Estimation of surface geometry on combustion characteristics of AP/HTPB propellant under rapid depressurization
Kixun Chen , Zhenwei Ye , Xiochun Xue ,*, Yonggng Yu
a School of Energy and Power Engineering, Nanjing University of Science and Technology, Nanjing 210094, Jiangsu, China
b School of Science, Hangzhou Dianzi University, Hangzhou 310018, Zhejiang, China
Keywords:AP/HTPB propellant BDP model Rapid pressure decay Burning surface geometry
ABSTRACT The 2D sandwich model serves as a potent tool in exploring the influence of surface geometry on the combustion attributes of Ammonium perchlorate/Hydroxyl-terminated polybutadiene (AP/HTPB) propellant under rapid pressure decay.The thickness of the sandwich propellant is derived from slicing the 3D random particle packing, an approach that enables a more effective examination of the micro-flame structure.Comparative analysis of the predicted burning characteristics has been performed with experimental studies.The findings demonstrate a reasonable agreement,thereby validating the precision and soundness of the model.Based on the typical rapid depressurization environment of solid rocket motor(initial combustion pressure is 3 MPa and the maximum depressurization rate is 1000 MPa/s).Atype(a flatter surface),B-type(AP recesses from the combustion surface),and C-type(AP protrudes from the combustion surface) propellant combustion processes are numerically simulated.Upon comparison of the evolution of gas-phase flame between 0.1 and 1 ms, it is discerned that the flame strength and form created by the three sandwich models differ significantly at the beginning stage of depressurization,with the flame structures gradually becoming harmonized over time.Conclusions are drawn by comparison extinction times: the surface geometry plays a pivotal role in the combustion process, with AP protrusion favoring combustion the most.
1.Introduction
Ammonium perchlorate/Hydroxyl-terminated polybutadiene(AP/HTPB)is considered as one of most crucial energetic materials in the applications of solid rocket motors for both ballistic missiles and aerospace engineering due to its availability, good ignition characteristics, and predictability of the burning rate.The main drawback of the solid rocket motor is its lack of flexibility comparison with liquid or hybrid propellants.However, with the increasing employment of AP/HTPB propellant in solid rocket motor propulsion, a successful and precise operation of terminating combustion rapidly on command is required(e.g.,at initial times of satellite launch, separation of Multistage Rocket Engines or when the rocket has reached its desired velocity).Rapid depressurization has been demonstrated to be an attractive technique to extinguish the burning AP/HTPB propellant.When the solid rocket motor is suddenly opened, allowing the high-pressure conditions in combustion chambers drop rapidly to ambient in a few milliseconds,which will lead to extinguishment.The flame extinction characteristics are dependent on combustion chamber geometry,various propellant properties, and the depressurization rate [1].
To achieve reliable depressurization quenching, a thorough investigation of the flame structure resulting from the combustion of AP/HTPB propellant is essential.The Beckstead-Derr-Price flame model stands as the most widely accepted approach for handling the combustion of heterogeneous AP propellants.Over the past forty years, this model has undergone continuous refinement by subsequent researchers,leading to enhanced predictive capabilities[2-7].From a microscopic perspective, the global reaction of the AP/HTPB propellant consists of multiple "micro-element flames".Exploring the micro-element structure flame is of significant importance in understanding the combustion mechanism of AP/HTPB propellant.Researchers have explored two distinct approaches when studying the flame structure of AP/HTPB propellant.Firstly,there is the examination of the flame structure formed by a single AP particle.Refs.[8-10]employ high-speed OH planar laserinduced fluorescence(PLIF)to capture images of the diffusion flame generated by a single AP particle.Gross and Beckstead [11-13]conducted numerical simulations of micro-flames formed by different AP particle sizes,corroborating the experimental findings of Refs.[8-10], and investigating the temperature and species distribution within the combustion field.Secondly, there is the exploration of sandwich propellant combustion.Considering the complex nature of the AP/HTPB micro-combustion process, which involves a multi-phase, chemically reacting system with strong temperature gradients and intricate geometry, researchers proposed the use of a 2-D configuration known as the laminae of AP and HTPB(sandwich model).Extensive experimental[5,7,11-15,16]and theoretical [5,7-10,14,17-19,20] research on the sandwich model has been conducted, providing a substantial database to validate numerical models.Interestingly, Vijay [19] employed the cutting method to connect the sandwich model with the threedimensional AP/HTPB propellant, yielding favorable predictive results.However, in the actual AP/HTPB propellant, the AP particle may protrude or recess from the burning surface.The influence of the burning surface shape on the physical information of the combustion field is shown in Refs.[21,22].
Discoveries of flame regimes are important for the development of the unsteady combustion of solid propellants.In transient combustion processes, there exists a time delay between an instantaneous change in pressure and the subsequent adjustment of the gas phase flame structure, resulting in deviations between the instantaneous burning rate of the compound and the corresponding pressure.This time delay is essentially responsible for transient combustion phenomena.Consequently, during the process of depressurization, the heterogenous blow-out effect manifests itself prior to the temperature distribution aligning with the steady-state combustion pressure.This effect arises from variations in surface temperature gradients and heat feedback from the flame attributable to their distance from the burning surface.When this phenomenon becomes pronounced, it can potentially lead to combustion extinction.Numerous experimental and theoretical investigations have been carried out on the transient combustion characteristics of AP/HTPB propellant.A brief review of the literature on this is presented below.
In the aspect of experiments, Ciepluch [23,24] first carried out transient experiments to simulate actual motor combustion conditions in 1961.It was recognized that there is a critical depressurization rate exists below which combustion keeps, and above which extinction occurs.Mchale [25] investigated the transient combustion process of three different solid propellants using an optical strand burner with fused silica windows (To minimize the corrosive burning effect and to cause one-dimensional ’cigarette’burning of the propellant).The critical extinction point(luminosity zero)was determined from the plot of dP/dt values vs p,the results show that the experimental results are in good agreement with the theoretical analysis.Merkle[26]used a similar experimental device(Extinction was considered to occur when zero light emission could be detected) to find that higher AP content, finer AP particles and propellant containing Al are more difficult to extinguish.At the same time, the influence of combustion chamber shape on the depressurization extinguishment of propellant was examined.
In theory, since Ciepluch et al.used the exponential depressurization method to study the transient combustion process in 1961,the maximum depressurization rate occurred at the very beginning of the transient combustion.This fact is erroneously interpreted to show a tight link between dynamic quenching process and the initial depressurization rate.Merkle [1] recognized that the dynamic extinction depends on the entire P(t) curve.At the same time,it is pointed out that Mchale[25]and Paul[26]misinterpreted the quasi-steady assumption of the gas phase as the thermal feedback itself is quasi-static, so a new quasi-static flame model is furnished.The critical quenching temperature was empirically introduced for simulations.Solid state pyrolysis reaction was considered too weak to occur deflagration waves below this temperature.Tien[27]attributed heat loss from the flame as the reason for the instantaneous burning rate lower than the corresponding pressure steady.At the same time, Tien argues that the critical quenching temperature introduced by Merkle is equivalent to the quenching burning rate and it has a strong correlation with heat loss and final pressure.Therefore,the numerical simulation of fixed critical quenching temperature for the specific propellant is not rigorous.Tien’s theory was extended by Mongia and Ambs [28],which considered that the heat release of the condensed phase and the finite time depends on the instantaneous properties near the combustion surface during transient combustion.Suhas and Bose[29]divided the quasi-static process of the gas phase by modifying the KTSS model and the unsteady gas phase continuity equation.The idea of using the linear thermal feedback law of the KTSS model to study extinguishment is relatively novel.Although the physical mechanism of the method is questionable,the team recognizes the importance of the finite time associated with the gas-phase process.
The theoretical research on transient depressurization and flameout of solid propellants mainly focuses on the prediction of flameout behaviours.At the same time,some experimental data of transient combustion of solid propellant are difficult to reproduce(e.g., the experimental determination of pressure limit P and flameout time T, because these data are easily affected by small perturbations including environmental disturbances).So solidpropellant dynamic still does not appear to be available from a scientific point of view,especially the evolution of the micro-flame structure of solid propellants under transient combustion and the prediction of quenching time.In recent years, some researchers began to use the sandwich model to study the unsteady combustion of AP/HTPB propellant.Xue et al.[30].established a onedimensional unsteady AP/HTPB transient combustion model using the critical quenching temperature to predict quenching time,which is in good agreement with the experimental results of Yu et al.[31].Subsequently, Cao [32] and Ye [33] studied the rapid depressurization quenching process of AP/HTPB based on the twodimensional sandwich model based on Xue.However,the previous literature lacks a systematic analysis of the quenching mechanism of transient combustion and the sandwich model they adopt does not consider the regression of the combustion surface.Since the temperature of the propellant combustion surface is not the same in the process of rapid depressurization, the shape of combustion surface will change with time, thereby affecting the distribution law of the multi-parameter of the combustion field.At the same time, the effects of initial combustion pressure, final combustion pressure, and depressurization rate on the unsteady combustion process of solid propellants are mainly reported in previous literature.However, Refs.[34,35] show that the AP particles may protrude or recess from the surface in the real combustion process,which will also affect the transient depressurization process of the combustion field.Therefore, it is necessary to study the effects of different combustion surface shapes on the transient combustion process.
The works of Xue [30], Cao [32] and Ye [33] have given insight into the process of extinction of AP/HTPB propellant by depressurization.But the mechanism of combustion field extinguishment caused by rapid depressurization is still missing.This paper aims to bridge this gap.Using the method proposed by Vijay[27],the threedimensional AP/HTPB propellant is transformed into a twodimensional sandwich physical model and the micro-scale irregular regression fluid-solid coupling transient combustion model of AP/HTPB propellant is established to investigate the transient depressurization combustion process of AP/HTPB.Finally,the influence of different combustion surface shapes of the sandwich model on the flame structure is explored based on the typical working conditions of the rocket engine (P = 3 MPa, dp/dt = -1000 MPa/s).
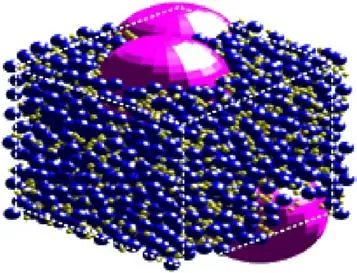
Fig.1.3-D SD III-22 propellant box.
2.Acquisition of 2-D sandwich model
In this paper,the SD III-22 propellant in Miller[16]is selected to generate 3-D random particle packing with AP particle loading rate of 86% by the Monte-Carlo method, as shown in Fig.1.Subsequently, the 2-D sandwich model is obtained by slicing the 3-D propellant pack according to Vijay’s method[19] (The thickness of AP and HTPB are 28 μm and 12 μm),as presented in Fig.2.In Fig.2,L1= 12 μm and L2= 40 μm, the height and depth of the computational zone above and below the surface are 700 μm and 500 μm.To simplify the calculation, the following assumptions are proposed:

Fig.2.2-D sandwich model.
(1) The experiments of Boggs and Hightower [36,37] indicate that the melting layer exists on the propellant surface.Gassolid phase heat transfer only considers the thermal conductivity.
(2) Oxidizer and binder are considered as two independent components with different thermal parameters due to the heterogeneous nature of AP/HTPB propellant.The zone where the chemical reaction occurs is very small and close to the burning surface.
(3) Ossen approximation is applied due to the thin width of the sandwich model.
(4) Assuming the species from oxidizer and binder are the ideal gas whose Lewis number and Prandtl number are 1 and constant, and thermal conductivity is the function of temperature.
(5) It is assumed that the products from the solid phase undergo adiabatic expansion during the rapid depressurization process.
(6) AP/HTPB propellant burns thoroughly under the high temperature and oxidizing environment, the effects of incomplete combustion and radiation could be ignored.
3.Micro-scale AP/HTPB combustion model
3.1.Modelling of solid phase
3.1.1.Solid phase control equation
It can be seen from assumption 1 that the solid phase heat transfer equation satisfies the following form:
where ρ(kg/m3), c (J·kg-1·mol-1),λ(W·m-1·k-1), and sc(W/m3)are density, the specific heat, thermal conductivity, and energy source term of the solid phase.
where Qc,AP(J/kg)and Qc,B are the decomposition heat of AP and HTPB; rb(cm/s) satisfies the following form:
In the expression of rb,rAPand rBare burning rates of AP and HTPB;Ts(K)represents the temperature of the burning surface.AAP(m/s),AB,EAP,EB(J/mol), TAP,S and TB,S represent pyrolysis rate constants,pyrolysis activation energy and burning surface temperature of AP and HPTB.
For the far field of the solid phase:
3.2.Modelling of gas phase
3.2.1.Kinetic details
AP/HTPB transient combustion includes two components pyrolysis and gas reaction process.
Under high-pressure transient combustion, AP undergoes the following pyrolysis process:

Chemical reaction rates R1and R2are described by Arrhenius law:
where D, n, P (atm), and E /Ru(K) are the pre-exponential factor,pressure index, ambient pressure,and reaction activation energy;[X ], [Y ],and [Z ]represent the mass fraction of components ˜X , ˜Y ,and ˜Z ; T is the gas temperature; subscripts 1 and 2 correspond to the first step and the second step reaction.
3.2.2.Gas phase control equation
The gas phase is described by continuity equation for a variable density gas, momentum equation, Navier-Stokes equation, and species equation.
The corresponding equations for temperature and components ˜X , ˜Y and ˜Z are as follows:
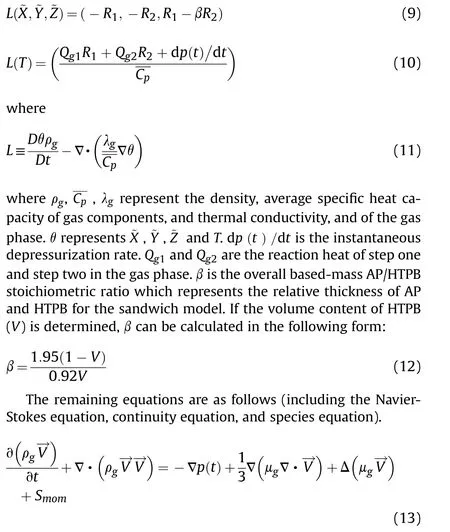
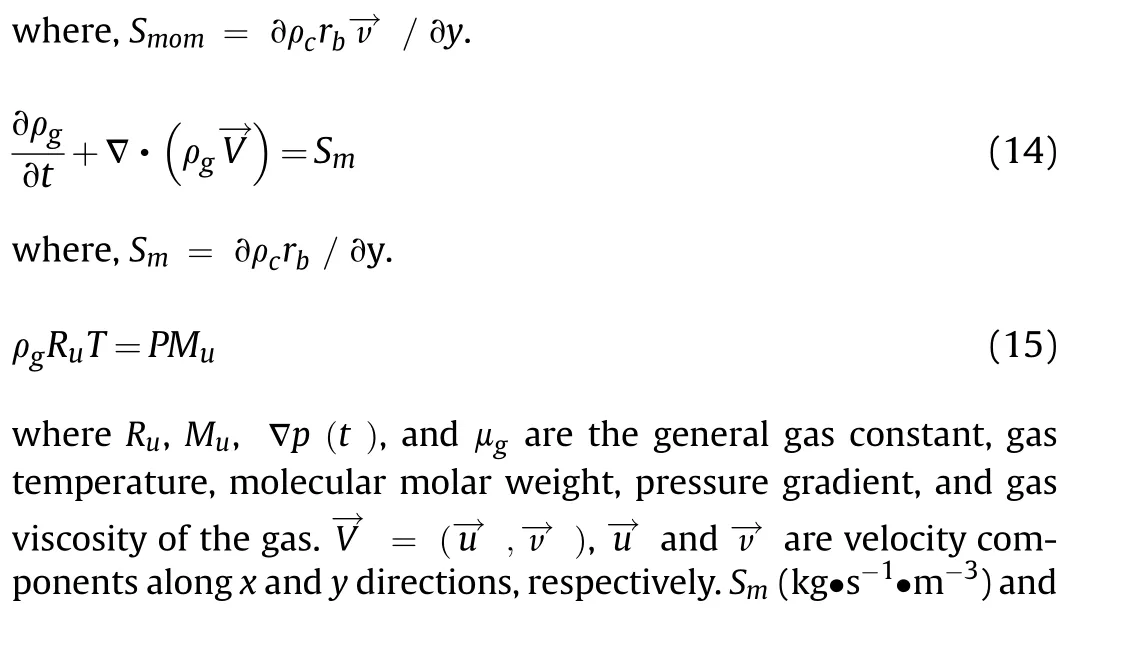
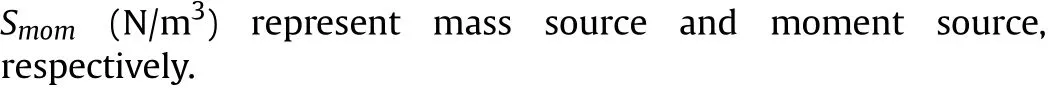
The thermal conductivity λgand gas viscosity μghave the following form:
3.3.The recessing law of burning surface
The burning surface moves downward with a speed rb.As can be seen from the expression of rb, the pyrolysis activation energies of fuel and oxidizer are large.So,the small change of temperature will influence the burning surface characteristics.In other words, the temperature of the burning surface dominates the propagation process of the surface.The handling method of dynamic surface in this paper is similar to the approach described in Ref.[18].
3.4.Connection conditions of solid-gas phase
In the propellant surface,the equilibrium relationship can refer to our previous work[38].
The species distribution above the burning surface is as follows:
3.5.Extinction criterion
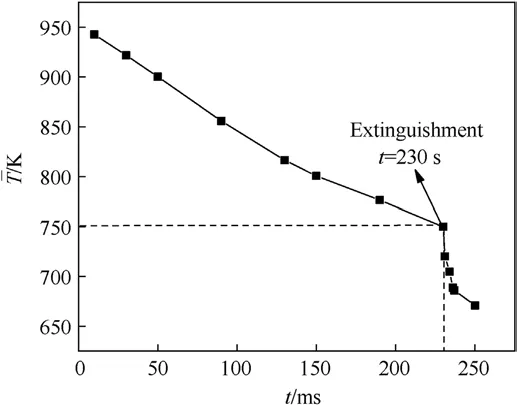
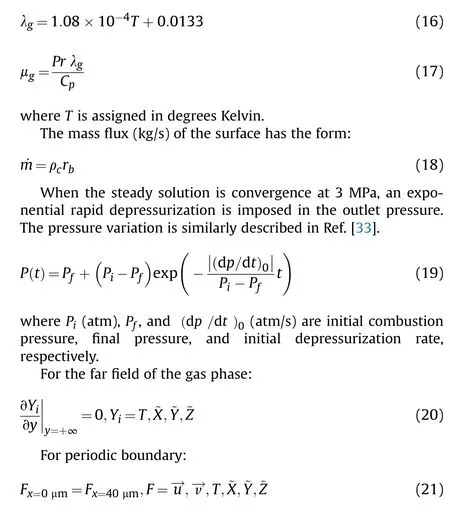
Fig.3.Comparison between predicted extinction time and experimental value [40].
Table 1 Grid independence verification.
During the transient depressurization combustion process of AP/HTPB propellant, the temperature of the burning surface decreases furtherly because of the heterogeneous blowing-out effect(i.e., the flame away from the burning surface, resulting in less thermal flux for solid phase pyrolysis.).When the average surface temperature is around 750 K [22], the thermal decomposition products of AP cannot provide sufficient oxidizing gas to sustain AP/HTPB propellant combustion.Thus, the combustion of AP/HTPB propellant under transient pressure decay may be quenched.Our team (referring to Ref.[39]) has carried out the extinction experiments of the AP/HTPB propellant and obtained that the AP/HTPB propellant was extinguished once the combustion temperature is less than 750 K.Also,the critical extinction temperature was always 750 K for whatever the content and locations of AP particles in AP/HTPB propellant were [39].Subsequently, we found that 750 K as the critical quenching temperature is also reasonable after a sequence of numerical tests.To qualitatively estimate the flameout effects under rapid depressurization, 750 K is introduced as the critical quenching temperature of AP/HTPB.
In this paper,the 20th experiment of Ref.[40](Initial pressure is 3.15 MPa, the maximum depressurization rate is 56.4 MPa/s) is selected to verify the reliability of selecting 750 K as the critical quenching temperature.Numerical simulation is carried out under this condition, and the predicted variation of surface temperature with time is shown in Fig.3.The burn surface temperature decreases monotonically from 942.68 K at 10 ms to 750 K at 230 ms as the depressurization process progresses.The relative error between numerical simulation and experimental result (240 ms) is only 4.16%, which shows that it is feasible to select 750 K as the critical extinction temperature.
3.6.Mesh partitioning and its independence verification
Dynamic mesh technology is used to simulate the transient nonplanar regression surface.The finer grids are used near the AP/HTPB interface where reaction rate and gradients are expected to be steep.Grid independence verification has to be carried out to save computing resources based on ensuring the accuracy of the calculation.In this paper, three sets of unstructured grids whose numbers are 10000 (coarse grids), 25000 (medium grids), and 50000(fine grids)are selected respectively to calculate the steadystate burning rate of 3.45 MPa.The calculation results of the three sets of grids are compared with the experiment [16], as shown in Table 1.
Fig.4.Local schematic with 25000 grids.
According to the data in the table above, comparing fine and coarse grids,the calculation result of medium grids is independent of the number of grids based on ensuring accuracy.To save computing resources, the medium grids are used for numerical calculation.The grids near the burning surface are shown in Fig.4(Due to the large aspect ratio of the grids,only the grid distribution near the burning surface is shown).
In actual AP/HTPB propellants, AP particles may protrude or recess from the burning surface, whereas HTPB acts not only as a fuel but also as a binder.Therefore,in the numerical simulation of sandwiches, HTPB is often considered as a plane while AP can be planar or non-planar, as shown in Fig.5.Refs.[34,35] indicate the distance from the AP particle to the plane has a great influence on the combustion characteristics,and its distribution function can beexpressed as, where a, b and c are constants.Recent literature on the effects of surface characteristics on flame properties has been examined by Zou [20] et al.However,apart from the burning rate, they don’t make extensive comparisons with experiments.The proposed model by Zou is still steady and doesn’t consider the evolution of pyrolyzing surfaces, thus limiting their work.Since the burning surface shape will affect the flame structure of the combustion field,the thermal feedback from the gas phase will make the irregular regression law of the burning surface different.However, Considering the micro-unsteady evolution of the regression surface with time and micro-unsteady combustion characteristics have been seldom examined so far.The objectives of this paper are devoted to bridging this gap.

Table 2 Grid independence verification for B-type sandwich.
This study keeps the width of AP and HTPB constant for facilitating the comparison.A-type (a flatter surface), B-type (AP recesses from the combustion surface), and C-type (AP protrudes from the combustion surface) sandwich models are numerically calculated to investigate the burning surface shape on the transient combustion properties.Similarly,three types of unstructured grids are used to test the grid independence to capture computational accuracy, which are shown in Table 2 and Table 3.As can be seen,the medium grids(i.e.,18697 grids for B-type sandwich and 19673 grids for C-type sandwich)can obtain good computational accuracy based on saving computational resources.The grids near the burning surface of B-type and C-type are shown in Figs.6 and 7.
3.7.Calculation method, convergence criterion and parameter selection
Finite volume discretization is used to solve transient numerical calculation, the second-order upwind scheme is used to solve density, mass, momentum, and composition equations, whereas the first-order accurate scheme is used for discretizing the time derivative.The time step is chosen as 1 μs.The transient calculation is used in the simulation.The continuity residual,velocity residual,and component residual are less than 1×10-3in each step,while the energy residual is less than 1 × 10-6in each step.The parameters used in the calculation are identical to those used by Buckmaster [41].
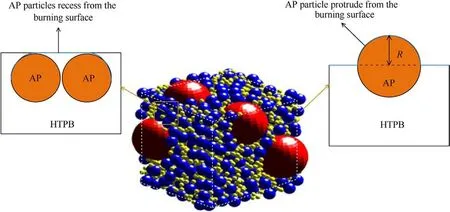
Fig.5.The schematic diagram of AP particles protruding or recessing from the burning surface.

Table 3 Grid independence verification for C-type sandwich.
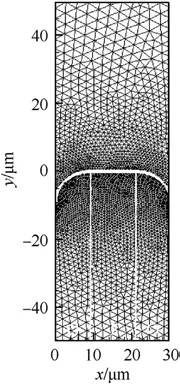
Fig.6.B-type grids distribution.
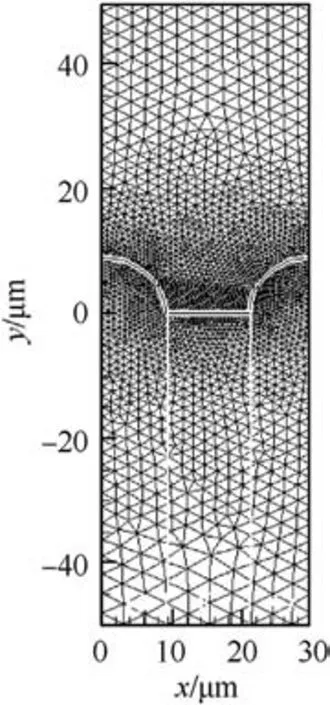
Fig.7.C-type grids distribution.
4.Validation of the model by experiments
4.1.Burning surface physical information verification
The comparison between predicted burning rates and Miller’s experimental results is shown in Fig.8.As can be seen,a reasonable agreement is obtained.Fitting the predicted burning rate at each pressure according to Eq.(24).where rbis the burning rate,a and n represent the burning rate at unit pressure and pressure index,respectively.The values of a and n obtained by fitting are shown in Fig.8, where the value of n coincides with the range of 0.4-0.6 given in Ref.[42].
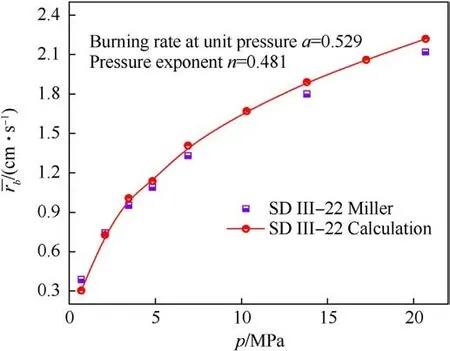
Fig.8.Comparison between predicted burning rate and experimental value.
4.2.Verification of burning surface characteristics
Fig.9 shows the comparison between the predicted combustion surface and the reported in Refs.[5,7].It can be seen from the figure that the theoretically predicted regression trend at HTPB is consistent with the experiment.The AP regression rate near the AP/HTPB interface is larger than that on the edge sides, and there are two small bands at the interfaces between AP and HTPB [5].
However,a periodic sandwich unit for modelling limits current work as the average total width of the experimental image is 3000 μm, while the thickness of the 40 μm sandwich model is used for modelling.A direct comparison is therefore not possible.Despite the difference between simulation and experiment, numerous important combustion and surface geometry characteristics in sandwich experimental cases can be replicated using a periodic sandwich unit.
5.Results and discussion
In this paper,the gas phase unsteady combustion and extinction characteristics are investigated at typical solid rocket pressure(3 MPa)with the maximum depressurization rate of 1000 MPa/s by using ’A’, ’B’ and ’C’ types of sandwich models.
5.1.Combustion field characteristics before quenching
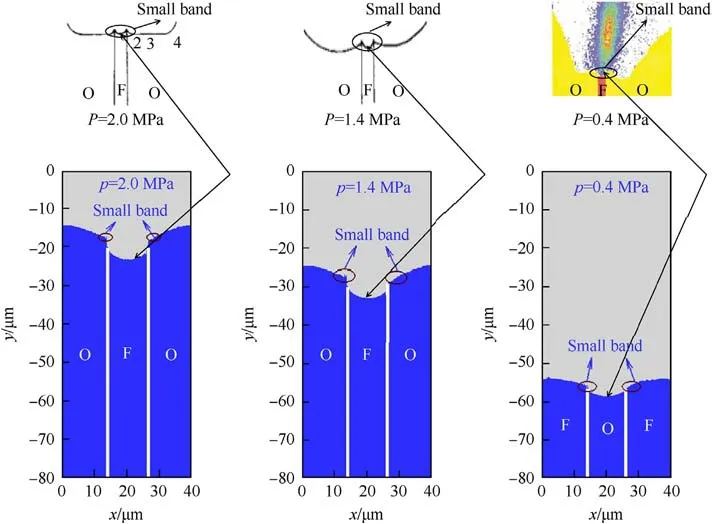
Fig.9.Comparison of surface shape.
In this section, the simulations of the ’A’ type sandwich model are taken as an example.The distribution characteristics of physical quantities in the combustion field at different times are examined to give insight into fluid dynamics and chemical kinetics on combustion during the rapid depressurization process.Fig.10 shows the spatial distribution of combustion field temperature, heat release and component ˜Z at 0.5 ms,1 ms,3 ms,and 9.7 ms.At t =0.5 ms,the combination of Eqs.(7) and (8) shows that the chemical reaction rate is positively correlated with the combustion pressure.The higher the pressure, the greater the chemical reaction rate.Currently, the faster first-step reaction R1pyrolysis extensive oxidizing component ˜Z , and some of which even diffuse above HTPB, resulting in the left and right components ˜Z merging.The oxidizing gas reacts with component ˜Y from the binder,releasing a large amount of reaction heat in the vast reaction zone, and its maximum value is 1.61 ×1013W.Because the gas reaction zone is close to the burning surface,where the temperature gradient tends to be significantly steep.The temperature above the centre of AP increases from 883 K to 2600 K at 20.8 μm,while the distance above HTPB reaches 2600 K only at 6 μm.At the same time, the AP is convex above HTPB, making it easier for the fuel vapor to diffuse into the oxidizer stream.It can be observed from Fig.10 that the chemical reaction at the AP/HTPB interface is particularly intense,so the temperature gradient near the interface is also the largest.At t=3 ms,the gas phase distribution area of component ˜Z is greatly reduced, which increases the difficulty of the gas phase chemical reaction.Numerically, the maximum value of the component ˜Z decreases from 0.57 in 0.5 ms to 0.44 in 3 ms.The decrease of the content of oxidizing gas inhibits the second step reaction R2,resulting in a weaker reaction strength.At t =1 ms,the peak value of heat release is 1.17×1013W,when t =3 ms,it decreases by 76%compared with t = 0.5 ms, which is 3.85 × 1012W.The extremely reduced heat release indicates that the temperature gradient near the oxidizer binder interface becomes smaller,and the distances of the above centre of AP and HTPB reaching 2600 K becomes 51.8 μm and 49.5 μm, respectively.It can be seen from the temperature contour of Fig.12 that the characteristic of contour lines makes a transformation from‘W’type at t =0.5 ms to’ID’type at t =3 ms,which shows that reactants are uniformly mixed before the reaction completes.At t=9.7 ms,component ˜Z is approximately onedimensionally distributed above the gas phase.It takes 12 μm to change the content of component ˜Z above HTPB from 0 to 0.1,and its maximum content is only 0.24.The smaller content of component ˜Z results in the chemical reaction tending to stagnate, this is the reason why the maximum heat release is only 1.76 × 1011W.The larger low temperature-zone is seen near the burning surface,which aggravates the heterogeneous blow-out effect.The average surface temperature is 750 K,indicating that the thermal feedback from the flame cannot maintain the thermal decomposition of the propellant, resulting in the occurrence of flameout.
The variation characteristics of the burning surface and its area are shown in Fig.11.In the initial stage (0.5-3 ms), the gas phase produces a large amount of energy, which leads to the rapid combustion of the propellant.The centre of HTPB moves 41.6 μm downward,accounting for 70%of the retreat distance of the whole combustion process.Subsequently,in the medium stage(3-5 ms),the regression rate of the burning surface slows down compared with the previous stage,and its area decreased from 5.4×10-5m2to 5.25×10-5m2,indicating that the regression rate of AP began to exceed that of HTPB.Finally, at t = 5-9.7 ms, the difference in burning surface temperature is nearly equal, which induces burning surface area keeping constant, and its average regression rate at the centre of HTPB is 3.2 μm/ms.
The steepness of the burning surface can reflect the distribution characteristics of the gas phase reaction zone to a certain extent.During the depressurization process, the height differences at the centre of AP and HTPB at 0.5 ms,1 ms,2 ms,3 ms,5 ms,8 ms,and 9.7 ms are 7.55 μm,7.97 μm,9 μm,7.6 μm,5 μm,3.1 μm,and 2.6 μm,respectively.The decreasing drop not only appears the gas phase reaction zone more spread out with time but also indicates that the heat flow from the gas phase is the primary driving force for solid phase pyrolysis.
To elucidate information about the transient combustion of AP composite propellant,the variations of gas maximum temperature,average burning surface temperature, heat flux, and mass flux are shown in Fig.12.It can be seen from the figure that the maximum temperature of the gas phase fluctuates with time.The reasons for this are the irregular change of the burning surface shape and the complex chemical reaction of the gas phase.The average burning surface temperature, mass flux, and heat flux decrease monotonously from 884 K, 57.97 kg/s, and 59.43 W at 0.5 ms to 750 K,3.28 kg/s, and 8.56 W at 9.7 ms.Their variation trends are consistent with the analysis in Fig.10.It is worth noting that although the downward trends of the three curves are different at the beginning of depressurization,all of the three curve slopes gradually decrease with the depressurization process,so it infers that the extinction of AP/HTPB transient combustion is a gradual altering process.
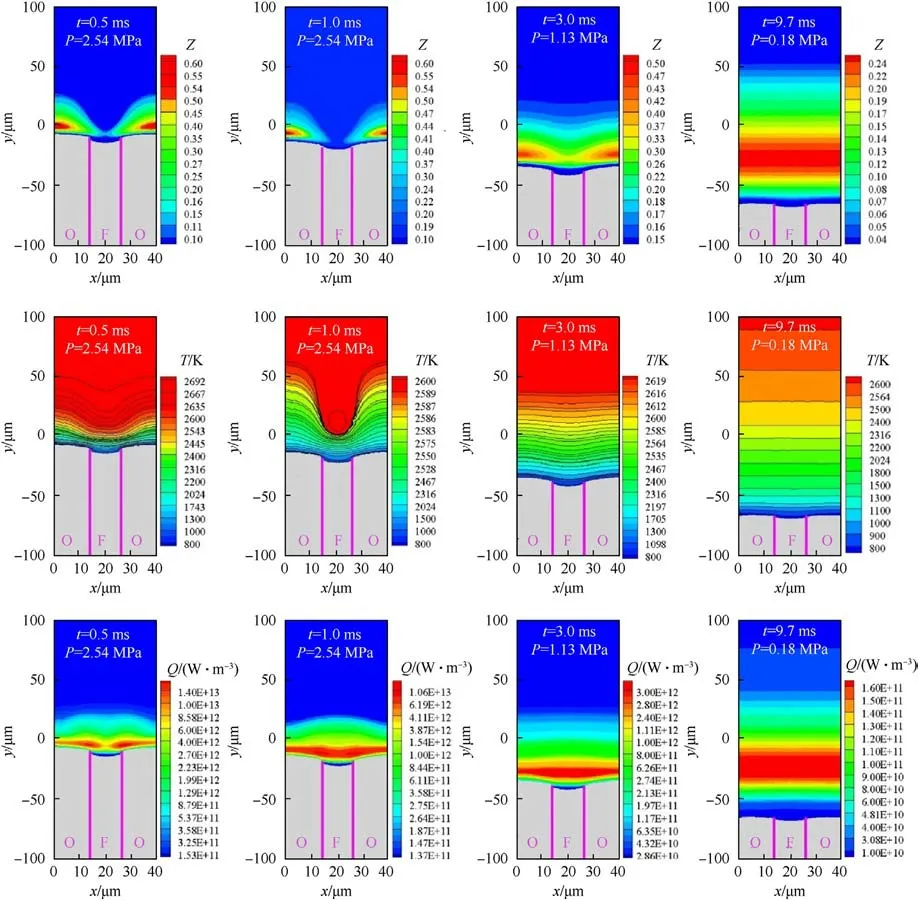
Fig.10.Combustion field characteristics under transient depressurization combustion.
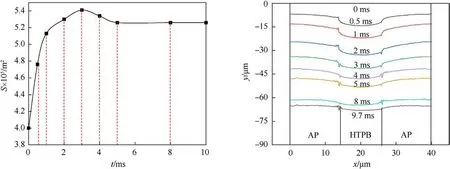
Fig.11.Changes of burning surface and its area during depressurization.
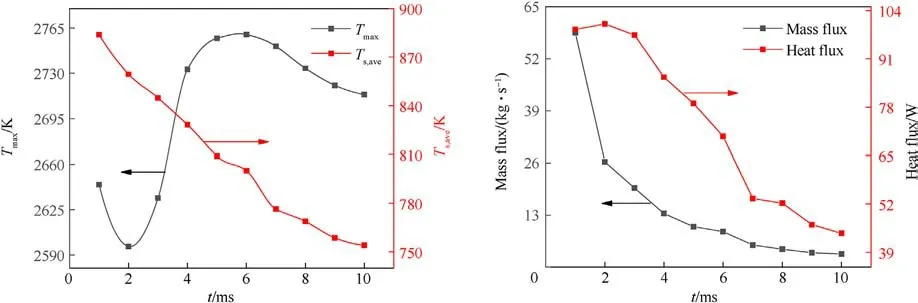
Fig.12.Burning surface information.
5.2.The effect of surface geometry on gas flame structure
In the initial stage of AP/HTPB rapid depressurization combustion, different combustion surface shapes will affect the diffusion and mixing process of components,thus forming different types of flame structures.At the same time, because the combustion pressure of AP/HTPB propellant decreases exponentially,it is speculated that the influence of combustion surface on flame structure mainly occurs in the early stage of rapid depressurization.To investigate the possibility, the flame is characterized in terms of heat release(Both of them have similar features, which confirms that it is reasonable to use heat release to study flame.).Its evolution processes of three different sandwich models during rapid depressurization (0.1-1 ms) as shown in Fig.13.At t = 0 ms, the component ˜Y from HTPB of ’A’ and ’B’ migrate to the AP side and react with the oxidizing component ˜X to form a separated diffusion flame structure.Compared with’A’and’B’,the reaction above HTPB of ’C’ is relatively intense.The reaction zone of reactants is restricted to the area in the vicinity of the HTPB,thus generating a merged flame.It is worth noting that the LEF of the three sandwich models near the position where the stoichiometric surface intersects the burning surface.The position, when the surface is flatter,is the location of the maximum local burning rate as well as the outside of the oxidizer binder interface.This trend is consistent with Shvab-Zeldovich’s theory and experimental results of Ref.[7].Furthermore, it is observed that the heat release cores of the’A’and’B’are located near the AP/HTPB interface,while the’C’has almost no chemical reaction in there.The reaction core is located on the AP side at a certain distance from the interface.This is due to the low temperature near the interface, which is not enough to maintain the chemical reaction,resulting in the upward movement of the flame structure.With the rapid depressurization process, at t = 0.1 ms, the convective diffusion effects along the x direction strength,while the diffusion transport ability along the y direction relaxes.The thin activity zone of components of’A’and’B’merged greatly, whereas the phenomenon of ’C’ tends to be not obvious.This is because the used small scaled sandwich models present a strong premixing effect [19].Since the regression rate of HTPB is higher than that of AP, the contact difficulty of pyrolysis gas is greatly reduced.The gas phase reaction bands of ’A’ and ’B’ are connected.However, due to the larger drop of combustion surface of’C’,the gas phase heat release is the largest,which is 2.1×1013W.When t=0.3 ms,the HTPB regression rate is faster than AP,and all the sandwich models become AP convex to the surface.Differences in the burning surface shape begin to become smaller than that before.The decreasing pressure further compresses the activity space of the reactants.The diffusion flame branches generated by’A’ gradually merge,and then gradually move from the edge of AP to the HTPB side.The energy production cores of the’B’and’C’are also constantly moving closer, showing premixed-diffusion flame characteristics.The maximum heat releases of three sandwiches are 1.7×1013W for’A’,1.73×1013W for’B’,and 1.81×1013W for’C’, respectively.The evolution of flame structure shows that the effect of surface morphology on combustion weakens gradually with time.When t=0.7 ms,the burning surface shapes are almost the same.However, due to the large influence of the burning surface on the gas phase flame at t = 0-0.3 ms, the flame structures are still different.At t = 1 ms, all the reaction zones have been merged completely.The decrease rates of the maximum heat release of’A’,’B’ and’C’sandwiches in 0-1 ms are 1.41×1013W/ms, 1.17 × 1013W/ms, and 1.08 × 1013W/ms, respectively.This indicates that AP protruding from the burning surface is more conducive to combustion [43].
Fig.14 shows the predicted combustion surface profile over time(0.1-1 ms) demonstrating how rapid depressurization affects the evolution of the burning surface.Combined with Fig.13, the chemical reaction above HTPB is more intensive than that of AP,resulting in the HTPB regression rate being faster than AP under three sandwich cases.The centre of HTPB of ’A’, ’B’, and ’C’ move 22.13 μm, 22.88 μm and 18.02 μm respectively within 1 ms, while the average moving distances on the edge AP of ’A’, ’B’, and ’C’ are 13.12 μm, 7.07 μm and 16.73 μm respectively.Meanwhile, the regression law of ’C’ compared with ’A’, and ’B’ gives indirect evidence of the fact that the heat flow on the burning surface of’C’is more uniform, corresponding to the results of Fig.13.The smaller regression distance on both edges of the’B’indicates that the local region above AP nearly no chemical reaction occurs, so’A’ is more beneficial to combustion than ’B’ during the t = 0.1-1 ms.
5.3.Influence of combustion surface morphology on extinguishment
Average surface temperature variation profiles are given in Fig.15 during the rapid pressure decay.The average surface temperature of’A’,’B’,and’C’decreases from 883 K,889 K,and 888 K at 0.5 ms to 750 K at 9.7 ms for ’A’,13 ms for ’B’ and 15.8 ms for ’C’,respectively.The predicted result manifests that non-planar propellant is more conducive to combustion.Therefore, the effect of burning surface morphology on combustion cannot be ignored.
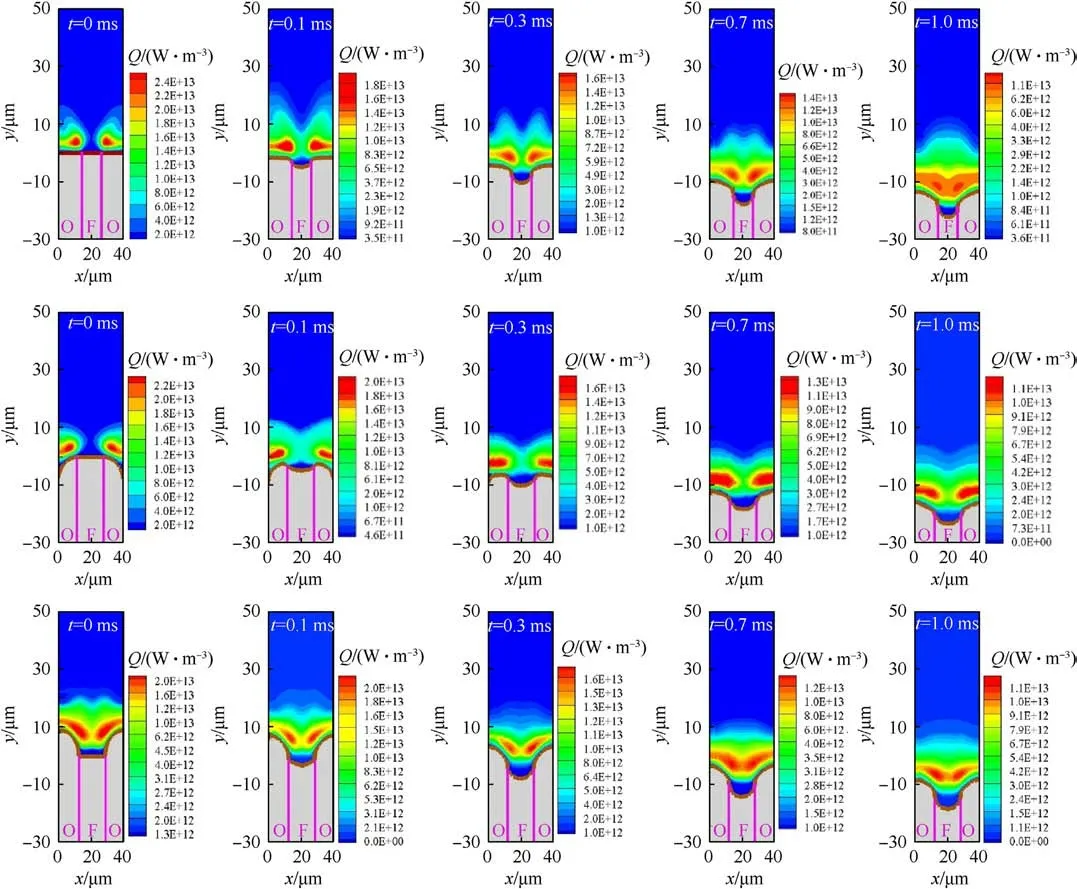
Fig.13.Gas-phase flame structure of sandwich model with different burning surfaces under rapid depressurization(0.1-1 ms).They represents the’A’,’B’,and’C’types of sandwich models, respectively.
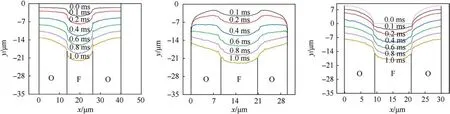
Fig.14.The changing trend of the burning surface under rapid depressurization combustion (0.1-1 ms): They represent the ’A’, ’B’, and ’C’types of sandwich model, respectively.
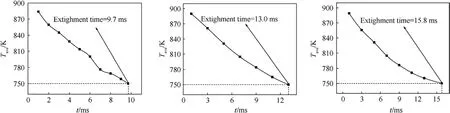
Fig.15.The variation of average burning surface temperature with time under different surface shapes: They represent the ’A’, ’B’, and ’C’ types of sandwich model, respectively.
6.Conclusions
2D periodic sandwich models are obtained by slicing the propellant pack using the method reported by Vijay.Many trends including burning rate, pressure index, surface geometry, and extinction time in experimental cases can be replicated using the proposed model.The sandwich models with different surface geometry during rapid depressurization are simulated to investigate their effects on the combustion and extinction characteristics.The following conclusions are reached:
(1) The effects of combustion surface geometry on AP/HTPB rapid depressurization combustion are mainly concentrated in the initial stage of depressurization (0.1-1 ms).At t = 0.1-1 ms, the burning surface shape mainly affects the fluid dynamic diffusion flow ability along the x direction,while the chemical reaction rate is almost independent of it.
(2) In the rapid depressurization process combustion, flame controlled by the diffusion mixing between the components at the initial stage of depressurization is transformed into the premixed flame dominated by chemical kinetics at the later stage of depressurization.That in turn weakens the gas reaction, then the propellant doesn’t get thermal feedback to maintain paralysis.Finally, the mass flow from the combustion surface to the gas phase smaller and smaller,which leads to flameout.
(3) When AP protrudes from the surface is beneficial to combustion, burning the longest time.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research is supported by the National Natural Science Foundation of China (Grant No.51176076).
- Defence Technology的其它文章
- Explosion resistance performance of reinforced concrete box girder coated with polyurea: Model test and numerical simulation
- An improved initial rotor position estimation method using highfrequency pulsating voltage injection for PMSM
- Target acquisition performance in the presence of JPEG image compression
- Study of relationship between motion of mechanisms in gas operated weapon and its shock absorber
- Data-driven modeling on anisotropic mechanical behavior of brain tissue with internal pressure
- The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin

