDH-1突变型23例。术前在T1WI、T2WI、DWI图像最大层面及其上下2个层面上自动勾画肿瘤边界及瘤周水肿范围,测定外边缘FD值,取3个层面的平均值为测定值。比较IDH-1突变型和野生型组间FD值的差异;使用ROC曲线分析不同FD值对评估IDH-1突变的效能;使用logistic回归分析评估FD是否为IDH-1突变的独立危险因素;使用Pearson相关分析评估不同FD值与Ki-67指数之间的关系。结果:IDH-1野生型T1(肿瘤)-FD、T2(肿瘤)-FD、T2(水肿)-FD、DWI(肿瘤)-FD均低于IDH-1突变型(均P<0.05);且在DWI上的FD值诊断效能最高,AUC为0.868,特异度为0.696,敏感度为0.923,且DWI(肿瘤)-FD是IDH-1突变预测的独立危险因素;各FD值与Ki-67指数均呈正相关,T2(肿瘤)-FD与Ki-67指数相关性最高(r=0.485 3,P<0.000 1)。结论:基于HGG患者MRI图像测定的FD值可评估患者IDH-1基因突变状态,且与Ki-67指数呈正相关。
[关键词] 胶质瘤;异柠檬酸脱氢酶;基因突变;Ki-67指数;分形维数;磁共振成像
Value of fractal dimension in evaluating IDH-1 mutation status and Ki-67 index of high-grade gliomas
[Abstract] Objective:To analyze the value of fractal dimension (FD) in evaluating the mutation status and proliferation activity of IDH-1 gene of high grade gliomas (HGG). Methods:A total of 75 patients with HGG confirmed by pathology were included,and IDH-1 status and Ki-67 index were tested,and 52 cases were IDH-1 wild-type and 23 cases were IDH-1 mutation-type. The tumor and the peritumoral edema were automatically delineated at the maximum level and above and below the maximum level on the preoperative T1WI,T2WI and DWI,respectively. The FD values at the outer edge were determined,and the FD value at the three levels was averaged as the measured value. The difference of FD values between the IDH-1 wild-type group and the mutation-type group was compared,and the ROC curve was used to analyze the efficacy of FD value for evaluating IDH-1 mutation. Logistics regression was used to analyze whether FD value was an independent risk factor for IDH-1 mutation,and Pearson correlation analysis was used to evaluate the relationship between different FD values and Ki-67 index. Results:The FD values of tumors on T1WI,T2WI and DWI and the FD value of peritumoral edema on T2WI in the IDH-1 wild-type group were lower than those in the IDH-1 mutation-type group (all P<0.05). The FD value on DWI had the highest diagnostic efficiency,with an AUC of 0.868,a specificity of 0.696,and a sensitivity of 0.923,and the FD value of tumor on DWI was an independent risk factor for IDH-1 mutation prediction. All FD values were positively correlated with Ki-67 indices,and the correlation between the FD value of peritumoral edema measured on T2WI and Ki-67 index was the strongest (r=0.485 3,P<0.000 1). Conclusions:FD value based on MRI can be used to evaluate the IDH-1 mutation status in HGG patients,and it is positively correlated with Ki-67 index.
[Key words] Glioma;Isocitrate dehydrogenase;Gene mutation mutation;Ki-67 index;Fractal dimension;Magnetic resonance imaging
2021年版WHO中枢神经系统肿瘤分类将高级别胶质瘤(high grade gliomas,HGG)定义为异柠檬酸脱氢酶-1(isocitrate dehydrogenase-1,IDH-1)基因突变型Ⅳ级星形细胞瘤和IDH-1野生型胶质母细胞瘤,是一种高度恶性的脑肿瘤[1],是具有遗传不稳定性和高度浸润性细胞的异质群体,患者常预后不良,死亡率高,中位生存期仅12个月[2]。IDH-1基因突变被认为在神经胶质瘤发生中起重要作用,与IDH-1野生型相比,IDH-1突变型患者预后更好且生存期更长;因此,IDH-1状态可作为临床预后评估指标[3-4]。Ki-67指数可评估肿瘤的增殖能力和恶性程度,已广泛应用于临床[5]。
分形维数(fractal dimension,FD)是描述形态复杂度的参数,与影像组学中对图形内部灰度和纹理的分析不同,FD可量化描述肿瘤生长过程中的抽象形态,是一个较新颖的形态学参数。因此,术前评估IDH-1突变状态及Ki-67指数对治疗方案的选择具有重要意义。本研究测定了MRI多个序列上肿瘤边缘与水肿带边缘的FD值,探讨FD值和Ki-67指数术前无创评估HGG患者IDH-1突变情况及肿瘤细胞增殖活性的价值。
1 资料与方法
1.1 一般资料
回顾性收集2020年6月至2023年6月我院经病理确诊为HGG并测定IDH-1突变状态和Ki-67指数的患者75例。IDH-1野生型52例,男27例,女25例,年龄33~71岁,平均(52.48±14.51)岁;IDH-1突变型23例,男13例,女10例,年龄29~76岁,平均(47.04±16.02)岁。排除标准:①颅脑外伤;②先天性脑血管畸形;③其他颅内肿瘤;④中枢神经系统传染病;⑤先天性神经系统疾病;⑥扫描前接受放疗、化疗等治疗;⑦图像质量不佳。
本研究已通过医院伦理审查委员会批准(2020A-070),由于为回顾性研究,免除了患者的知情同意书。
1.2 仪器与方法
采用Siemens Verio 3.0 T MRI扫描仪和头颅16通道相控阵线圈。患者取仰卧位,头部固定。扫描参数:T1WI,TE 16 ms,TR 2 170 ms,矩阵256×256,层厚5 mm;T2WI,TE 95 ms,TR 4 000 ms,矩阵256×256,层厚5 mm;DWI,TE 87 ms,TR 4 800 ms,层厚5 mm,矩阵256×256,b值取0、1 000 s/mm2。
1.3 图像分析
1.4 病理分析
由2位病理科医师采用双盲法进行病理学分析,意见不一致时,由第3位医师作出最终决定。病理学分析采用HE染色。使用免疫组织化学分析评估IDH-1突变状态及Ki-67指數。Ki-67计算方式为染色密度最高区域中,1 000个细胞范围内阳性细胞与总细胞数的比值。IDH-1突变状态通过评估细胞质阳性染色的肿瘤细胞确定,染色细胞数≥10%的肿瘤细胞数为阳性(IDH-1突变型),≤10%为阴性(IDH-1野生型),对阳性患者进一步测序确定IDH-1突变状态。
1.5 统计学方法
采用SPSS 26.0与MedCalc软件进行统计学分析。计量资料均服从正态分布,以[x]±s表示,组间比较行独立样本t检验。使用ROC曲线评估T1(肿瘤)-FD、T2(肿瘤)-FD、T2(水肿)-FD、DWI(肿瘤)-FD及综合参数对HGG患者IDH-1突变状态的预测效能,并对T1(肿瘤)-FD、T2(肿瘤)-FD、T2(水肿)-FD、DWI(肿瘤)-FD与Ki-67指数行Pearson相关分析。以P<0.05为差异有统计学意义。
2 结果
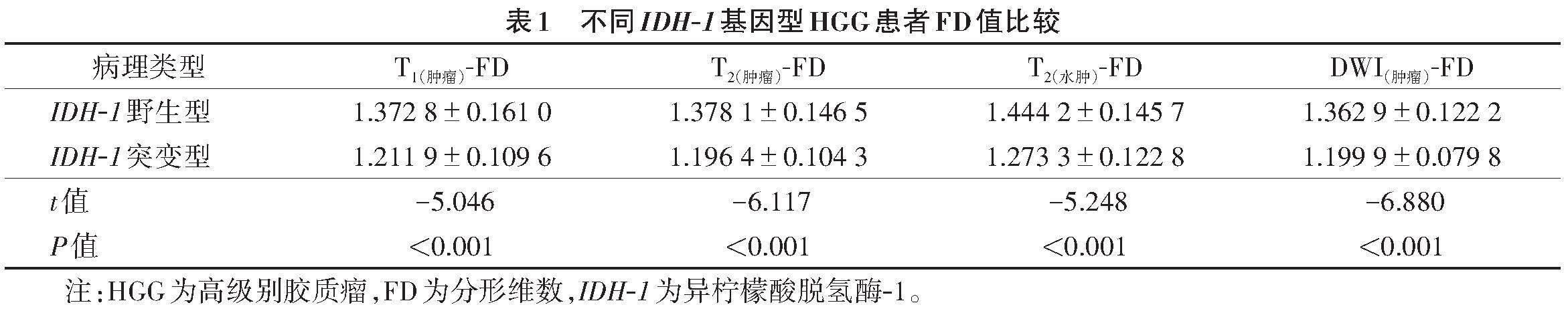
2.1 不同IDH-1基因型HGG患者的FD值比较
各序列测得的IDH-1野生型FD值均高于IDH-1突变型(表1)。
2.2 不同FD值对IDH-1突变的预测效能
使用ROC曲线分析不同FD值对IDH-1突变的预测效能,其中DWI(肿瘤)-FD效果最好,AUC为0.868,预测截断值1.305 5(表2,图2)。综合4个参数对IDH-1突变预测的AUC为0.892。多因素logistic回归分析显示,DWI(肿瘤)-FD是IDH-1突变的独立危险因素,OR=3.962(1.296~12.111)(表3)。
2.3 不同FD值与Ki-67指数的相关性
T1(肿瘤)-FD、T2(肿瘤)-FD、T2(水肿)-FD、DWI(肿瘤)-FD与Ki-67指数均呈正相关(r=0.471 3,0.464 1,0.485 3,0.329 8;均P<0.05),当Ki-67指数增加,FD值也随之增加(图3)。
3 讨论
HGG是一种预后极差的恶性神经系统肿瘤,其主要治疗方法有手术切除、放疗和化疗等[7-9]。尽管治疗方式较多,但HGG患者由于病程进展快、术前评估复杂、术后效果差,生存期仍较短[10],因此术前准确评估肿瘤状态尤为重要。检测IDH-1突变或O6-甲基鸟嘌呤-DNA甲基转移酶基因启动子甲基化状态等可指导临床诊断和治疗HGG[11-12]。有学者通过影像组学或ADC值等评估胶质瘤基因突变情况或预后,为临床提供了较多信息,但这些方法均为分析图像内部特征或肿瘤直径、截面面积、肿瘤与正球体之间相似性等简单形态特征,并未考虑肿瘤生长时的整体形态特征[13-14]。FD则可描述肿瘤的整体生长形态,由于肿瘤细胞呈明显异型性改变,肿瘤组织的力学参数和周围组织往往不同,因此导致肿瘤界面呈复杂且不规则的几何形状,如影像中常见的分叶、成角或弥漫浸润等。而胶质瘤的生长形态又与其内部基因突变关系密切[15],加之MRI可提供具有出色空间分辨力和质量的详细几何信息,可简单快速地对肿瘤或瘤周水肿的FD进行测定,为临床医师提供更多信息。因此,本研究基于MRI图像对肿瘤及瘤周水肿进行分形分析,使用FD值描述肿瘤表面的结构复杂度并量化肿瘤形态,进而评估肿瘤IDH-1突变状态,探究Ki-67指数与FD值之间的关系。
本研究发现,IDH-1野生型和突变型患者中,前者各序列测定的FD值均高于后者,可能与IDH-1野生型胶质瘤肿瘤微血管形成且血管通透性增加、肿瘤广泛浸润周围脑组织及肿瘤增殖能力强有关[16-18]。由于肿瘤恶性程度高,更易形成微血管、出现分叶等改变,导致肿瘤边界出现更多细微的褶皱、凸起或引起更大范围的水肿,使得肿瘤表面的形态复杂度明显增高。高级别肿瘤中细胞结构的增加限制了组织中水分子的运动,因此具有较低ADC值的组织在DWI上呈高信号[19]。HGG中IDH-1野生型的肿瘤细胞恶性程度高,细胞增殖旺盛,细胞密度增加,导致肿瘤细胞水分子弥散明显受限。因此,在DWI图像上勾画的ROI即为弥散受限的肿瘤细胞高密度区[20],相较于IDH-1突變型,IDH-1野生型细胞密度进一步加大,侵袭性进一步增强。因此,本研究中DWI(肿瘤)-FD的预测性能更优,且为IDH-1突变的独立风险因素,这与Jiang等[21-22]预测IDH-1的结果相同。
另外,本研究还发现随着肿瘤Ki-67指数增加,各序列测定的FD值也会增加,且测定的T2(水肿)-FD与Ki67指数相关性最高。Ki-67是一种肿瘤细胞增殖指数,广泛用于定量评估胶质瘤生长和患者预后。有研究表明,Ki-67 10%已被用作区分低级别胶质瘤与HGG,并可用于预测患者的生存率[23]。Ki-67的高表达与肿瘤生长及对大脑的高侵袭率相关,这与本研究及Yang等[23]的结论一致。Ki-67高表达导致肿瘤瘤周水肿带快速增大,水肿带的形态也更加扭曲,并形成HGG中典型的指压样水肿形态,使得T2WI测定的瘤周水肿带FD值明显增加。
本研究存在的不足:由于纳入、排除标准严格,导致样本量小,且仅包括IDH-1野生型胶质母细胞瘤和IDH-1突变型Ⅳ级星形细胞瘤。未来应扩大样本量,行多中心合作,分析不同组织成分肿瘤的形态特征,进行简单快捷的量化分析,为临床医师提供更多肿瘤信息。
综上所述,基于MRI图像对肿瘤及瘤周水肿的分形分析揭示了更多的胶质瘤固有的微观结构和病理信息,可在术前无创评估HGG患者IDH-1突变和Ki-67指数,为制订更精准的治疗方案提供依据。
[参考文献]
[1] LOUIS D N,PERRY A,WESSELING P,et al. The 2021 WHO classification of tumors of the central nervous system:a summary[J]. Neuro Oncol,2021,23(8):1231-1251.
[2] DIETTERLE J,WENDE T,WILHELMY F,et al. The prognostic value of peri-operative neurological performance in glioblastoma patients[J]. Acta Neurochir (Wien),2020,162(2):417-425.
[3] VEIKUTIS V,BRAZDZIUNAS M,KELERAS E,et al. Diagnostic approaches to adult-type diffuse glial tumors:comparative literature and clinical practice study[J]. Curr Oncol,2023,30(9):7818-7835.
[4] LI D,PATEL C B,XU G,et al. Visualization of diagnostic and therapeutic targets in glioma with molecular imaging[J]. Front Immunol,2020,11:592389.
[5] 赵君. 构建MRI影像组学及深度学习模型预测少突胶质细胞瘤TERT启动子状态的研究[D]. 兰州:兰州大学,2023.
[6] SURYADEVARA C M,VERLA T,SANCHEZ-PEREZ L,et al. Immunotherapy for malignant glioma[J]. Surg Neurol Int,2015,6(Suppl 1):68-77.
[7] TORRE M,WEN P Y,IORGULESCU J B. The predictive value of partial MGMT promoter methylation for IDH-wild-type glioblastoma patients[J]. Neurooncol Pract,2022,10(2):126-131.
[8] JASPERS J P M,TAAL W,VAN NORDEN Y,et al. Early and late contrast enhancing lesions after photon radiotherapy for IDH mutated grade 2 diffuse glioma[J]. Radiother Oncol,2023,184:109674.
[9] JUSUE-TORRES I,LEE J,GERMANWALA A V,et al. Effect of extent of resection on survival of patients with glioblastoma,IDH-Wild-Type,WHO Grade 4 (WHO 2021):systematic review and meta-analysis[J]. World Neurosurg,2023,171:524-532.
[10] SILVANI A. New perspectives:glioma in adult patients[J]. Tumori,2023,109(4):350-355.
[11] DUBINSKI D,WON S Y,RAUCH M,et al. Association of isocitrate dehydrogenase (IDH) status with edema to tumor ratio and its correlation with immune infiltration in glioblastoma[J]. Front Immunol,2021,12:627650.
[12] YANG J,ZHANG X,GAO X,et al. Fiber density and structural brain connectome in glioblastoma are correlated with glioma cell infiltration[J]. Neurosurgery,2023,92(6):1234-1242.
[13] 尹娣,陳国丹,盛玉瑞,等. 常规MRI联合扩散峰度成像的影像组学模型对脑胶质瘤分级的预测[J]. 中国中西医结合影像学杂志,2022,20(2):117-121,136.
[14] 张萌,耿瑞雯,白源,等. 动脉自旋标记和扩散张量成像评估脑胶质瘤IDH1基因表型的价值研究[J]. 磁共振成像,2023,14(10):58-64.
[15] SARKAR N,CHAUDHURI B B. An efficient differential boxcounting approach to compute fractal dimension of image[J]. IEEE Transactions on Systems,Man,and Cybernetics,1994,24(1):115-120.
[16] LI Y,QIN Q,ZHANG Y,et al. Noninvasive determination of the IDH status of gliomas using MRI and MRI-based radiomics:impact on diagnosis and prognosis[J]. Curr Oncol,2022,29(10):6893-6907.
[17] HAOPENG P,XUEFEI D,ZENGAI C,et al. High-resolution diffusion-weighted imaging of C6 glioma on a 7 T biospec MRI scanner:correlation of tumor cellularity and nuclear-to-cytoplasmic ratio with apparent diffusion coefficient[J]. Acad Radiol,2022,29(Suppl 3):80-87.
[18] BOBHOLZ S A,LOWMAN A K,BREHLER M,et al. Radio-pathomic maps of cell density identify brain tumor invasion beyond traditional MRI-defined margins[J]. AJNR Am J Neuroradiol,2022,43(5):682-688.
[19] CAO M,WANG X,LIU F,et al. A three-component multi-b-value diffusion-weighted imaging might be a useful biomarker for detecting microstructural features in gliomas with differences in malignancy and IDH-1 mutation status[J]. Eur Radiol,2023,33(4):2871-2880.
[20] KOLAKSHYAPATI M,ADHIKARI R B,KARLOWEE V,et al. Nonenhancing peritumoral hyperintense lesion on diffusion-weighted imaging in glioblastoma:a novel diagnostic and specific prognostic indicator[J]. J Neurosurg,2018,128(3):667-678.
[21] JIANG J S,HUA Y,ZHOU X J,et al. Quantitative assessment of tumor cell proliferation in brain gliomas with dynamic contrast-enhanced MRI[J]. Acad Radiol,2019,26:1215-1221.
[22] THERESIA E,MALUEKA R G,PRANACIPTA S,et al. Association between Ki-67 labeling index and histopathological grading of glioma in indonesian population[J]. Asian Pac J Cancer Prev,2020,21(4):1063-1068.
[23] YANG X,HU C,XING Z,et al. Prediction of Ki-67 labeling index,ATRX mutation,and MGMT promoter methylation status in IDH-mutant astrocytoma by morphological MRI,SWI,DWI,and DSC-PWI[J]. Eur Radiol,2023,33(10):7003-7014.

