Interface property of dissimilar Ti-6Al-4V/AA1050 composite laminate made by non-equal channel lateral co-extrusion and heat treatment
Juan Liao, Mengmeng Tian, Xin Xue
School of Mechanical Engineering and Automation, Fuzhou University, Fuzhou 350116, China
Keywords:Shear strength Co-extrusion Heat treatment Microstructure Intermetallic compounds
ABSTRACT The purpose of this paper is to examine the effect of processing parameters and subsequent heat treatments on the microstructures and bonding strengths of Ti-6Al-4V/AA1050 laminations formed via a non-equal channel lateral co-extrusion process.The microstructural evolution and growth mechanism in the diffusion layer were discussed further to optimize the bonding quality by appropriately adjusting process parameters.Scanning electron microscopes(SEM),energy dispersive spectrometer(EDS),and Xray diffraction (XRD) were used to characterize interfacial diffusion layers.The shear test was used to determine the mechanical properties of the interfacial diffusion layer.The experimental results indicate that it is possible to co-extrusion Ti-6Al-4V/AA1050 compound profiles using non-equal channel lateral co-extrusion.Different heat treatment processes affect the thickness of the diffusion layer.When the temperature and time of heat treatment increase, the thickness of the reaction layers increases dramatically.Additionally, the shear strength of the Ti-6Al-4V/AA1050 composite interface is proportional to the diffusion layer thickness.It is observed that a medium interface thickness results in superior mechanical performance when compared to neither a greater nor a lesser interface thickness.Microstructural characterization of all heat treatments reveals that the only intermetallic compound observed in the diffusion layers is TiAl3.Due to the inter-diffusion of Ti and Al atoms, the TiAl3 layer grows primarily at AA1050/TiAl3 interfaces.
1.Introduction
Dissimilar bimetal laminates are formed by the composite connection of two metals with contrasting materials and properties.Ti/Al composite laminates have grown in popularity due to their low cost and excellent properties:titanium alloys have a high specific strength, excellent high-temperature properties, and corrosion resistance,while aluminum alloys have a low density and high thermal conductivity.As a result, bimetallic laminates have a broad range of engineering applications in a variety of industries,including aerospace, marine, and heat exchangers [1-5].Because titanium and aluminum are two metals with obviously different physical and chemical properties,these differences impose stricter requirements on the co-extrusion and heat treatment processes used to create the Ti/Al composite laminate.
At the moment, the primary methods for preparing bimetal laminates are explosive compounding [6], welding compounding[7,8],diffusion welding[9,10],casting compounding[11,12],rolling compounding[13,14],and extrusion compounding[15-18],each of which has distinct advantages and disadvantages.For example,rolling compounding has the disadvantage of interface oxidation;explosive compounding and diffusion welding have the disadvantages of environmental pollution and hazardous operation; castrolling compounding has the advantages of low cost, low energy consumption, and fewer processes; however, the precision of the laminate is difficult to control and requires rolling process assistance.This work proposes a bimetal non-equal channel lateral coextrusion connection technology based on traditional equal channel corner extrusion.The die structure and plastic forming process are improved and optimized based on equal channel corner extrusion are proposed.
The non-equal channel lateral co-extrusion method is a novel material processing technology that combines aluminum alloy extrusion and titanium alloy pressure welding.It utilizes the extrusion force and temperature field of the aluminum's severe plastic deformation to create a composite of the two metals,as well as further interface bonding under a suitable heat-treatment process.The co-extrusion firstly is able to realize the deformable metal isolation from the air, which effectively ensures the oxide-free formation and unpolluted bonding interface.This means that it is possible to remove surface oxides and form an adequate bond during the co-extrusion process.In the other hand,the co-extrusion is an improved equal channel angular pressing process (ECAP),which is known for promoting diffusion bonding by reducing the activation energy.Currently,the co-extrusion process has become a favorite joining method for dissimilar metals,especially for bimetal composite rod[19,20].There are few reports of this new technology being used to create bimetallic composites.At the moment, only Grittner's team at the University of Hannover in Germany is investigating the use of non-equal channel transverse co-extrusion forming technology to create a composite of pure titanium and aluminum alloy Al99.5 [16].However, few studies have been conducted on the forming and heat treatment processes involved in the composite extrusion joining process of the Ti/Al composite laminate [21,22].Few systematic studies have been conducted on the mechanism of intermetallic layer growth during solid-state reactions at a temperature between 500°C and 600°C [9].

Fig.1.Illustration of a general non-equal channel lateral co-extrusion method.
The purpose of this study is to determine the effect of processing parameters and post-heat treatments on the microstructures and bonding strengths of Ti/Al laminations fabricated via a non-equal channel lateral co-extrusion process.The microstructural evolution and growth mechanism in the diffusion layer are discussed further to optimize the bonding quality through process parameter adjustment.
2.Materials and methods
Fig.1 illustrates a general non-equal channel lateral coextrusion method for dissimilar metal compound.The objective of this work is to develop a process for manufacturing asymmetric Ti/Al compound profiles with continuous titanium reinforcing elements.To accomplish this, a modified equal channel angular pressing die with lateral feed was designed and installed for titanium profiles, as shown in Fig.2.The die structure is mainly composed of five parts:the upper die,lower die,ram,AA1050 billet,and Ti-6Al-4V alloy sheet.The tool design enables the titanium profile to be fed laterally at a 90°angle.In this case the titanium profile is not formed, but the aluminum is pressed laterally to the titanium.Due to two processes a connection occurs: At first a mechanical clamping takes place by pressing the aluminum into bumps of the titanium surface.This creates an intimate material contact, secondly, due to the prevailing temperature and pressure conditions in the contacting zone a diffusion process starts, which is a prerequisite for an adhesive bond.
To investigate the bonding behavior via co-extrusion, combinations of commercially available AA1050 and Ti-6Al-4V were chosen.The chemical components are listed in Table 1.Due to the chemical similarities between the Al-Ti-Si-Fe systems intersections, the dissimilar AA1050 and Ti-6Al-4V alloys can be compounded under certain conditions.Aluminum alloy was cut into cylindrical pieces measuring Φ20 mm in diameter and 76 mm in length.Titanium alloy sheet was cut into 98 mm × 10 mm × 0.8 mm squares.A steel brush was used to roughen the titanium alloy sheets,and the aluminum alloy bar and titanium alloy sheet were ultrasonically cleaned in acetone.The process was carried out via direct extrusion, utilizing a 200 kN extrusion press.For 2 h,the die preheating temperatures was set to 480°C,500°C,520°C,580°C,respectively.All billets were finished with a 12 mm final width and a 3 mm thickness.All tests were conducted at a speed of 2 mm/s.

Fig.2.The experiment device for lateral co-extrusion of Ti/Al composite laminates: (a) Geometric model; (b) Experimental die.

Table 1 Chemical compositions in weight percent of the AA1050 and Ti-6Al-4V (mass fraction, %).
Cross-sections were prepared transverse to the extrusion direction for microstructural and SEM examinations.The types of SEM,ESB and XRD are as follows:The scanning electron microscope(SEM) of back-scatter electron mode (BSE) (Nova NanoSEM 230 type) was employed, as well as the energy-sensitive back-scatter electron mode (ESB) of a field-emission scanning electron microscope(Quanta 250 type).The X-ray diffraction(XRD)type of X pert 3 and Empyrean was used to determine the phase of samples.The main working conditions are the SEM voltage of 10 kV,the focused distance of 5 mm, and the EDS voltage of 20 kV.Fig.3 shows the used types of microstructural instruments, and some other conditions are as follows:
(1) Beam landing energy:500 V - 30 kV.
(2) Resolution at optimum working distance in HiVac:1.0 nm at 15 kV (TLD-SE) and 1.6 nm at 1 kV (TLD-SE).
(3) Probe current:0.6 pA to 100 Na.
(4) The angle range of phase detection:the was 20°-90°and the step size was 0.01°.
(5) The scan velocity: 5°/min.
Additionally,some samples were heated to investigate the effect of heat treatment on the evolution of the bonding zone's morphology.Compound samples produced at a co-extrusion temperature of 500°C were used in this investigation.As listed in Table 2, the following heat treatment parameters are chosen.520°C - 2 h with furnace cooling, 520°C - 4 h with air cooling,520°C-8 h with water cooling,560°C-2 h with air cooling,560°C-4 h with water cooling,560°C-8 h with furnace cooling,600°C-2 h with water cooling, 600°C - 4 h with furnace cooling and 600°C-8 h with air cooling are denoted by the letters L1,L2,L3,L4,L5, L6, L7, L8, and L9, respectively.The scanning electron microscope (SEM) and energy dispersive spectrometer (EDS) were used to characterize the microstructure.

Table 2 Parameters governing the heat treatment process.
Generally, there are some standards used for performing bond strength in diffusion bonds such as ASTM A 264 and GB/T6396-2008 standard.And, the shear strength test is one of commonly used method.In this work, since the effective size of the coextruded Ti/Al laminate sample is two small (i.e., the effective length only 20 mm) and not enough for the standard sample, a particular experimental set-up of simple shear test was developed.Fig.4 depicts a schematic diagram of the shear experiment device.The standard sample length for shear and bending tests requires more than 65 mm and 150 mm, respectively.However, the proposed shear test for bimetal composite laminate is reliable and acceptable.Because the method is able to make the sample uniform deformation in the interface and obtain a consistent mechanical data after several repeated tests.The composite board was tested using a universal testing machine at a crosshead speed of 1 mm/min (Instron 5967).SEM and XRD were used to examine the fracture surfaces.
3.Results and discussion
3.1.Microstructure and phase composition of co-extruded Ti-6Al-4V/AA1050 samples

Fig.3.(a) The Nova NanoSEM 230 field emission scanning electron microscope; (b) The Quanta 250 tungsten filament scanning electron microscope; (c) The Xpert 3 X-ray polycrystal diffractometer.

Fig.4.Shear strength test diagram for the prepared Ti/Al composite laminates.
Co-extrusion experiments with three extrusion temperatures demonstrate that it is possible to co-extrusion Ti/Al compound profiles via lateral angular co-extrusion, as shown in Fig.5.Due to relative movement within the die, the fed titanium profile was drawn into the die, allowing for close contact with the pressed aluminum.Due to the materials’ different flow rates, the AA1050 exited the die earlier but with no gap between the joining partners.
Fig.6 illustrates the microstructure of Ti/Al composite profiles co-extrusion with different extrusion processes.As illustrated in Figs.6(a) and 6(c)~6(e), smooth profiles with no crack or void are obtained in joints between titanium and aluminum layers.The Ti/Al composite interface is uneven and has a small wave shape, as shown in Fig.6(b), due to the uneven diffusion of atoms at the interface during the 480°C-0.5 mm/s extrusion process.As illustrated in Fig.6(d), a diffusion layer begins to form at the Ti/Al composite interface of the sample at 520°C, with a very thin thickness.As illustrated in Fig.6(e), a continuous layer approximately 1.86 μm thick exists at the interfaces of titanium and aluminum layers in specimens.The thickness of interfacial layers increases with increasing extrusion temperature.At a lower extrusion speed, the Ti/Al composite surface exhibits a more uniform structure.Due to the subsequent exploration of the influence of the heat treatment process on the thickness of the Ti/Al diffusion layer,the sample without the diffusion layer was selected.There is no intermetallic phase formed at the interfacial layer, as determined by XRD(Fig.7).When the co-extrusion temperature is 480-580°C, the kinetic and thermodynamic conditions for the formation of intermetallic compounds are insufficient, and the primary component of the composite interface is Ti/Al solid solution.
Aluminum undergoes strain hardening during the extrusion process.At higher extrusion temperatures, strain hardening produces crystal defects such as dislocations,which provide additional diffusion channels and increase the thickness of interfacial layers.Additionally, at lower temperatures, the thermal activity of the materials is insufficient,resulting in a low atomic diffusivity.In this case, the atomic inter-diffusion across the interface may decrease,resulting in the formation of a weaker bond between the layers.The increase in temperature promoted atom diffusion between titanium and aluminum,resulting in the formation of a diffusion layer at 580°C.The laminated composite manufactured at a higher temperature(580°C)has a stronger bond between the layers than the laminated composite manufactured at a lower temperature(480°C) under the same conditions.
EDS analyses were performed at the interface of two alloys using the linear scan method,as illustrated in Fig.7.EDS analyses reveal that there is no discontinuity in the interfacial regions of the composite profiles produced at 480°C,520°C,and 580°C,implying that the layers have a strong bond at both temperatures.As shown in Fig.6, the transition from Ti to Al at the bimetal's interface deformed at 580°C is more gentle and wider than at 480°C.There was no evidence of a plateau,which is typically associated with the formation of an intermetallic phase at the interface.According to Shehabeldeen et al.[23],the quality of the weld between two alloys was directly proportional to the bond width at their interface.Additionally,Li et al.[24]demonstrated that the effective interfacial joint between the substrates and clad materials was highly dependent on the thickness of the diffusion layers in the substrates and clad materials.Plaine et al.[25] demonstrated that a more gradual transition between alloys and a larger bond width could result in a stronger weld.
3.2.The microstructure and phase composition of samples of Ti-6Al-4V/AA1050 that have been heated
Heat treatment was performed on samples using an extrusion process of 500°C, 0.2 mm/s (extrusion temperature is 500°C,extrusion speed is 0.2 mm/s).In Fig.8, typical back-scattered electron micrographs of diffusion couples after various heat treatments are shown.As a result of the heat treatment, a dense intermetallic layer formed between the aluminum and titanium.By comparing Figs.8(a) and 8(b), it is clear that when the heat treatment temperature is lowered or the time is shortened,the interface morphology is essentially identical to that of the preparation;that is,no obvious diffusion layer is formed.When the temperature and time of heat treatment are increased,the thickness of the reaction layers increases dramatically.Heat treatment of 8 h at 600°C enables the growth of a layer to a thickness of between 2.56 μm and 3.03 μm.The SEM images of F1, F2, F4, and F5 samples are not included in the figures because the microstructure does not change significantly,and the only observation is an increase in the width of the intermetallic layer.

Fig.5.The profile of co-extruded Ti/Al laminate is schematically depicted: (a) Practical laminate nose end; (b) Bimetal interface.

Fig.6.BSE images of the interface region with varying degrees of co-extrusion:(a)480 °C,0.2 mm/s;(b)480 °C,0.5 mm/s;(c)500 °C,0.2 mm/s;(d)520 °C,0.2 mm/s;(e)580 °C,0.2 mm/s.

Fig.7.The XRD pattern of the reaction zone of a Ti/Al composite laminate during a coextrusion process at a temperature of 580 °C and a speed of 0.2 mm/s.
The Ti/Al interlayer is formed as a result of bimetal mechanical mixing and Ti/Al composition mixing at the Ti-6Al-4V/AA1050 composite interface.The lateral angular co-extrusion processes produce a premixed Ti/Al interlayer with a lamellate microstructure that facilitates effective Ti and Al inter-diffusion within the interlayer during the heat treatment process.Due to the premixture of Ti/Al interlayer formed during lateral angular co-extrusion, the Ti/Al reaction-diffusion layer generated during heat treatment eventually forms a thick diffusion-dissolution layer of intermetallic compound.Additionally, heat treatment processes affect the diffusion-dissolution layer's thickness and structure.As the heat treatment temperature and duration increase, the amount of atomic activation energy available for the diffusion of Ti/Al atoms increases,as does the number of diffused atoms.When the diffused atoms reach a critical concentration, an apparent diffusion layer forms [10].The growth of intermetallic compound in diffusionbonded Ti/Al laminated composite can be further describes as follows:First,the smooth interfaces of titanium and aluminium layers can avoid the crack or the void during the forming processes.The kinetic exponents and activation energy of Ti/Al layer growth at different annealing temperatures and times can be calculated according to the interdiffusion in Ti/Al binary system.Both Al and Ti are diffusing species at different annealing temperatures, but the dominant component is Al and the growth of Ti/Al layer has generated mainly at the interface.Additionally, Lee et al.[26]demonstrated that the homogeneously solidified microstructure existed in the cast Al alloy substrate side,while wrinkles were not formed in the Ti alloy side.The Ti/Al interface was clearly observed,but did not con contain any pores, cracks, or lateral delamination.The intermetallic compound formed at the Ti/Al interface can be deteriorated bonding strength because of its brittle characteristics.
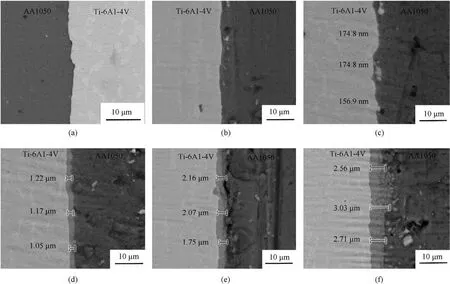
Fig.8.SEM back-scattered electron images illustrating the specimen's interfacial layer following subsequent heat treatment:(a)Co-extruded sample;(b)L7;(c)L3;(d)L8;(e)L6;(f)L9.
Table 3 summarizes the orthogonal experiment results for various heat treatment methods.SS,DF,MS and F are expressed as sum of square, degree of freedom, mean square and F-test.The larger the F value, the greater the influence of the heat treatment process on the thickness of the diffusion layer.The results indicate that,under specific heat treatment conditions,the time of the heat treatment has the greatest effect on the thickness of the Ti/Al interface,followed by the heat treatment temperature.The cooling method has the least effect on the diffusion layer thickness,and the difference between the cooling methods is primarily in the cooling rate.As illustrated in Fig.9, the heat treatment temperature and time are positively correlated with the thickness of the Ti/Al interface.The thickness of 600°C - 8 h-air cooling reaches its maximum value according to the results of the orthogonal experiment.It should be noted that the number of variance level is narrow, there are to some extent limitation on the sensitivityanalysis.We try to interpret the effect of main heat treatment process parameters on the intermetallic diffusion layer thickness as follow:the growth of the series of Ti/Al phases in the intermetallic diffusion layer prefer to induce by the heating process rather than cooling process.This is due to the most of metal elements including Ti and Al are more active during the elevated temperature than at a relatively low temperature.The diffusion layer for the 600°C-8 hfurnace-cooled Ti/Al composite laminate is predicted to be the largest in the entire experiment.

Table 3 Variance analysis of the thickness of the diffusion layer as a function of the heat treatment process.
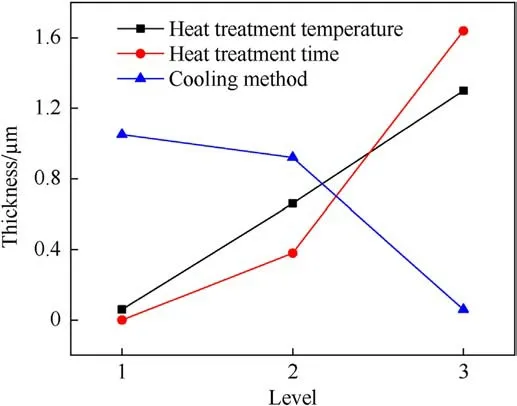
Fig.9.Orthogonal experimental results demonstrating the effect of the heat treatment process on the diffusion layer thickness.
To determine the distribution of elements at the compound's interface,an XRD sample was chosen.The results of a point analysis performed on bimetal following 8-h heat treatment at 600°C are shown in Fig.10.Spectrum B contained approximately 65.91 at.%aluminum and 25.13 at.%titanium.The oxygen content,in that case,was 8.96%.Spectrum A was distinguished by a lower aluminum content (51.3 at.%) and a higher titanium content (35.48 at.%) in comparison to the previous Spectra.According to the EDS linescanning result (Fig.10(b)), a TiAl3phase interlayer with the composition gradient characteristics was formed as a result of Ti/Al inter-diffusion.Additionally, based on the EDS detection results, a small number of island-shaped TiAl3particles were formed in the upper Al-cladding near the diffusion-dissolution layer.It was believed that under these heat treatment conditions, the diffusion of Ti into the upper Al-cladding was enhanced, resulting in the formation of a small amount of TiAl3phase.Fig.11 shows the XRD spectra of the L3 and L9 samples fracture surfaces on the Al and Ti sides, respectively.There are diffraction peaks for the Ti and Al elements in the L3 sample, but no intermetallic compounds form.TiAl3is detected for the first time in the L8 sample.The formation of TiAl3was also confirmed by XRD analysis, and subsequent experiments suggested indicated that TiAl3was the only compound observed after reactive diffusion between Ti and Al for time up to 8 h at temperatures 520-600°C.
According to the EDX and XRD analysis results within the interface zone(Table 4),the stoichiometric proportion of Ti to Al is approximately 1:1 at points B and C,which are assumed to contain TiAl3phase and Ti solid solution,respectively,due to the occurrence of reaction-diffusion according to the Ti/Al phase diagram;whereas the stoichiometric proportion of Ti to Al is approximately 1:3 at points D, which is assumed to contain TiAl3phase.The results obtained here were also compared to those obtained in Xu et al.[9]multi-laminate's Ti/Al diffusion couple experiment.The vacuum hot-pressed laminates were annealed for varying periods at temperatures ranging from 793 K to 923 K.The continuous intermetallic layers formed were entirely composed of TiAl3phase.Additionally, Grittner et al.[15] demonstrated that the only intermetallic compound detected at the Ti/Al interface was TiAl3.The thickness of the TiAl3layer increased as the annealing temperatures and times were increased.

Table 4 The distribution of EDS elements in selected locations of A-B is depicted in Fig.8(a).
The phases evolved during bonding of Ti/Al bonds can be explained as follows: With increasing the constitutive ratios of aluminium to titanium are in the following sequence:Ti3Al →TiAl→TiAl3,.In addition, the diffusion coefficient relation ofexists in the studied temperature range.With increasing the constitutive ratios of aluminium to titanium, the diffusion of aluminium is easier than that of titanium, which is limited by the growth of reaction layer.Thus, the required activation energy for aluminium diffusion in TiAl3is lower than Ti3Al.Therefore,aluminium is regarded as the dominant diffusion species.

Fig.11.XRD pattern of a reaction zone in a Ti/Al laminates: (a) L3; (b) L9.
3.3.The formation mechanism of the intermetallic compounds Ti-6Al-4V/AA1050
As illustrated in Fig.12,the reaction region's X-ray map analysis reveals the inter-diffusion of Al and Ti atoms.Ti and Al are both diffusing species, but the difference in the number of diffused atoms at the reaction zone is obvious.According to Assari et al.[10],the difference in diffusion is due to the diffusion coefficients.According to Arrhenius law, the diffusion coefficient is primarily determined by the activation energy of diffusion,and Al diffusion in TiAl3requires lower activation energy than Ti diffusion [27].As a result, Al diffuses more easily than Ti.Al is considered to be the dominant diffusion species, which is consistent with the experiment's results.Additionally, Loo et al.[28] asserted that Al atoms diffuse much faster than Ti atoms in TiAl3.Since the activation energy for aluminium and titanium diffusion titanium aluminide compounds depends on constitutive ratios of aluminium to titanium.
According to the Ti-Al phase diagram and aluminum-rich regions of the Ti-Al phase diagram (Fig.13) [27,29], the maximum solubility of Al in Ti is approximately 11.7 at.%and the solid solution of Al in Ti exists over a broad range of composition, whereas the solubility of Ti in Al is approximately 0.12 at.%and the solid solution of Ti in Al exists over a narrow range of composition.As illustrated in Fig.14(a), the solid solution of Ti in Al is expected to saturate more rapidly than the solid solution of Al in Ti in nucleating TiAl3.Inter-diffusion of Ti and Al atoms via reaction layers is required during the growth of the TiAl3layer, as illustrated in Fig.14(b).Similarly, on both sides of the diffusion layer, the density of TiAl3nuclei is different.On the Al side, we anticipate a much more uniform distribution of TiAl3nuclei due to the extremely low solubility; on the Ti side, TiAl3nuclei may form at the grain boundaries of the Ti foils when the solubility is locally exceeded.
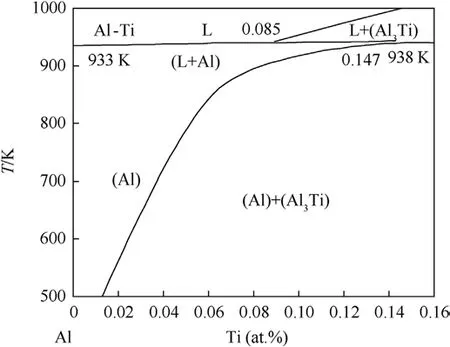
Fig.13.The region of the Ti-Al phase diagram that is aluminum-rich [27].
Fig.15 illustrates the diffusion process of the Ti/Al atoms.During co-extrusion,a significant number of dislocations are created under the influence of the extrusion force.At the welding temperature,the dislocations can move rapidly along the glide plane and settle with the least systematic energy,resulting in the formation of new sub-grain boundaries that enhance the element diffusion at the Ti/Al interface.The plastic deformation and welding heat increase the kinetic energies of the Ti and Al atoms.Due to the increaseddiffusion routes and kinetic energy,the initial recrystallized grains and interfacial diffusion layers are formed.When elements diffuse further due to heat treatment,TiAl3with a negative ΔGfis formed,resulting in a lower interfacial Gibbs free energy.When the Ti/Al inter-diffusion continues,the TiAl3grains and recrystallized grains grow further, resulting in a continuous TiAl3layer at the Ti/Al interface.

Fig.12.Map analysis of specimen morphology after 8 h at 600 °C:(a)Micromorphology of the Ti/Al composite interface;(b)Elemental distribution of the diffusion layer;(c)Al atom distribution; (d) Al atom distribution.
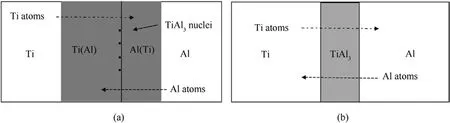
Fig.14.Schematic illustration of the Ti/Al diffusion process: (a) During the TiAl3 nucleation stage; (b) During the TiAl3 layer growth stage.

Fig.15.Standard free energy of formation(ΔGf)and effective heat of formation(ΔHm)of the Ti-Al intermetallic compounds:(a)ΔGf of the intermetallic compounds;(b)ΔHm of the intermetallic compounds.
TiAl3is the only intermetallic phase observed at the interfacial layer, as confirmed by XRD (Fig.12) and EDS (Fig.11) analyses,which is consistent with previous investigations [9,10,15,16,30].Numerous intermetallic compounds exist as equilibrium phases in the Ti-Al binary system: TiAl3, Ti2Al5, TiAl2, TiAl, and Ti3Al.The Gibbs free energy of formation expressions for titanium aluminide compounds as a function of temperature obtained by Kattner et al.[31].Among these,the formation of Ti2Al5and TiAl2requires TiAl as a starting material [32].As a result, Ti2Al5and TiAl2are omitted from our discussion here.As illustrated in Fig.15(a), TiAl3is considered to have the lowest formation free energy of all phases.However, the sequence in which intermetallic phases form is determined not only by thermodynamics but also by the system's diffusion kinetics.Kale et al.[33]proposed a modified effective heat of formation (MEHF) model that takes diffusion kinetics into account.It was successfully used by Xu et al.[9] to predict the formation of the first phase in a Ti-Al binary system.As shown in Fig.15(b)[34],TiAl3has the lowest effective heat of formation and is the first phase capable of nucleating and growing at Ti/Al interfaces,which is consistent with experimental observations.
As previously stated, TiAl3should form first at the Ti/Al interfaces during the heat treatment.The shear experiment demonstrates that shear fracture of the Ti/Al composite interface formed by the diffusion layer after heat treatment occurs on the partial aluminum side,resulting in a composite plate with a greater shear strength than the aluminum side and the aluminum side forming more brittle and hard phases.The TiAl3layer has grown primarily at the Al/TiAl3interfaces.
3.4.Mechanical properties and mechanisms of formation of metallurgical bonding interfaces Ti-6Al-4V/AA1050
3.4.1.Ti-6Al-4V/AA1050 mechanical properties
Three specimens from each heat treatment process were shear tested to determine the Ti/Al laminates' failure loads.Table 5 summarizes the shear strength of the Ti/Al composite interface after various heat treatment processes.The shear strength of the Ti/Al composite interface is proportional to the diffusion layer thickness (Fig.16).Without the formation of a diffusion layer at the interface, Ti/Al atoms diffuse unevenly along with it, resulting in a weakly bonded region at the interface, lowering the bonding strength.
At this point,test specimens fractured at the interface.When the diffusion layer is 1.15 μm thick, the shear strength of the Ti/Al composite interface increases to 70.6 MPa, exceeding the shear strength of the base material AA1050 alloy (60 MPa).The sample fractured at this point on the aluminum side of the interface.The laminates’ shear strength decreases as the interface thickness increases.The shear strength of the Ti/Al laminate is 64 MPa,and the interface layer width is currently 1.99 μm.When the interface layer is 2.77 μm wide,the shear strength is reduced to 62 MPa once more.It is concluded that a medium interface thickness results in superior performance when compared to neither a larger nor a smaller interface thickness.

Table 5 Shear strength of Ti/Al laminates subjected to various heat treatment processes.

Fig.16.The evolution of the shear strength of Ti/Al laminates as the interface thickness increases.
As a result of insufficient heat input and insufficient atomic diffusion,the interface of Ti/Al is weaker than the surrounding area,resulting in relatively weak mechanical-metallurgical bonding.In the case of the Ti/Al laminate obtained at a high heat treatment temperature and a prolonged heat treatment time,Al and Ti alloys react at the interface, resulting in a stronger mechanicalmetallurgical bond.However, once the intermetallic compounds form over the proper content, the sample fractures along with the TiAl3layer,and the shear strength decreases proportionately.It can be interpreted as follow: Firstly, the element diffusion may be insufficient due to inadequate heating input.This results in the interface bonding strength of Ti/Al laminate is less than that at the surrounding area of the interface.It is a relatively weak mechanical-metallurgical bonding.Although it was previously believed that intermetallic compounds would weaken the Ti/Al laminates and reduce their shear strength,it was demonstrated in this study that an intermetallic compound layer of sufficient thickness could benefit the interface's strength, which was consistent with the findings of Zhou et al.[35].
The microstructure of the laminates'fracture surfaces is similar under various heat treatment processes.Thus, Fig.17 shows SEM micrographs of the laminates'typical fracture surface morphology.As illustrated in Fig.17(a), when the non-diffusion layer was formed, the laminate fractured primarily at the Ti/Al interface; at the fracture surface, the cleavage planes and tearing ridge characteristic of brittle fracture were visible.Alternatively,when the Ti/Al interface formed the diffusion layer's thickness, the sample fractured on the aluminum side of the interface.The fracture surface is dotted with shallow dimples, indicating that the fracture on the local part exhibits ductile failure characteristics, as illustrated in Fig.17(b).Additionally, at the fracture toughness, island-shaped TiAl3fragments can be found.
3.4.2.Mechanisms of formation of Ti-6Al-4V/AA1050
At a certain temperature and pressure,the atoms on the contact surface of the titanium alloy and the aluminum alloy undergo a series of reaction processes such as activation,migration,diffusion,and recrystallization, and a metallurgical bond with a specifiedconnection strength is formed.Through the non-equal channel cavity, the aluminum alloy undergoes violent plastic deformation.Under the action of the extrusion force, the contact area between the aluminum alloy and the titanium alloy, which continuously produces a new surface,continues to grow.Because the aluminum alloy fills the mold cavity,the contact area between the aluminum alloy and the titanium alloy is maximized.At this point, the aluminum alloy and titanium alloy's contact surface has undergone some micro-plastic deformation.As the co-extrusion process progresses, the aluminum alloy and titanium alloy are combined and moved together in the extrusion direction into the working belt by the extrusion force and friction force.At this stage,the microscopic plastic deformation of the aluminum alloy and titanium alloy contact surface increases further.Simultaneously, the titanium alloy and aluminum alloy contact interface atoms continue to absorb energy due to the effect of the extrusion temperature.When an atom absorbs enough energy enough to overcome the constraints imposed by the existing forces between atoms,the atom is activated; the activated atom then departs from its initial equilibrium position and interacts with other activated atoms to form chemical bonds.
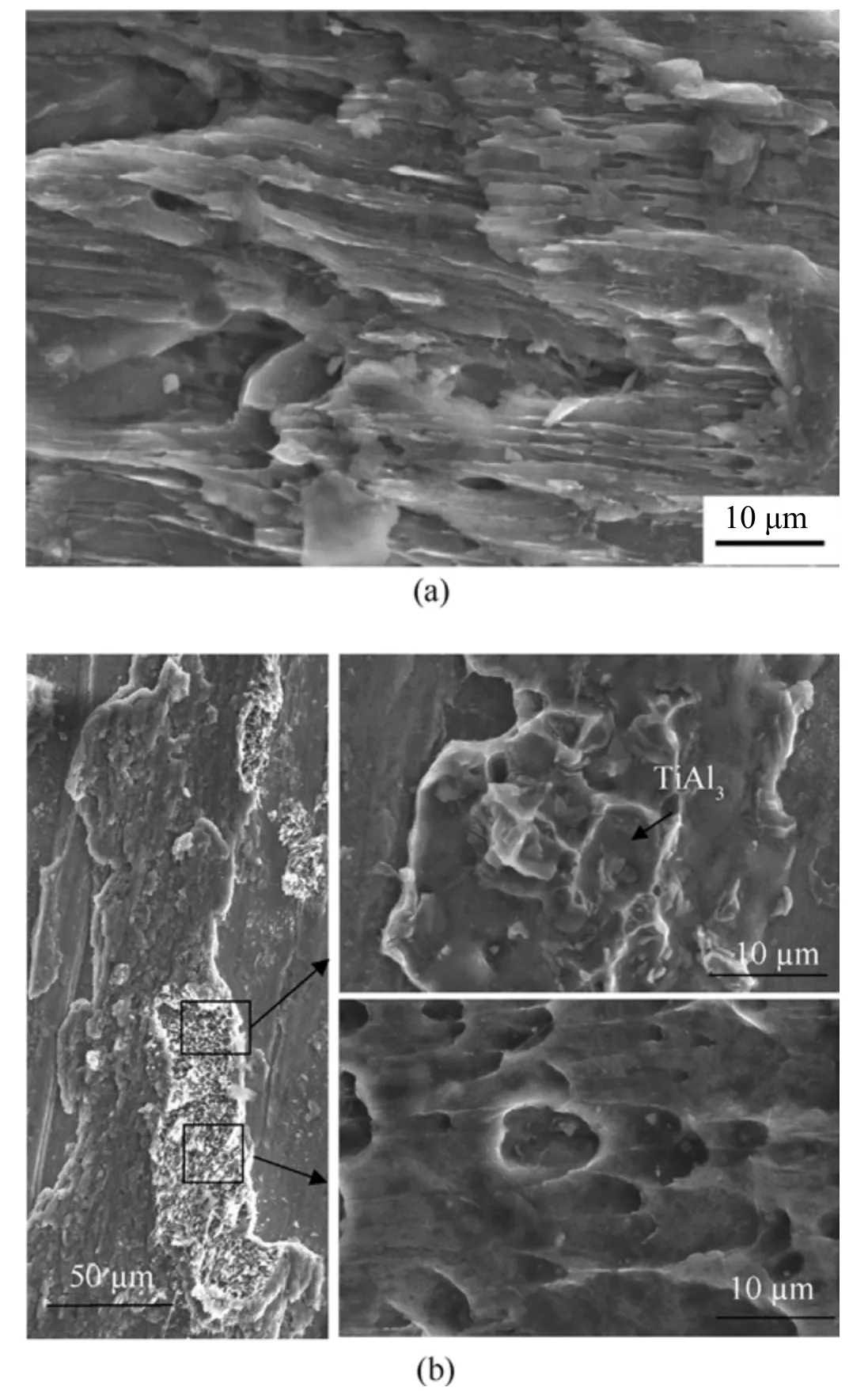
Fig.17.SEM micrographs of the fracture surfaces:(a)After cooling to 520 °C-8 h with water; (b) After cooling to 600 °C - 8 h with air.
We also focused on the heating generations required for interlayer formation during co-extrusion to elucidate the forming mechanisms.Thermal sources include (i) heat-generated during the Warm-up process;(ii)frictional heat generated at the interface between Al and its lower Ti substrate; (iii) material interior heat released during plasticized deformation and fracture of AA1050 at the Ti-6Al-4V/AA1050 interface.Al's maximum co-extrusion processing temperature has been determined to be approximately 0.7-0.8 times its melting point[36].The heat transfer from the processed Al plate to the Ti/Al interface reduces the resistance force required for the Ti alloy to deform plastically.Thus, the thermal-mechanical effects of co-extrusion act at the Ti/Al interface,resulting in the formation of a relatively thick and dense Ti/Al interlayer structure with laminated characterization.Co-thermalmechanical extrusion's effect can completely realize metallurgical bonding at the Ti-6Al-4V/AA1050 interface under the right process conditions.
Mechanical mixing of the bimetal Al and Ti in the resulting interlayer pre-homogenizes the chemical compositions.Prehomogenization is preferred to ensure that the Ti-6Al-4V/AA1050 composition is sufficiently homogenized during the heat treatment procedure.The interlayer's Ti/Al laminated structure significantly reduces the Ti/Al inter-diffusion distance.After heat treatment with a suitable heat treatment method, a relatively homogeneous diffusion-dissolution layer of Al/TiAl3dual-phase structure is easily formed.The Al/TiAl3diffusion-dissolution layer at the Ti/Al interface can enhance bonding strength by providing good interfaces between Al and its adjacent TiAl3phase regions.However, the Al/TiAl3interlayer's bonding performance degrades due to the presence of too many TiAl3phase regions.
4.Conclusions
The non-equal lateral channel co-extrusion process was used to fabricate defect-free Ti-6Al-4V/AA1050 composite laminates.After heat treatment, the microstructure and mechanical properties of the Ti-6Al-4V/AA1050 interface regions were investigated.The following conclusions were reached as a result of this study:
(1) It is possible to achieve seamless interfaces between titanium and aluminum layers that are free of cracks or voids.There is an obvious diffusion layer,measuring approximately 1.86 μm at the extrusion temperature of 580°C.The duration of the heat treatment has a significant effect on the thickness of the diffusion layer.When heat treatment temperatures and times increased, the diffusion layer thickness increases.At 580°C-8 h-air cooling, the diffusion layer reaches a maximum value of approximately 1.95 μm.
(2) The shear performance is initially enhanced by the formation of intermetallic compounds at the interface.With a 1.15 μm interface layer, the maximum shear strength of the Ti-6Al-4V/AA1050 laminates is 70.6 MPa.When the thickness of the interface is increased further,it is discovered that the maximum shear load decreases again.It is a ductile fracture that occurs when intermetallic compounds are formed, and the sample fractures on the aluminum side of the interface.
(3) At the interface of Ti-6Al-4V/AA1050,the only intermetallic compound observed is TiAl3.While both Al and Ti are diffusing species under various heat treatment conditions,Al is the dominant component, and the TiAl3layer has grown primarily at the AA1050/TiAl3interfaces.Under suitable anneal conditions, a well-composed homogenized intermetallic compound layer improves the laminate bonding strength.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work received the financial support by the National Natural Science Foundation of China (No.12272094, 51805087 and 51705080)and the Natural Science Foundation of Fujian Province of China (No.2022J01541).
- Defence Technology的其它文章
- Evolution of molecular structure of TATB under shock loading from transient Raman spectroscopic technique
- MTTSNet:Military time-sensitive targets stealth network via real-time mask generation
- Vulnerability assessment of UAV engine to laser based on improved shotline method
- Free-walking: Pedestrian inertial navigation based on dual footmounted IMU
- Investigation of hydroxyl-terminated polybutadiene propellant breaking characteristics and mechanism impacted by submerged cavitation water jet
- Estimation of surface geometry on combustion characteristics of AP/HTPB propellant under rapid depressurization

