The effect of reactive plasticizer on viscoelastic and mechanical properties of solid rocket propellants based on different types of HTPB resin
Tihomir Kovčević , Slvko Mijtov , Jelen Gržetić , Suzn Ckić ,Bojn Fidnovski , Sš Brzić
a Military Technical Institute, Ministry of Defence, Ratka Resanovića 1, Belgrade, Serbia
b Faculty of Technology, University of Niš, Bulevar oslobo enja 124, Leskovac, Serbia
Keywords:HTPB-based composite propellants Castor oil Reactive plasticizer Gel permeation chromatography Sol-gel analysis Mechanical and viscoelastic properties
ABSTRACT Conventional plasticizers deteriorate mechanical and viscoelastic properties of the propellants due to their migration upon aging and long-term storage, which affects reliability and safety properties during exploitation.To address this issue,conventional plasticizer,dioctyl adipate(DOA),is replaced by reactive one, castor oil (CO).In addition, three different types of HTPB were used to obtain propellants with designed viscoelastic and mechanical properties.The CO increased propellants viscosity, without a significant impact on the propellant processability, regardless to the type of prepolymer.Conversely, mechanical properties were different depending on the type of resin, which were further analyzed by gel permeation chromatography (GPC).Addition of CO formed a denser polymer network and shifted Tg to higher values, compared to the compositions with DOA.The tensile strength of CO-containing propellants was lower at +20 °C and +50 °C compared to the reference compositions, while the strain at maximum load and strain at break were significantly increased with pronounced plastic deformation,especially for samples at-30 °C.The inclusion of CO in the propellants composition gives more room for adjusting a wide range of mechanical properties.
1.Introduction
Complex mixture of solid rocket propellants is composed of a polymeric binder,plasticizer,antioxidant,bonding agent and solids,which include oxidizer and metallic fuel.These ingredients are homogenized and then the resulting slurry is casted directly into the rocket motor case or thermal insulation/inhibitor.It is required that the uncured propellant possesses acceptable viscoelastic properties that enables easy handling and extended pot life.Pot life is the time the propellant slurry remains liquid,which allows easy manipulation and casting into rocket motor chamber [1].To improve viscoelastic properties of the composite propellant,which is mainly manifested through reduced viscosity, plasticizers are employed [2].In addition, the use of a plasticizer increases solids loading and thus improves the inner ballistic properties of the composite propellants [3].
Plasticizers, a low-molecular weight compounds, improve the processing properties of solid propellant by physically inserting between binder chains, which reduces their mutual interactions,i.e.decreasing the cohesive forces and glass transition temperature(Tg) of the system [4,5].Furthermore, non-reactive plasticizers,which are not chemically bonded to the polymeric matrix can easily migrate through the propellant due to concentration gradient,even after curing process [6].This phenomenon causes deterioration of the mechanical characteristics of propellant during long-term storage and aging [6-8].In addition, the plasticizer diffusion, primarily caused by storage conditions,leads to its enrichment at the interface between propellant and liner (case-bonded) or inhibitor(free-standing)within the grain and thus affects adhesion resulting in debonding[9,10].Finally,according to Zhang et al.,the migration of plasticizer can also cause decrease of the energetic properties of the propellant, affecting the combustion stability during motor start, especially when energetic plasticizers are used [11].
Jeremić showed that although ballistic requirements dictate grain configuration, the structural integrity and viscoelastic properties of the propellant are a key parameter in defining its reliability and use lifetime [12].The mechanical properties of elastomerbased composite propellants are a function of the prepolymer properties,i.e.molecular weight,hydroxyl value,functionality,etc.Selection of a proper curing agent to form the three-dimensional binder network is also essential.Other ingredients such as solids(primarily oxidizer and metallic fuel), bonding agents and plasticizers are normally included in the binder and are also beneficial in improving the propellant mechanical properties.In addition,De La Fuenete et al.confirmed that solids shape, particle size and distribution as well as the mutual interaction with binder significantly affect the mechanical properties of the propellant [13].The interactions at binder - filler interface, which can potentially create the chemical bonding, are not favorable due to their physical and chemical characteristics(non-reinforcing fillers).This shortcoming is overcome by introducing bonding agent within the propellant compositions, which possesses several functional groups reacting with these from prepolymer and curing agent(OH and NCO)as well as with filler surface (reinforcing fillers), which was confirmed by the research of Azoug et al.[14].
The propellant grain in a rocket motor is exposed to extreme conditions (high pressure and temperature, rapid acceleration,rotation and so on) during the launch and flight and therefore its mechanical stability is essential.Moreover, the aging of the propellant upon prolonged storage,which occurs due to combinations of chemical and physical processes, affects its properties during ignition, according to Wang et al.[15].In detail, when the propellant cannot withstand generated stresses,it collapses,which leads to cracks and defects within the grain.According to Kohga et al.this is a very dangerous scenario due to an abruptly increase in the burning surface area, which further leads to a motor failure and explosion [16].Therefore, scholars have focused on the development of new methods and materials to prevent or reduce the migration of plasticizer.Some of those techniques include introducing a polyester layer between the propellant and the liner[10,11], using a high molecular weight energetic plasticizer [17],including a graphene oxide in the liner composition [18,19] or adding an unsaturated plasticizer that contains ester groups,which establish covalent bonds with the polymer binder from the propellant[20].Another approach to address this issue is employing of reactive plasticizer,which has a multiple role within the propellant.In brief, it softens the propellant enabling easy processing, participates in the curing reaction, which results in inclusion into the network and, finally, acts as a cross-linker as well as a chain extender.In this way, the final product has a compact structure where the plasticizer is unilaterally anchored to binder, while, on the other side, the loose hydrocarbon segment provides elasticity and flexibility of the polymer-based propellant.Materials that can meet previous requirements to be an adequate replacement for conventional plasticizers are bio-based compounds,non-toxic and readily available.Indeed,among these compounds,castor oil(CO)is recognized as suitable since it is a low-cost natural material with secondary hydroxyl groups capable of participating in the curing reaction with isocyanates.
CO has long non-polar fatty acid chains with three secondary hydroxyl groups at position 12,which give it the potential to bind to the base polymer matrix, while eight more C-atoms hang as a pendant providing flexibility to a final product(plasticizing effect).Due to previous, CO has been extensively used for preparation of polyurethane matrices and bio-based plasticizer for PVC [21-25].However, in the field of energetic materials, CO and its derivatives have rarely been used, mostly as a binder instead of hydroxylterminated polybutadiene (HTPB) [3,26] or as a bonding agent and liner [27,28].
In this study,HTPB,provided from three different suppliers,was used as a prepolymer for the preparation of composite propellants where conventional plasticizer, dioctyl adipate (DOA), was substituted with reactive one, CO.Specific objectives of this work were evaluation of the impact of HTPB type and presence of CO within the propellants on the mechanical and viscoelastic properties of the final products.Furthermore, the objective of this study was to investigate to what limits the strain capability of a chosen baseline propellant can be improved without substantially decreasing the tensile strength.All the obtained results were considered in relation to the reference ones, with DOA as a plasticizer.
2.Experimental
2.1.Materials
Three different HTPB resins:S-Sartomer(USA,viscosity at 30°C:5000 mPa·s, OH value: 47.1 mg KOH·g-1, hydroxyl functionality:2.4-2.6, average molecular weight, Mn: 2800 g·mol-1, glass transition temperature, Tg: 75°C), R-45M (USA, viscosity at 30°C:5500 mPa·s,OH value:44-51 mg KOH·g-1,hydroxyl functionality:2.4, average molecular weight, Mn: 2900 g·mol-1, glass transition temperature, Tg: 80°C) and Shanghai Rongau Enterprises -SRE(China, viscosity at 40°C: 3500 mPa·s, OH value: 0.7382 mmol KOH·g-1,hydroxyl functionality:2.2,Mn:2850 g·mol-1)were used as a binder.Castor oil (CO-Interhem Ltd, Republic of Serbia, hydroxyl functionality: 3.0, Mn: 933 g·mol-1) was used as a reactive plasticizer while commercial one, dioctyl adipate (DOA), was procured from Merck, Germany.Bonding agent, triethylenetetramine(TETA), was purchased from Merck, Germany.Antioxidant (2,2′-bis(4-methyl-6-tertbutyl) phenol, commercially available as a product called AO 2246 and curing agent,isophorone diisocyanate(IPDI), were supplied from Sigma Aldrich, Germany, and Acros Organics, Belgium, respectively.Copper chromite, used as burn rate modifier, was procured from Hershaw, USA.
2.2.Sample preparation
The analyzed propellant compositions were homogenized using a laboratory 1-gallon Baker-Perkins planetary mixer at a temperature of 60°C.The homogenization process started with a premix phase where prepolymer, plasticizer, bonding agent (liquids) and antioxidant, burn rate modifier and metal fuel (solids), excluding oxidizer and curing agent, were placed in a mixer and homogenized.The next step included adding the oxidizer in three equal portions.The final step, after thorough homogenization, was the addition of a curing agent.
Propellants classified according to the used binder,in following groups:propellant group 1-based on SRE resin,propellant group 2-based on S resin and propellant group 3-based on R-45M resin.The amount of the main liquid ingredients of the analyzed propellant are shown in Table 1.In addition to the tabulated components,propellants contained bimodal mixtures of metallic fuel(Al)and oxidizer(AP).The amount of AP is 69.5%for propellants group 1 and 2 and 73.0% for propellant group 3, while for Al it is 15.0% for propellants group 1 and 2 and 12.0% for propellant group 3.The amounts of antioxidant and bonding agent were 0.17%-0.19% and 0.04%, respectively.In addition, propellants from group 3 contain 0.32 wt%of copper chromite as a burn rate modifier.The reference compositions, SRE1, S1 and R-45M1 contain DOA as a plasticizer within their structure (without castor oil).
The casting process was performed under low pressure(vacuum) with simultaneous vibration to remove residual air,moisture and possibly present low boiling components.Afterwards,curing of prepared propellant mixtures was carried out for 3 days at(70±2)°C.The procedure for preparing propellant is shown in Fig.1.
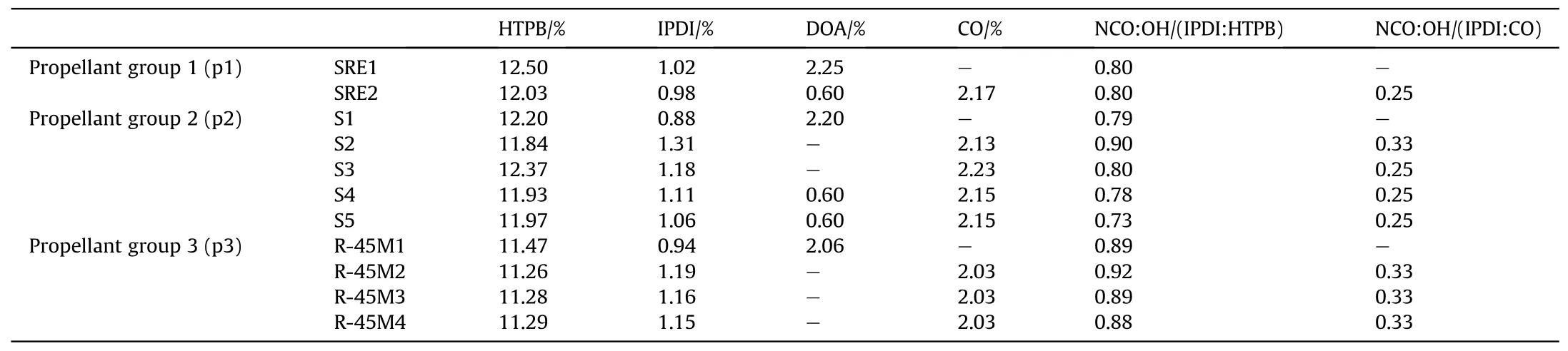
Table 1 The main liquids quantities in composite propellants and the NCO:OH ratio.
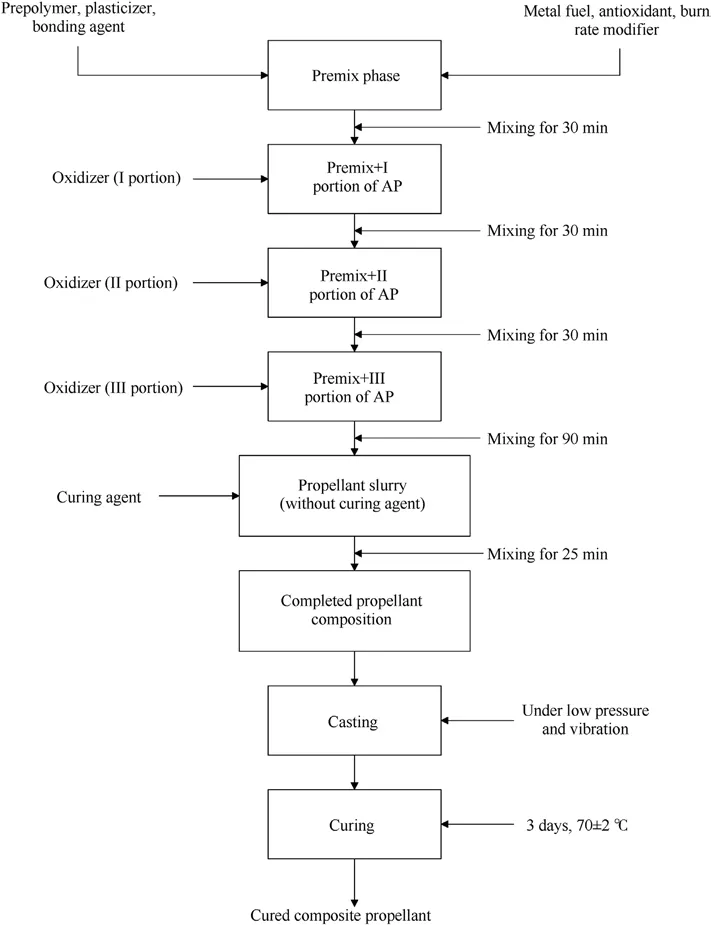
Fig.1.Schematic diagram of the propellants preparation process.
2.3.Characterization methods
The viscosity of the uncured propellants was measured at the 60°C by HBT type Brookfield viscometer with a T-C spindle at a rotation speed of 5 rpm.
Structural characterization of propellant containing CO was determined by Nicolet™iS™10 FT-IR Spectrometer(Thermo Fisher SCIENTIFIC) with Smart iTR™Attenuated Total Reflectance (ATR)Sampling accessories.Spectrum was recorded in transmittance mode,within a range of 400-4000 cm-1,at a resolution of 4 cm-1and in 20 scan mode.
The molecular mass distribution of the binders were determined by gel permeation chromatography (GPC), using Agilent 1100 Series system with refractive index.Prior to test, all samples were dissolved in tetrahydrofuran(THF).The flow rate of the carrier solvent was 0.5 cm3·min-1.The concentrations of analyzed samples were 10 mg·cm-3and 4 mg·cm-3.The average molecular weights,Mn,Mwand polydispersity index PI were determined using software Agilent ChemStation.
The dynamic mechanic analysis (DMA) of cured propellants(rectangular bar with dimensions as follow: length: 54.0 mm,width: 10.0 mm and thickness: 4.0 mm) was done by Modular Compact Rheometer MCR-302 (Anton Paar GmbH, Austria) equipped with standard fixtures SRF12, temperature chamber(CTD-620)which has high temperature stability(±0.1°C)and with automated cooling accessories using liquid nitrogen.DMA measurements were performed at temperature range-85°C to+60°C with the heating rate of 5°C·min-1and the single angular frequency of 6.28 rad·s-1.In addition, DMA parallel plate tools were used to characterize the rheological behavior of CO and HTPB in the reaction with IPDI.The samples were non-isothermally analyzed in temperature range from 50°C to 175°C at the heating rate 1°C·min-1.The initial plate gap was 0.5 mm.
The sol content of the polymer matrix, Spoly, was performed by extraction with dichloromethane using three Soxhlet extraction unit.Extraction lasted 16 h and each extraction unit contained approximately 150 ml of solvent.The polymeric part, Spoly, of the total extracted material, Etotal, was calculated according to Eq.(1).
where, Spolyis the polymeric moiety of the extracted part of the propellant; Etotalis the total quantity of the extract, g; Epolyis the polymeric moiety of the extract,without nominal parts of DOA,AO 2246 and CuCr2O4, g; A is weight of the sample prepared for the extraction, g; Ak-Pis weight of the sample prepared for the extraction without the nominal parts of AP, Al, DOA, AO 2246 and CuCr2O4,g;PAPis nominal part of AP;PAlis nominal part of Al;PDOAis nominal part of DOA;PAO2246is nominal part of AO 2246;PCuCr2O4is nominal part of CuCr2O4.
The mechanical properties of the cured propellant specimen at -30°C, +20°C and +50°C were determined employing an Instron 1122 uniaxial tensile test machine.JANNAF ''C'' dog-bone shaped samples (length: 120.6 mm, width: 25.0 mm and thickness: 8.0 mm) were used in the test.The crosshead speed of the uniaxial tensile test machine jaws was 50 mm min-1.Five samples were tested for each propellant composition and the average value was calculated,along with the standard deviation.
The homogeneity of the cured propellants was confirmed by radiographic examination with an industrial X-ray device with a maximum voltage of 300 kV (Teledyne ICM, Belgium).Tests were performed at 80 kV and a retention time of 1000 ms by recording 10 images.
3.Results and discussion
3.1.Viscosity measurements
The effect of CO on the end-of-mix (EOM) viscosity of the uncured propellants depends on the molar ratio between the reactive groups from the polymer matrix (prepolymer, reactive plasticizer and bonding agent) and the curing agent.Compositions SRE1 and SRE2 have a slightly higher value of EOM due to presence of greater amount of curing agent(SRE1)and CO(SRE2),but its increase is too slow indicating a great pot life (Tables 2 and 3).The main disadvantage of SRE1 and SRE2 propellant formulations is low reactivity of the polymer matrix, which has not cured even 10 days after placing in the curing chamber (SRE2).SRE resin has the lowest functionality in comparison to the other two, which is one of the reasons for the manifested phenomenon even though a reactive plasticizer is introduced in its structure.
The reference composition for the propellant 2 (S1) has a functional NCO:OH group ratio (ratio between the isocyanate moieties on the IPDI to the hydroxyl moieties on the HTPB) 0.79,showing that the propellant has a high pot-life, which provides good castability (Tables 2 and 3).However, the high pot-life indicates the poor reactivity of such material, which means that the formation of the solid polymer network,i.e.cross-linking,requires a long curing time, where the consumption of electric energy and the engagement of the necessary equipment is not optimal.Furthermore, extended pot-life can cause sedimentation of the solids, which is reflected in uneven density, and thus mechanical and inner-ballistic properties along the propellant grain.This is the main reason,in addition to the possibility of tuning of mechanical properties, why the commercial inert plasticizer (DOA)is replaced with the reactive one.
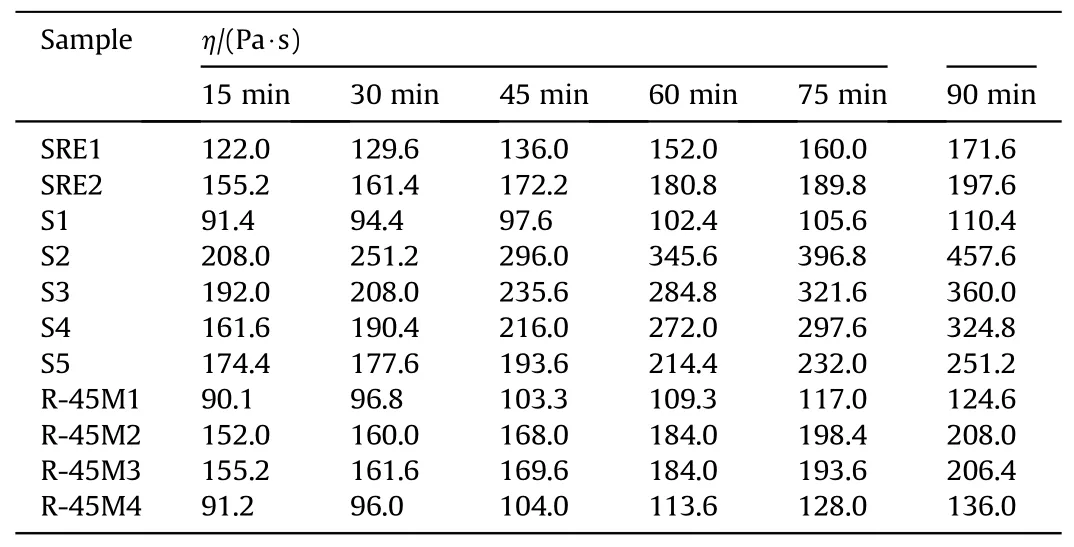
Table 2 Viscosity of the tested propellants.
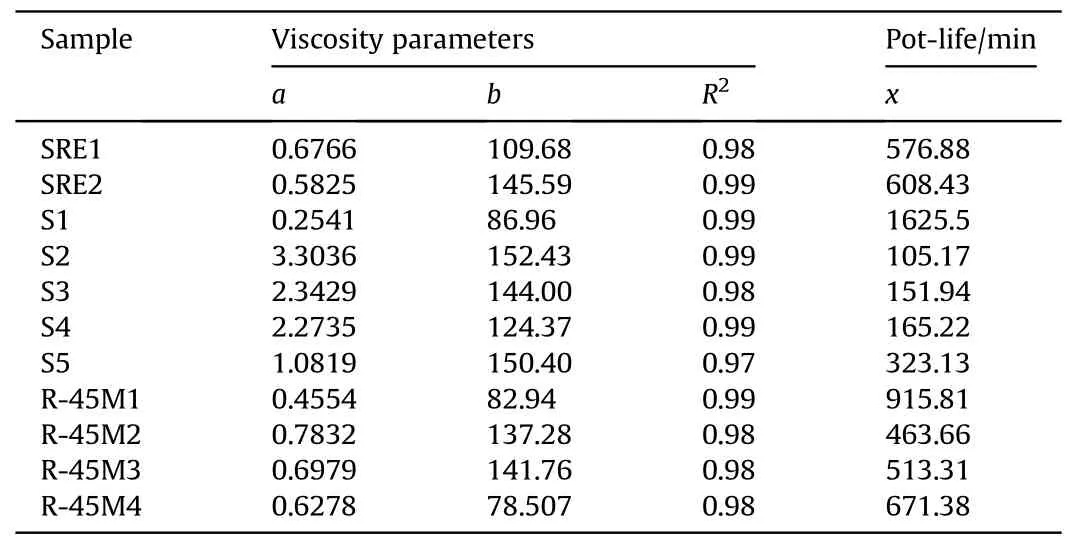
Table 3 Values of model parameters of linear time-dependent viscosity and pot-life of tested propellant compositions.
Composition S2 has a functional group ratio 0.90 (HTPB:IPDI)and 0.33 (CO:IPDI) where the resulting propellant is easily casted,but the increase in EOM is very rapid(Table 2).To address this issue,the amount of curing agent is reduced to 0.80(HTPB:IPDI)and 0.25(CO:IPDI) in S3.The obtained pot-life is optimal with moderate increase of the viscosity.A practical issue with this composition is cleaning of tools for the casting process due to sudden increase in viscosity upon cooling and stickiness of the propellant caused by the presence of CO within the polymer matrix.To further lowering the viscosity,with preservation of the mechanical properties,5 phr DOA is added to propellants S4 and S5,while the amount of curing agent is reduced to 0.78 (S4-HTPB:IPDI)and 0.73 (S5-CO:IPDI).
The reference composition, containing DOA as a plasticizer, R-45M1 has a functional group ratio 0.89, which results in a propellant with good processability (long pot-life), but slow increase in viscosity(poor curing).It is prepared for comparative analysis with propellant containing CO(primarily for comparison with R-45M3).The addition of CO significantly increases the viscosity of the propellants due to a substantially enhance in the amount of curing agent.Based on the technical data sheet of R-45M and S resins,both require the same amount of curing agent, i.e.a functional group ratio between HTPB/CO and IPDI should be the same.However,empirical tests show great differences because some prepared compositions do not cure or the obtained material is too stretchy,almost like a pure polymer matrix.The somewhat acceptable results are obtained when the functional group ratio is 0.86(HTPB:IPDI) and 0.33 (CO:IPDI) (viscosity after 15 min and 90 min was 85 Pa·s and 110 Pa·s, respectively).This slow increase in viscosity is confirmed by the resulting rubbery and sticky material even after 4 days of curing.The chosen formulations have the NCO:OH ratio(HTPB:IPDI)in range of 0.88-0.92(R-45M2-0.92,R-45M3-0.89 and R-45M4-0.88),while CO:IPDI is fixed at 0.33.These propellants have acceptable viscosity and satisfactory curing kinetics (Table 2).
Compositions with viscosity up to 500 Pa·s are completely acceptable for propellants casting[1].In addition,IPDI is chosen as a curing agent due to its moderate reactivity, which gives a lower initial viscosity and its slower increase making these propellants suitable for casting process [29].
In general, the presence of CO in the propellants results in higher EOM viscosity, with a more expressed change in this value with time.Numerical models of the dependence of viscosity on time for the examined propellant compositions are derived by regression analysis (Fig.2) using Eq.(2) as follows:
where,y is the maximum value of viscosity(500 Pa·s),x is the potlife, and a and b are coefficients.
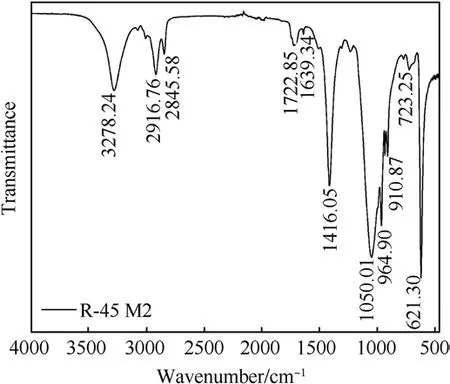
Fig.3.FTIR analysis of the propellant containing CO.
In the tested propellant compositions with CO, a linear dependence of the change in viscosity with time is remarked.High values of correlation coefficients (from 0.97 to 0.99) indicate that the experimental values are quite well approximated by the appropriate linear model.In other words, the change in viscosity at any moment can be described by a first-order reaction.The values of the rate constant of the cross-linking reaction are calculated from the slope of the approximate line, while the values of the initial viscosity are determined from the intersection with the ordinate.The values calculated in this way are shown in Table 3.
The calculated pot-life values, which correspond to the measured viscosity values(Table 2),are in the range of 105 h to 27 h(1625 min)at a temperature of 60°C.In practice,all values obtained exceed the time required for casting propellant grains of various dimensions in semi-industrial conditions,except possibly for the S2 batch.In general,pot life design primarily depends on the size and number of the propellant grains as well as construction of the casting tool, which dictates the dynamics of the casting process[30].
3.2.FTIR analysis of the propellant containing CO as a plasticizer
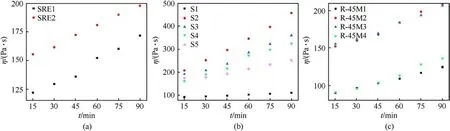
Fig.2.Time dependences of viscosity of corresponding compositions: (a) Propellant group 1; (b) Propellant group 2; (c) Propellant group 3.
FTIR analysis is employed to examine the effect of CO on the qualitative composition of the analyzed propellants (Fig.3).The peaks at about 3278 cm-1and 1416 cm-1originate from OH groups stretching and bending vibrations from CO and HTPB, which remain unreacted, respectively.Overlapped symmetric and asymmetric vibrations of -CH3and -CH2groups are noticed at about 2916 cm-1and 2845 cm-1.Fig.3 also shows that there is no characteristic N=C=O peak at 2260 cm-1, suggesting that curing reaction between CO and IPDI is successfully performed.Two peaks at about 1722 cm-1and 1639 cm-1are associated with the carbonyl part of the urethane group and the ester group,respectively[31,32].A strong tape at about 1050 cm-1is related to C-O stretching vibrations in CO, while the peak at about 964 cm-1originates from C=C bending vibrations, present in both,CO and HTPB.
3.3.GPC analysis
GPC analysis is employed for a deeper insight into the characteristics of the resins, primarily due to the behavior of the SRE binder.Fig.4(a)shows the GPC molecular weight distribution of all three resins at the same concentration(10 mg cm-3).The SRE resin exhibits a broad, multimodal distribution of the molar mass, indicating heterogeneity within its structure.The differences in the polymer chains length is significant,causing uneven curing within the material making the longest chains unattached to the polymer network (low viscosity).Hereupon, it is very difficult to adjust the mechanical properties of the propellant containing SRE resin as a binder due to the predominantly linearity and thus great anisotropy of the final material.The low curing rate and determined molecular structure make this resin unsuitable for preparation of the propellant formulations, which have strict demands with regard to viscoelastic and mechanical properties.That is the reason why this resin is excluded from further research.
R-45M and Sartomer resins have a monomodal distribution of molar mass,whereby the peak for the former is lower and slightly wider (Fig.4(a)).However, there is no significant difference between these two resins, although the behavior and physicochemical characteristics of the final compositions differ in practice.The resin is primarily responsible for the physical characteristics of the propellants and remarked differences may be related to the method of polymerization during their synthesis [33].Therefore, the GPC analysis has been repeated with a lower samples concentration,4 mg/cm3, (Fig.4(b)), whereby the R-45M resin displays bimodal,broad distribution of the molar mass in comparison to Sartomer,whose GPC curve is narrow.
The obtained graphs are numerically supported by values of Mn,Mwand PI(Table 4).It is well known that viscosity depends on the average molecular weight, i.e.as molecular weight increases, viscosity displays higher value(see Table 2)[34].However,molecular weights are calculated directly from the calibration withpolystyrene standard and, consequently, the obtained values have relative significance.Conversely, PI, which represents the ratio of Mwto Mn, is a useful measure of the width of the chain size distribution[35].Sartomer resin has the lowest value of PI,while SRE possesses the highest polydispersity index, which is in good correlation with analyzed characteristics of resulting propellants.
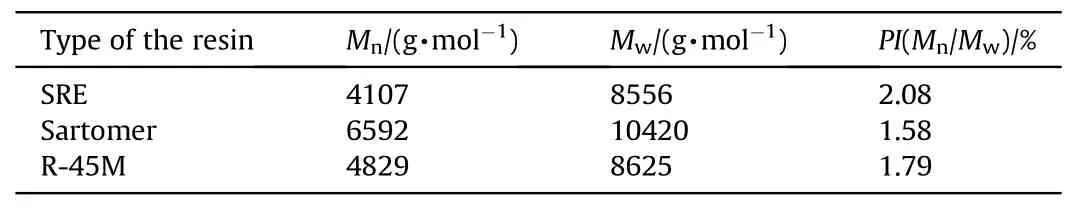
Table 4 Average molecular weights and polydispersity index of the HTPB resins.
3.4.DMA analysis of the corresponding propellant formulations
DMA is a useful technique for investigation the viscoelastic characteristics of the polymer materials and is employed to quantify the changes in storage and loss moduli(G′and G′′,respectively)as well as damping factor tan(δ) in the temperature range from -80°C to +60°C (Fig.5).Fig.5 shows that the G′exhibits a similar trend for all analyzed propellants, where the maximum values are reached below the glass transition temperature zone(-80°C to -70°C).As temperature continues to rise, G′drops abruptly, which is a consequence of the greater mobility of the macromolecule segments[36].A significantly higher value of G′in the temperature range from -70°C to -30°C for propellants containing CO(S4 and R-45M3)indicates the establishment of good intermolecular interactions due to its incorporation into the polymer network (chemical linkage).In this way, the movements of polymer segments are hindered,which is a consequence of denser and more branched network, as well as rigid and less mobile structure, because the domains between the two junctions are quite shorter compared to propellants containing an inert plasticizer [32].There is substantial difference in G′in the rubbery plateau where propellant R-45M1 exhibits quite higher value in comparison to composition S4.This phenomenon is related to the structure of the polymer network caused by the presence of CO in the composition S4 and the type of the resin,as well as the amount of curing agent within the propellants structure.Namely, composition S4 has a smaller amount of curing agents per functional unit of the polymer matrix (HTPB and CO) then propellant R-45M1,making it more mobile and flexible for the cooperative motions of the macromolecule segments between the cross-linked junctions.
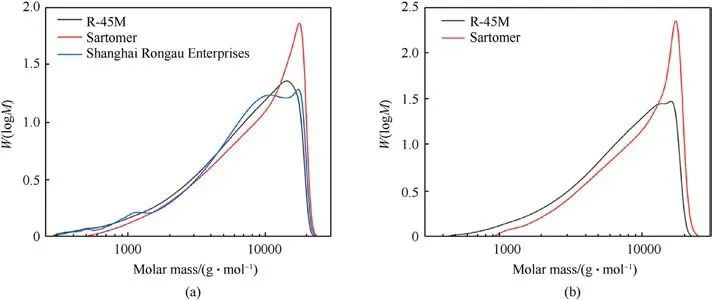
Fig.4.GPC molecular weight distribution curves of the HTPB resins: (a) Concentration of 10 mg/cm3; (b) Concentration of 4 mg/cm3.
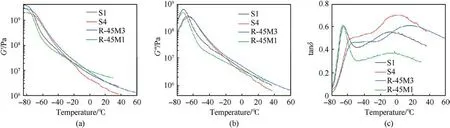
Fig.5.Temperature dependences of the analyzed propellants: (a) G'; (b) G′′; (c) tan(δ).
G′′increases to its maximum in the glass transition region,which is caused by energy dissipation occurring in the interaction between the solid phase and the polymer matrix [37,38].These maxima shift to higher values in the case of propellants containing CO (for about 7°C), which is caused by the formation of a tighter network and, consequently, a smaller free volume for the movement of polymer segments.Afterwards, G′′decreases as the macromolecule segments become moveable due to the absorption of heat energy provided by the temperature increase[39].There is a similar pattern of G′′as for G′in the temperature region from-72°C to-25°C,which is dictated by the presence of CO,as it mentioned above.Such a similarity is also remarked in the area of the rubbery plateau,where the highest values are obtained for the propellants containing DOA as a plasticizer due to great linearity manifested by HTPB, which disables the conformational flexibility of the entire network and thus gives substantial rigidity of the system.
The viscoelastic behavior of the polymers can be determined through tan(δ) since it reflects structural composition, type and number of relaxation process as well as morphology in the case of the polymer-based composites [32].The tan(δ) profile for propellants containing CO is not very different compared to those belonging to compositions where DOA is used as a plasticizer.COfree compositions(S1 and R-45M1)display two peaks,belonging to the soft and rigid domains within the structure, which is a special feature of the HTPB-based polyurethanes [40].The first one, positioned between-80°C and-50°C,with a maxima at-65.5°C(S1)and -66.2°C (R-45M1) originates from the primary relaxation process,α,which expresses the Tgin the soft segment regions(Tsoftg)of the corresponding propellants.The polymer network based on HTPB has a large length of macromolecule segments between crosslink junctions, which gives it high mobility at the higher temperatures [32,41].The second transition area, remarked between -50°C and +30°C, with peaks at -8.6°C (S1)and -10.7°C (R-45M1) represents the second relaxation process(β), which is attributed to the movements of polymer segments within hard moieties(T)[40].The second peak is also related to the motions of chains that are restricted due to their interactions with the surface of the solid particles, e.g.adsorption of the polymer matrix onto AP surface, whereby hydrogen bonds are established which prevent the mobility of macromolecule segments[13].
The primary relaxation process within the propellants containing CO is significantly shifted towards higher temperatures compared to CO-free compositions due to motion hindrance arising from the nature of the established polymer network as well as the high filler content.These phenomena together with bonding properties of CO provoke a moderate broadening of the relaxation regions within composites containing reactive plasticizer(Fig.5(b))[28].Furthermore, the increase in the intensity of the secondary relaxation process may be caused by friction between filler particles or filler and polymer as well as damping excess associated with thermal stresses or a change in the conformation of macromolecule in vicinity of interface [13].In addition, the tan(δ) peak heights are lower due to the denser structure particularly manifested through the higher amount of hard moieties (Fig.5(b) and Table 5).This indicates that fewer polymer chains participate in the glass transition.Peaks positioned between -34°C and +37°C for S1 (maximum at +1.27°C) and -25°C and 60°C for R-45M3(maximum at 15.2°C),represent the sum of the second relaxation process of the HTPB moiety and primary relaxation process of the CO domain,which overlap almost in the entire temperature range.
Table 5 shows Tgvalues and the amount of hard segments and Tgfor all analyzed propellants.The amount of hard segments (ξ) is calculated as the sum of wt% of IPDI, which is consumed by OH groups from CO and HTPB (total amount of urethane bonds).
The remarked differences in Tgbetween the propellants with and without CO, within the same group, originate from the resin itself.That variation in Tgis more pronounced in the case of propellant group 3 (about 13°C) compared to propellant group 2(about 7°C).Sartomer resin shows higher reactivity than R-45M,which required less cross-linker in the design of the propellant to obtain optimal mechanical characteristics (see later).CO has a lower molecular weight than the resin and thus greater mobility of the chains, which causes a better orientation of the OH functional groups from the castor oil towards the NCO groups from the crosslinker,which leads to the incorporation of its structural units into a three-dimensional polymer network.The consequence is that a large number of macromolecular segments from the resin itself remain "hanging" and plasticizing the final product, which is best reflected by lower Tgvalues.In propellant group 3, the amount of cross-linkers is significantly higher (about 12%) compared to propellant group 2, which results in the creation of a stiffer network where the movements of macromolecular segments are hindered.The mentioned phenomena are manifested as an increased Tgvalue of propellant group 3 compared to propellant group 2.Nevertheless, the detected increase in the glass transition area does notcompromise the practical application of the obtained propellants since the lowest temperature limit is -40°C.
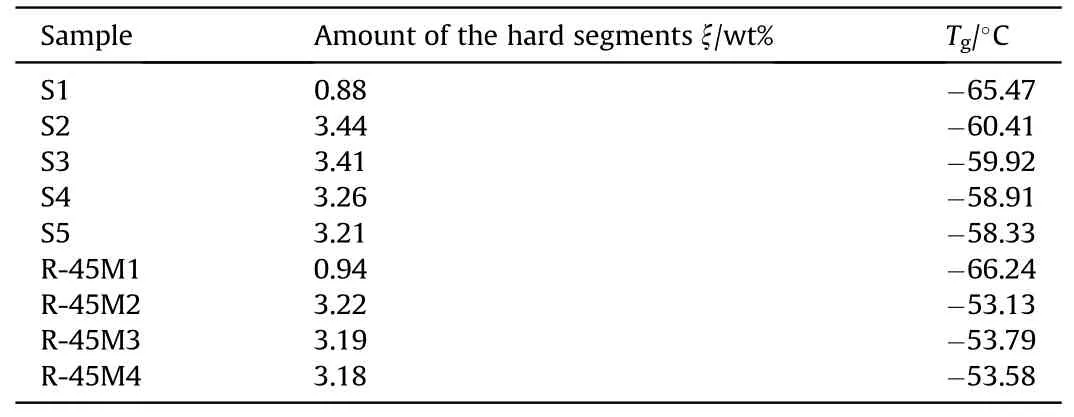
Table 5 Calculated amount of hard segments and Tg for corresponding propellants.
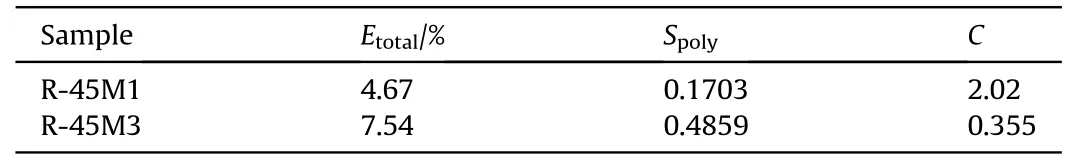
Table 6 Etotal,Spoly and C of the compositions R-45M1 and R-45M3.
3.5.Sol-gel analysis
Sol-gel analysis has been performed to evaluate the effect of CO on the behavior of the polymer network regarding to viscoelastic and mechanical characteristics.Table 6 shows values of Spolyas well as the cross-linking density (C) of the polymer matrix based on R-45M resin (Sartomer shows similar behavior), calculated using Charlesby-Pinner equation (Eq.(3)) [40]:
The presence of CO within the propellant has significant impact to physical and chemical structure of polymer network.It is well known that sol fraction contains unlinked binder chains and plasticizer, i.e.in this case HTPB and CO/DOA [42].Table 6 shows that extractable part of propellant R-45M3 is 61.4%higher compared to the CO-free composition, and it contains for 185% more polymeric part than the R-45M1 extract.These results suggest that both propellants do not contain enough curing agent to establish a complete polymer network, but this phenomena is considerably more pronounced in case of R-45M3 composition (R-45M1-NCO:OH=0.89, R-45M3- NCO:OH=0.66).A significant part of the reactive components(HTPB and CO)remains unconnected with the polymer network,which is also exhibited through the cross-linking density where C for R-45M1 propellant is 469%higher compared to the R-45M3 composition.Regardless to the previous,the amount of hard segments within the CO containing propellant is significantly higher due to a more reactive system, which requires a considerably greater quantity of curing agent (see Table 5).
In brief, the introduction of CO into propellant formulations opens more rooms for scholars and engineers to respond to greater technological demands, especially regarding the design of compositions with a large niche of mechanical properties.
3.6.Mechanical properties
Prior to investigation of mechanical properties, resulting propellants are recorded by radiographic examination in two distinctive projections (Fig.6).The obtained images show a uniform composition of the propellant without voids and air gaps.Such a result is the consequence of the two parameters, raw material storage preparation (solid hygroscopic ingredients are thoroughly dried prior to mixing) and the propellant preparation conditions(see section Sample preparation).
HTPB based propellant is a particle-filled material, which exhibits viscoelastic behavior and its mechanical characteristics highly depend on temperature.Considering this, it is very important to perform thoroughly investigation of the mechanical properties of composite propellants at wide temperature range,primarily extreme conditions, to simulate end evaluate work of solid rocket motor [43].
The tensile characteristics of analyzed composites,expressed as tensile strength (σt), strain at maximum load (εm), strain at break(εb) as well as Young's modulus (E), at three different temperatures,-30°C,+20°C and+50°C,are shown in Table 7 and Fig.7 (only at +20°C).The mechanical characteristics of the propellant directly depend on the density of chemical bonds,i.e.on the content of hard segments of the resulting polymer network,which can be increased by adding diols/triols, providing more sites for cross-linking[44].Table 5 shows that the introduction of a reactive plasticizer leads to a significant increase in hard segments within propellants structure (maximum 291% - propellant group 2 (S2)and 242%-propellant group 3(R-45M2),compared to the reference compositions).Nevertheless, Table 7 shows that the tensile strength values at 20°C decrease, which is most pronounced in composition S5 (95.2%), where the amount of hard segments is higher by 264.7% compared to the reference formulation (S1).Conversely, the propellants based on S resin manifest higher elasticity, which is reflected in higher strain at maximum load and strain at break (maximum increase is 92.0% and 115.7% for composition S5, respectively).
A similar trend is observed for propellant group 3, with thedifference that the decrease in tensile strength is less pronounced(maximum 68% - propellant R-45M3 compared to composition R-45M1).Contrary to this,strain at break and strain at maximum load increase substantially,which is significant from the point of view of propellant application in real rocket motors.The increase in strain at maximum load is 200.5%,while the increase in strain at break is 245.6% for propellant R-45M4 compared to reference composition R-45M1.These results are reflection of castor oil ability to change conformation of macromolecular chains within a threedimensional polymer network.In brief, during the impact of the external force,there is a redistribution of stress,which gives greater deformability of the material, making it mechanically more resistant to plastic deformation.In addition, plastic response is also in good correlation with the obtained value for sol fraction, which does not contribute to elastic deformation[42].
Fig.6.Rendgen images of the cured propellants: (a) Planar projection; (b) Orthogonal projection.
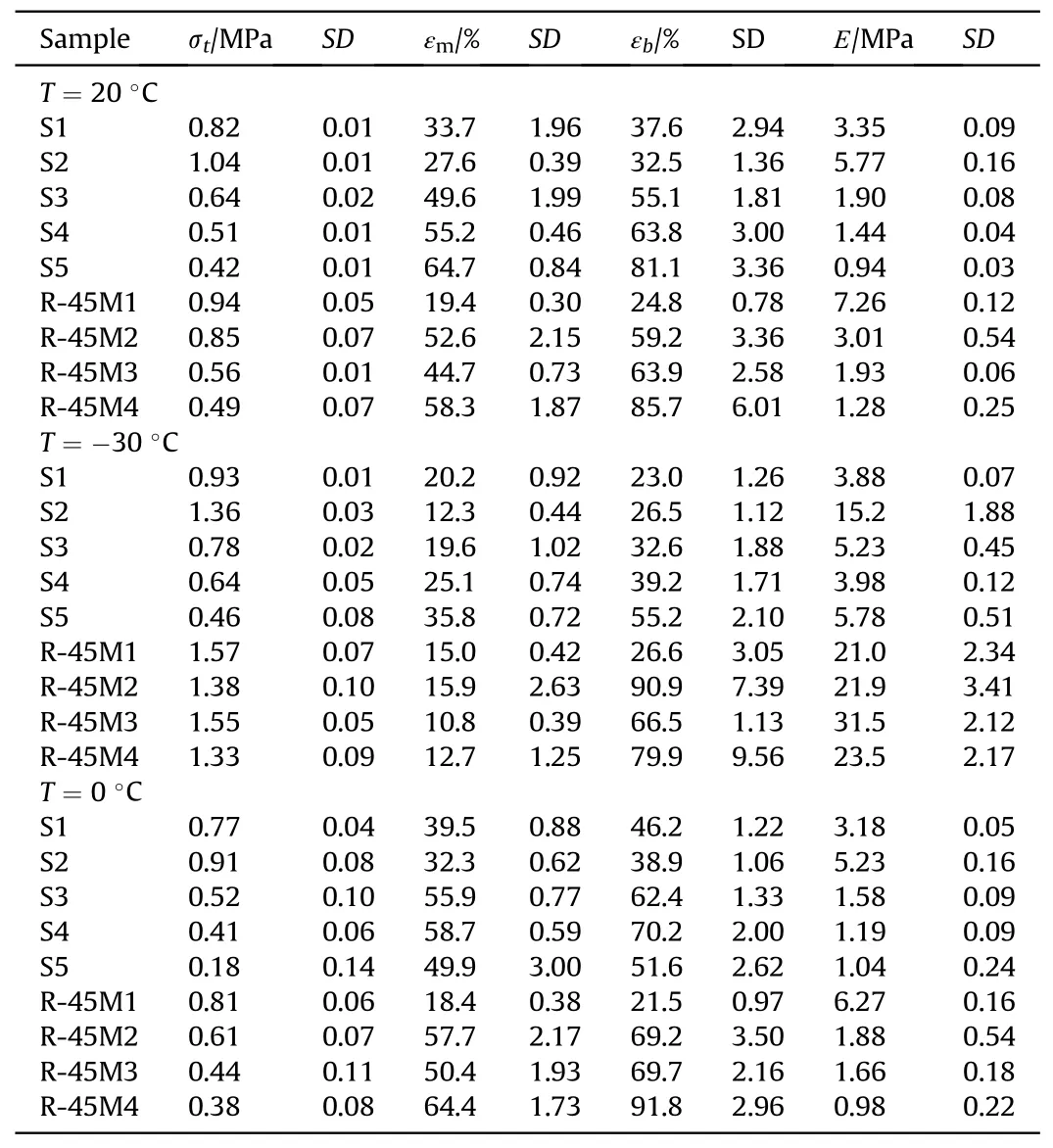
Table 7 Average values of tensile properties of corresponding propellants at+20 °C,-30 °C and +50 °C.
A decrease in temperature leads to an increase in stiffness, i.e.the tensile strength and modulus of elasticity of the corresponding propellants, with a simultaneous decrease in elasticity, which is reflected in lower strain at maximum load and strain at break.The reference composition from propellants group 2 has a 13.4%higher tensile strength compared to the value obtained at +20°C.Propellants within the same group, which contain CO, behave similarly,where the highest increase in tensile strength is remarked for composition S2 (30.8%).Contrary to this, the elasticity of the corresponding propellants decreases significantly, whereas the greatest drop in values of strain at break and strain at maximum load is obtained for composition S3,153.1%and 69.0%,respectively.The increase in tensile strength within propellant group 3 at low temperature exhibits a similar trend to propellant group 2, but is more pronounced.Namely, the highest increase of 176.8% is remarked for composition R-45M3, compared to the result obtained at +20°C.The strain at maximum load decreases significantly (maximum reduction of 359.0%-propellant R-45M4), while the strain at break increase for all analyzed propellants except R-45M4.Nevertheless,the differences between these two strains are quite remarkable (for 75.0% - R-45M2).In general, lower temperatures give a tough propellant, which can withstand more load,reflecting in higher tensile strength.The hindered mobility of the polymer segments together with the high cross-linking density and matrix-filler interactions within the CO containing propellants are the reason for the results tabulated in Table 7[45].In contrast,high elasticity at reduced temperature is not a common feature of this type of material.The remarked phenomenon is a consequence of the presence of reactive plasticizer,which participates in the curing process.Table 5 shows that the amount of hard segments within CO containing propellants is high,which leads to the formation of stiff and rigid material at a reduced temperature.Such a material withstands the applied load,during which the bonds between the polymer and the solids become weaker [46] and the matrix takes on the generated stress.The polymer matrix contains numerous loose macromolecule chains and a three-dimensional structure that relieves the entire material which is expressed through a high interval of plastic deformation until the collapse of the specimen(strain at break).
An increase in temperature decreases the tensile strength and modulus of elasticity with a concomitant increase in strain at maximum load and strain at break.Both groups of propellants show similar behavior where the greatest decrease in tensile strength is 133.3%(S5)and 39.3%(R-45M2)compared to the values at+20°C.This reduce in tensile strength for S5 propellant is due to the lower amount of curing agent, i.e.the number of cross-linking junctions under the threshold value, making the polymer network looser and more mobile when the temperature increases.The reduction of strain at maximum load and strain at break is from 12%to 20%for both propellant groups compared to the same values at +20°C.In general, the stiffness and strength of polymer based materials tend to decrease as the temperature increases due to more energy which the macromolecule segments posses, making them mobile and the overall material softer[47].
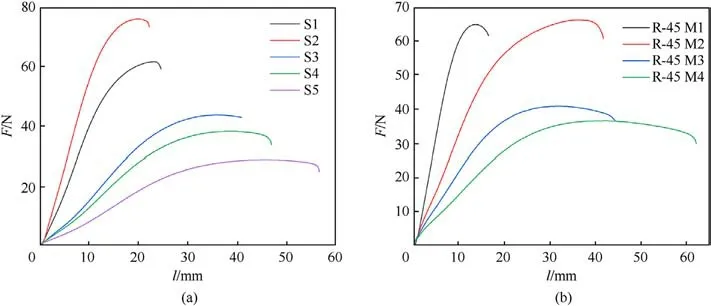
Fig.7.Mechanical properties of the analyzed composites at +20 °C.
Use of the CO, in addition to increasing the amount of hard segments, also increases the contents of soft moieties, which is a phenomenon manifested by the chemical structure of castor oil itself.Namely,the amount of curing agent is theoretically calculated so that 2 out of 3 hydroxyl groups in the CO structure remain free,i.e.unattached (for propellant group 2, the amount of IPDI is not enough to crosslink even one whole OH group from binder).This assumption is investigated by the parallel plate tools DMA test,which determines the reactivity of the OH groups from CO and HTPB (Sartomer) with IPDI.A plots G′and G′′versus temperature show that the initial values of these parameters are much higher for HTPB considering its greater molar mass compared to CO.As temperature increases, G′rises while G′′decreases and, in one moment, these two curves intersect giving the gel point of the corresponding polyurethanes(125.1°C for HTPB-IPDI and 142.6°C for CO-IPDI,respectively).The gel point for the HTPB-IPDI system is lower, but this does not mean that the OH groups from HTPB are more reactive than those from CO.The real indicator is primarily the G′value at the end of the reaction,which is substantially higher in the case of CO-IPDI polyurethane system.Another parameter for such conclusion is the slope of the G′curves, as they increase abruptly, which can be easily remarked from Fig.8 (1126.8 and 241.5 for CO-IPDI and HTPB-IPDI, respectively).CO possesses smaller molecules with dense arrangement of OH groups where the potential for the successful contact with isocyanate groups from IPDI is much higher than in the case of HTPB.
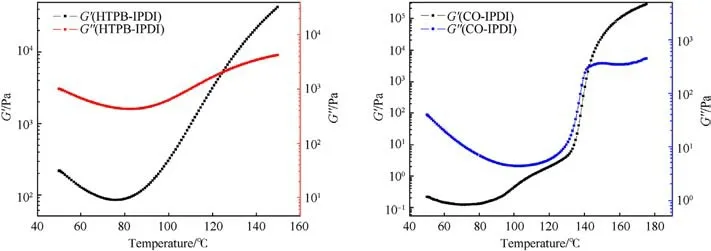
Fig.8.Reactivity of the OH groups from (a) HTPB and (b) CO with the izocyanate groups from IPDI.
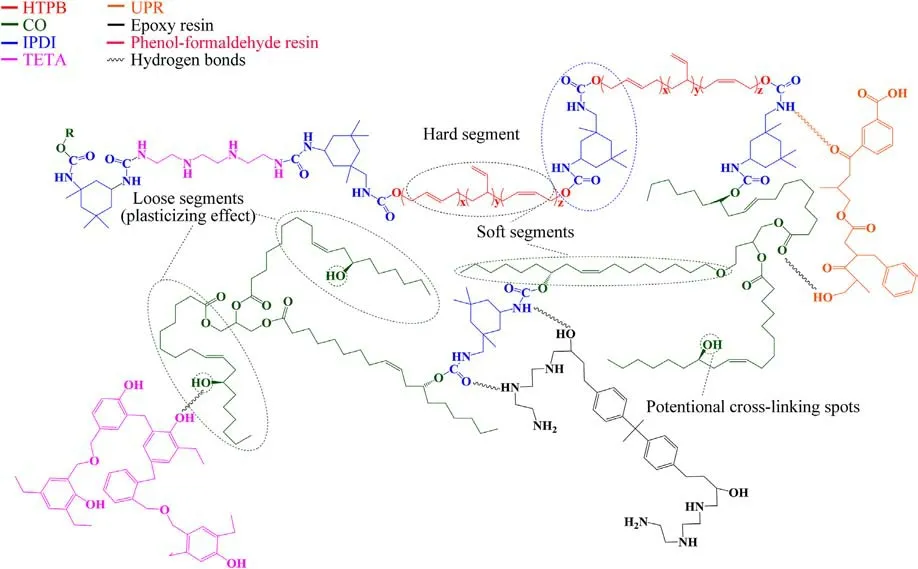
Fig.9.The potential structure of the formed polymer network within the corresponding propellants and the hydrogen interactions with the resins used as thermal insulation or inhibitor.
Fig.9 shows the potential structure of the created polymer network, where available OH groups from CO and HTPB create covalent bonds with isocyanate groups from the curing agent.It can also be remarked that the available N, O and H atoms from the polymer network are capable to establish hydrogen bonds with polar part of polyurethane base liner [48,49] or unsaturated polyester resin (UPR), epoxy resin or phenol-formaldehyde resin(resole type), which are used as thermal insulation (case-bonded grain) or inhibitor (free-standing grain).The practical meaning of these interactions is improved adhesion between the propellant and the liner/inhibitor,which enables regular motor operation and prevents its failure and,potentially,severe consequences for people and equipment.
Fig.9 shows that the bonding agent also participates in the cross-linking reactions,forming the urea bond between amino and isocyanate groups from TETA and IPDI,respectively.The amount of TETA is small, which is the reason why its effect on mechanical properties has not taken into account regarding the calculation of the quantity of the curing agent needed for the propellants solidification.
In addition,designed total NCO:OH ratio indicates that a certain amount of HTPB and CO remains partially free contributing to the additional flexibility of the polymer network.Free fatty acid chains from CO and loose macromolecules from HTPB cause the establishment of a disordered conformation giving higher deformability of the materials [50,51].The mentioned effects, along with the existence of a flexible ester bond in castor oil, contribute to obtaining strain at maximum load and strain at break as in Table 7.The presence of soft segments as well as loose chains (plasticizing effect) overcomes the higher amount of hard segments (crosslinking sites) within the propellants since the increase in elongation is more pronounced than in tensile strength.
So they were married, and in the middle of all the festivities and rejoicings the bride s father came home and was not a little surprised at finding his daughter celebrating her wedding
The mechanical integrity of the propellant,both in case-bonded or free-standing grains,is a very important characteristic because it is exposed to thermal stresses during its service life and firing.Such environment provokes significant strains caused by great differences in the linear thermal expansion coefficient between propellant and steel motor chamber or composite thermal insulation/inhibitor.The practical meaning of the above is that cured propellants have to withstand generated strains, i.e.it needs to be viscoelastic.Such properties can be tuned by an adequate binder,which is capable of receiving a huge amount of solids [52].
The benefit of using CO, practically as a component of binder,provides two essential characteristics to the polymer network that is beneficial for better elongation capabilities.The long chain molecular structure of the CO molecule serves as chain extender in the polymer network, improves the elongation capability of the polymer network.The second important characteristic of the CO molecule is that it has substantially more degrees of freedom(more flexibility).
4.Conclusions
The aim of this research was to examine the influence of CO,as a reactive plasticizer,on the viscoelastic and mechanical behavior of composite rocket propellants based on three different type of HTPB resin.The obtained results indicated the following.
(1) The introduction of a reactive plasticizer into propellant formulations enhances the initial viscosity as well as its increase over time,which is pronounced when Sartomer resin is used.In practice, all analyzed compositions cast easily,except possibly for the S2 batch.
(2) GPC analysis shows that SRE resin has multimodal, while Sartomer and R-45M have a monomodal distribution of the molar mass, at the same sample concentration.Using lower concentration, R-45M resin displays a bimodal, broad distribution of the molar mass in comparison to Sartomer,whose GPC curve is narrow.These results are supported by the polydispersity index, where SRE has the highest and Sartomer resin the lowest value.
(3) DMA analysis shows an increase in Tgfor propellants containing CO in comparison to reference samples.That variation in Tgis more pronounced in the case of propellant group 3(about 13°C)compared to propellant group 2(about 7°C),which is a consequence of the binder used.
(4) The designed compositions,which contain CO,have a higher amount of extractable fraction, according to the sol-gel analysis.Such results suggest that a significant part of the reactive components (HTPB and CO) remains unconnected with the polymer network, giving flexibility to the final material.
(5) Use of the CO, in addition to increasing the amount of hard segments,also increases the contents of soft moieties,which is a phenomenon manifested by the chemical structure of castor oil itself.The inclusion of CO in the propellants composition gives more space to adjust a wide range of mechanical properties at all three applied temperatures.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors acknowledge the support of this research from the Serbian Ministry of Education, Science and Technological Development (Grant No.451-03-68/2023-14/200325) and Ministry of Defense (Grant No.VA-TT/1/22-24).
- Defence Technology的其它文章
- Evolution of molecular structure of TATB under shock loading from transient Raman spectroscopic technique
- MTTSNet:Military time-sensitive targets stealth network via real-time mask generation
- Vulnerability assessment of UAV engine to laser based on improved shotline method
- Free-walking: Pedestrian inertial navigation based on dual footmounted IMU
- Investigation of hydroxyl-terminated polybutadiene propellant breaking characteristics and mechanism impacted by submerged cavitation water jet
- Estimation of surface geometry on combustion characteristics of AP/HTPB propellant under rapid depressurization

