Simple preparation of C (CS)/g-C3N4/Co carbon aerogel and its catalytic performance for ammonium perchlorate
Yujie Yan, Bo Jin, Rufang Peng
State Key Laboratory of Environment-friendly Energy Materials, School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang, 621010, China
Keywords:g-C3N4 Chitosan Co Ammonium perchlorate Pyrolysis kinetics
ABSTRACT Biomass chitosan(CS)was used as a template,graphitic phase carbon nitride(g-C3N4)with high nitrogen content and certain catalytic activity was used as a dopant, and nano-transition metal cobalt (Co) was used as a catalytic center point.The carbon aerogel (C (CS)/g-C3N4/Co) with a three-dimensional network-like structure was prepared by assembling the three materials through experimental operations such as freeze-drying and high-temperature carbonization.It was demonstrated by scanning and transmission characterization that the CS in the carbon aerogel could provide more active sites for the cobalt nanoparticles,and the doping of graphite-phase carbon nitride as a template dispersed the cobalt nanoparticles and changed the conductivity of the CS.To investigate the catalytic effect of carbon aerogel on ammonium perchlorate (AP), it was investigated by differential thermal analyzer and TG thermal analysis.This carbon aerogel was very effective in catalyzing AP, and the 10 wt% content of the catalyst reduced the AP pyrolysis peak from 703.9 to 595.5 K.And to further investigate the synergistic effect of the three materials, further carbon aerogels such as C (CS)/Co, g-C3N4/Co were prepared and applied to catalyze AP,and the same ratio reduced the AP pyrolysis peak by 98.1 °C and 97.7 °C.This result indicates a synergistic effect of the assembly of the three materials.
1.Introduction
As the main energy source for missile and rocket propellants in the military field, solid propellants convert chemical energy into thermal energy in the process of combustion [1].Ammonium perchlorate (AP), as a strong oxidizer, is an important part of the solid propellant system due to its good compatibility,large amount of released gas, and stable combustion performance.Moreover, its mass ratio reaches 60%-90% of the propellant system [2],depending on the combustion characteristics of AP (AP thermal decomposition rate, high temperature decomposition peak value,and activation energy), i.e., the decomposition is completed at the lowest possible temperature[3,4].However,the biggest obstacle to the thermal decomposition of AP is the high temperature of the pyrolysis peak.So, the main way to improve the thermal decomposition performance of AP is to reduce the pyrolysis peak of AP[5,6].The main way to reduce the peak value of decomposition peak at high temperature is to reduce the particle size of AP crystals or add combustion catalysts.However, the preparation process of ultrafine AP is more difficult and dangerous; so, the addition of combustion catalysts is more reasonable and applicable than ultrafine AP[4].
For the past few years, numerous studies have shown that transition metals, transition metal oxides, and MOF materials are effective in AP catalysis [7].Transition metals are promising combustion catalysts due to their low activation energy and high reaction rate.A mass of cobalt compounds have been reported in the field of AP catalysis,such as Co(OH)2[8],cobalt alginate[3],Co3O4[9], and others.However, transition metals have large interfacial specific surface area (unsaturation and instability, as well as small size effect)and often occur as agglomerates,which is not conducive to the catalytic effect of metals.To cross-linking of cobalt metal particles with sodium alginate as a templating agent led to the enhancement of cobalt-catalyzed AP [3].
As one of the 3D carbon-based materials,chitosan(CS)is widely used in the fields of adsorption, catalysis, and medicine due to its large specific surface area,low cost,and good biocompatibility[10].CS contains a large number of amino and hydroxyl groups in its molecular chain,which can chelate with metals[11].CS can act as a carrier for metal centers to accelerate charge propagation.It can act as a precursor to disperse metal particles to improve their catalytic activity.CS can be used as a carrier for metal centers to accelerate charge propagation and as a precursor to disperse metal particles to improve their catalytic activity;the transfer rate of metal particles modified by carbon-based materials can be further enhanced [12].
The doping of heteroatoms in carbon-based materials may improve the electrical conductivity of carbon-based materials.This received wide attention due to the higher electronegativity of N that can affect the charge distribution of electrons and the similar size of N atoms and carbon atoms,which makes the destruction of the carbon skeleton relatively unlikely in the process of N substitution for C[13,14].g-C3N4is a new type of polymer semiconductor with high nitrogen content and thermochemical stability [15].It can be obtained by calcining urea, melamine or thiourea at high temperature.According to the literature [16], g-C3N4has certain catalytic properties for ammonium perchlorate at a temperature increase rate of 10 K/min.Therefore, the introduction of g-C3N4with high nitrogen content into CS increases the electrical conductivity of CS and further increases the catalytic properties of the system.Wan et al.[17] used g-C3N4as a templating agent to disperse CuFe2O4and applied it to the AP system by immobilizing CuFe2O4on the surface of g-C3N4by in situ solvothermal method.AP + CuFe2O4/0.5% g-C3N4reduced the pyrolysis peak of AP from 403.7°C to 311.7°C and AP + CuFe2O4from 403.7 to 331.8°C.
Based on these experimental investigations,a ternary system of CS, g-C3N4and cobalt nitrate was constructed by in situ growth method.The obtained samples were subjected to a series of subsequent treatments such as freeze-drying and high temperature carbonization to obtain carbon aerogels for the sake of increasing the specific surface area of the samples and forming more pores.The final experimental results showed that the carbon aerogel had a great catalytic effect on AP.
2.Experiment
2.1.Experimental drugs and instruments test conditions
Urea (CAS 58069-82-2), chitosan (CAS 9012-76-4) and cobalt nitrate hexahydrate (CAS 10141-05-6) were purchased from Aladdin Company and Macklin (Shanghai, China) respectively.The morphological analysis of the prepared C (CS)/g-C3N4/Co was performed by scanning electron microscopy (Germany ZEISS Sigma 300) and transmission electron microscopy (FEI Tecnai G2 F20,USA).The crystallographic analysis of C (CS)/g-C3N4/Co was performed by powder X-ray diffractometry.Surface analysis of C(CS)/g-C3N4/Co by X-ray photoelectron and the calculation of the specific surface area of C (CS)/g-C3N4/Co by Brunauer-Emmett-Teller(BET) were performed.The corresponding pore types were obtained.The catalytic effect on ammonium perchlorate was analyzed by means of thermal analysis, such as DTA and TGA.The experimental conditions were as follows:the heating rates were 5 K/min,10 K/min,15 K/min,and 20 K/min,and the temperature range of the tests was 20-500°C.The test atmosphere of DTA was air with highpurity α-Al2O3as the reference.The temperature range and temperature rise rate of TGA test were the same as the DTA test conditions, but nitrogen was used as the atmosphere during the TGA test (WRT-1D), and the crucible used was a quartz crucible with a size of 5 mm × 5 mm without a lid.
2.2.Preparation of g-C3N4
According to the preparation process in the literature [10], g-C3N4was prepared by loading 10 g of urea into a covered crucible under air atmosphere in a tube furnace, heating it to 550°C at a heating rate of 5 K/min, and keeping it for 3 h to obtain a paleyellow powder.
2.3.Preparation of C (CS)/g-C3N4/Co
g-C3N4(0.2 g) was dispersed in 20 mL of distilled water and sonicated for 2 h.CS was dispersed in 2% acetic acid solution and stirred continuously for 2 h.g-C3N4and CS were mixed and stirred,and cobalt nitrate solution was added dropwise during stirring.The obtained solution was left for a period of time, placed in a refrigerator at -18°C for 12 h, and then freeze-dried.The lyophilized samples were carbonized in a tube furnace with an argon atmosphere.They were warmed up to 550°C at a heating rate of 5 K/min and held for 3 h to obtain C (CS)/g-C3N4/Co carbon aerogel.The preparation flow chart is shown in Fig.1.
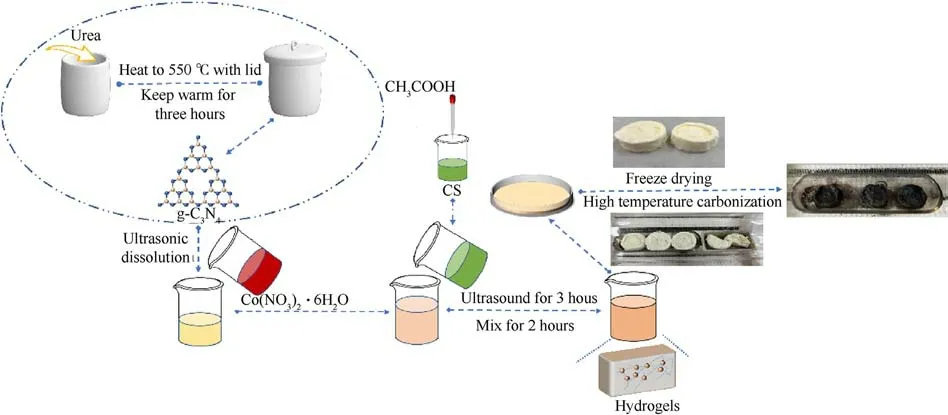
Fig.1.Preparation of C (CS)/g-C3N4/Co carbon aerogel.
2.4.Preparation of C (CS)/Co, g-C3N4/Co
Cobalt nitrate (0.2 g) was dispersed in CS solution with continuous stirring,freeze-dried after uniform dispersion,and carbonized at high temperature.The carbon aerogel obtained was named C(CS)/Co.g-C3N4(0.2 g) was dispersed in 20 mL of distilled water,sonicated for 2 h, and mixed by adding cobalt nitrate solution dropwise.Then, it was freeze-dried and carbonized at high temperature,and the carbon aerogel obtained was named g-C3N4/Co.The carbonization conditions of C (CS)/Co and g-C3N4/Co were the same as those of C (CS)/g-C3N4/Co.
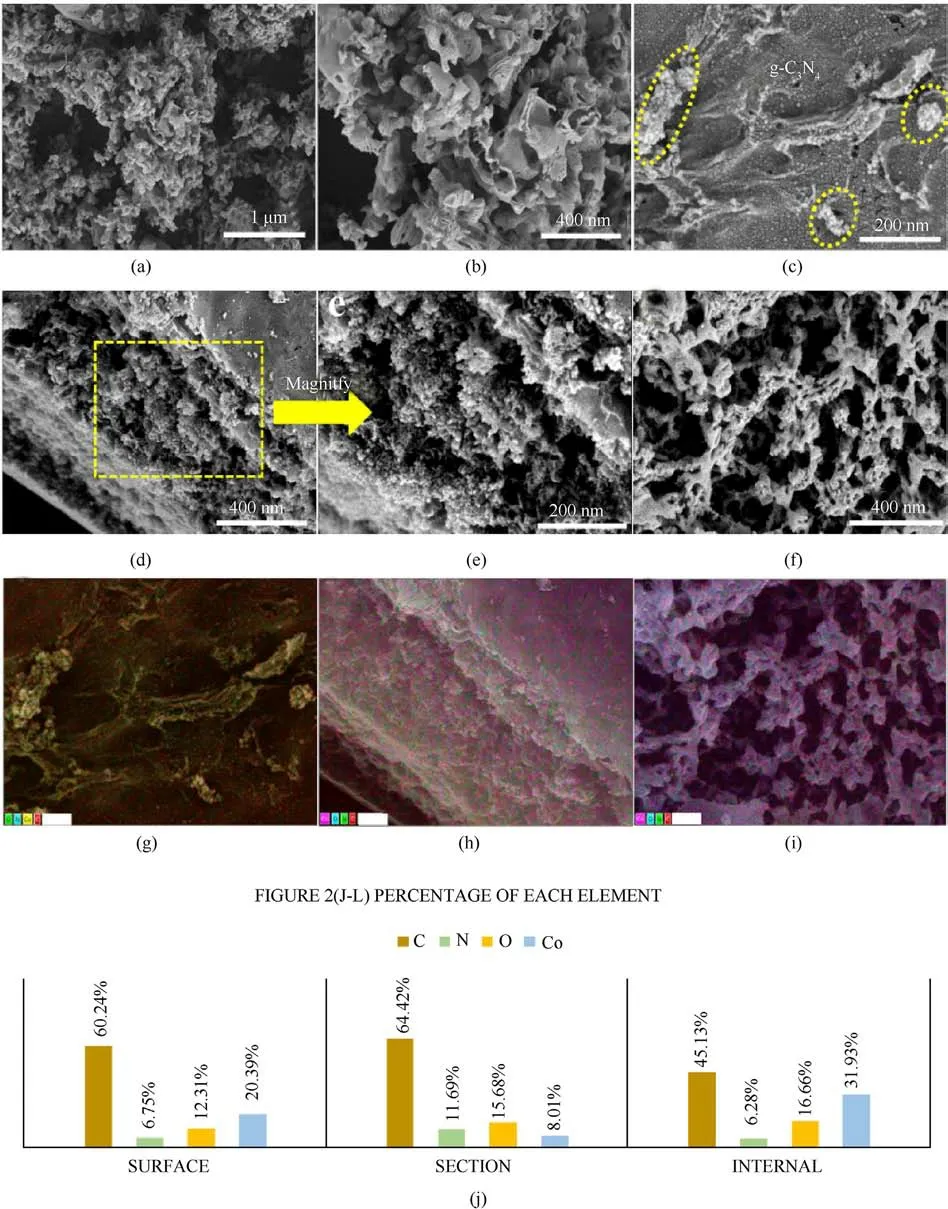
Fig.2.(a)and(b)SEM images of g-C3N4;(c)SEM image of C(CS)/g-C3N4/Co surface;(d)and(e)SEM images of C(CS)/g-C3N4/Co section;(f)SEM image of C(CS)/g-C3N4/Co internal;(g)-(i) mapping images of Figs.(c)/(d)/(f); (j) percentage of each element of Figs.2(g)-2(i).
3.Results and discussion
3.1.Morphological and compositional analysis
3.1.1.SEM
Figs.2(a) and 2(b) shows the morphology of g-C3N4.It is composed of many irregular bending layers,showing that g-C3N4is a typical graphite lamellar structure and forms clusters.The irregular assembly form of these bending layers leads to the formation of a porous structure.Porous structures are caused by gas spillage during the decomposition of urea, this phenomenon favors the increase of specific surface area [18].According to the Ref.[19],porous structures are more easily obtained when urea is used as a precursor.Fig.2(c) shows the scanning images of the surface of C(CS)/g-C3N4/Co nanocomposites.Clusters of g-C3N4are present,and uniformly dispersed particles can be observed in the CS matrix.The success of the nanomaterial composite is tentatively judged.Figs.2(d)and 2(e)show the scanned images at the cut surface of C(CS)/g-C3N4/Co.Fig.2(e)shows the enlarged image of Fig.2(d),and the presence of a large amount of mesh structure can be observed.Dense particles can be observed in the mesh structure.Fig.2(f)shows the internal structure of C(CS)/g-C3N4/Co.The presence of a large number of CS reticulated chains after carbonization can be observed.Uniformly dispersed particles can be observed on the reticulated chains.The energy spectra of Figs.2(c)and 2(d)and 2(f)were tested, and the plots corresponded to Figs.2(g)-2(i).The uniformly dispersed metal Co particles can be seen in the EDS plots,and their contents were tested.The results corresponded to Figs.S1-S3.Fig.2(j)showed that the contents of Co particles inside were more compared with those at the surface and the cut surface,presumably because the internal reticular chains could provide more specific surface area and more active sites for metal Co.
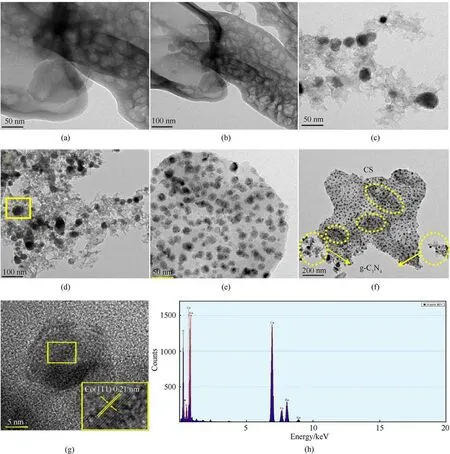
Fig.3.(a) and(b) TEM images of g-C3N4; (c) and(f) TEM images of C (CS)/g-C3N4/Co; (g) HRTEM image of C (CS)/g-C3N4/Co; (h) EDS pattern of C (CS)/g-C3N4/Co.
3.1.2.TEM
Figs.3(a) and 3(b) shows the TEM of g-C3N4.g-C3N4is a thin lamellar morphology with folds and more pore structures can be observed[10,20].This morphology is due to the formation of pore structures caused by the gas overflow formed during the thermal decomposition of urea;this can be consistent with the SEM results[21].Nearly spherical particles with some dispersion on the g-C3N4layer can be observed in Figs.3(c)and 3(d).The qualitative analysis of the spherical particles in Fig.3(d) can identify the spherical particles as metallic Co particles, further verifying that g-C3N4can be used as a template agent for nanoparticles.The copper element that is present in the energy spectrum is an additional metal that appears under the test conditions.Figs.3(e)and 3(f)shows the TEM of C (CS)/g-C3N4/Co.In Fig.3(f), part of g-C3N4is loaded on the surface of CS and part of g-C3N4is dispersed in the carbon aerogel matrix.A large amount of metallic cobalt particles is uniformly dispersed in the CS, and a small amount of Co is uniformly distributed to the g-C3N4.The carbon aerogel has a large number of active sites for Co use, increasing the catalytic activity of Co [22].The measured value of the lattice stripe of TEM in Fig.3(g) is 0.21 nm, which matches the value of the crystalline spacing appearing on the crystalline surface of Co(JCPDS 15-0806)(111)in XRD [4].
3.1.3.XRD
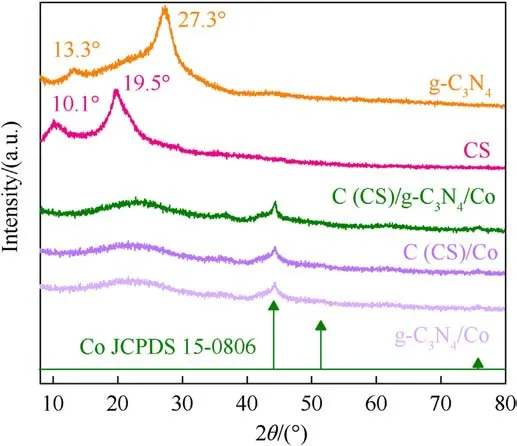
Fig.4.XRD images of various composite materials.
As shown in Fig.4, the crystallographic surface analysis of g-C3N4, CS, C (CS)/g-C3N4/Co, C (CS)/Co, g-C3N4/Co was performed using XRD test means.g-C3N4has two diffraction peaks at 13.3°and 27.3°.The weaker diffraction peak near 13.3°is attributed to the repeating triazine unit in g-C3N4, whereas the stronger diffraction peak at 27.7°is attributed to the interlayer stacking of the aromatic units of C-N[16,19].The characteristic peaks of CS appear at 10.1°and 19.5°, which are crystalline properties that appear due to the molecular hydrogen bonding,which in turn is caused by the amino and hydroxyl groups contained in CS[15].However,the crystalline structure of g-C3N4, CS was not observed in the XRD pattern of C(CS)/g-C3N4/Co.It is highly likely that the structures of CS and g-C3N4were disrupted during the high temperature carbonization process.The strongest diffraction peak appearing in C(CS)/g-C3N4/Co can only correspond well to the (111) peak face of Co (JCPDS 15-0806) presumably at a lower intensity due to the low addition of Co in the C (CS)/g-C3N4/Co nanocomposite (mass ratio CS:g-C3N4:Co = 5:1:1).The same is true for C (CS)/Co and g-C3N4/Co,which have some correspondence with Co (JCPDS 15-0806) This finding indicates that the metal Co particles are successfully compounded.
3.1.4.XPS
XPS was used to study the elements contained in the substances and their valence states.The results are shown in Fig.5(a), where the presence of specific Co 2p in the XPS spectrum of C(CS)/g-C3N4/Co indicates that the composite was successfully compounded with Co.In Fig.5(b), four peaks of 284.2 eV, 285.0 eV, 286.2 eV and 288.7 eV were detected in the spectrum of C 1s; 284.2 eV corresponds to C-C, the peak at 285.0 eV is associated with C-N-C present in g-C3N4,286.2 eV is associated with the C-O bond in CS,and 288.7 eV corresponds to the C-N=C present in the g-C3N4triazine unit[17,23,24].For the N 1s spectrum(Fig.5(c)),four peaks at 397.3 eV, 398.7 eV, 399.5 eV, and 400.7 eV are associated with C-N=C, C-NH-C, bridge N in N-(C)3, and N in aromatic heterocycles,respectively[10,25].As for the O 1s spectrum(Fig.5(d)),two peaks of 532.8 eV and 530.8 eV were detected,corresponding to the O atom signal in the -OH group and the Co-O bond [26], respectively,further confirming the presence of the Co element.For the Co 2p spectra(Fig.5(e)),the four detected peaks at 802.8 eV,796.3 eV,785.4 eV, and 780.4 eV correspond to the satellite peaks, Co 2p1/2,satellite peaks, and Co 2p3/2, respectively.The presence of the satellite peaks indicates the presence of cobalt in the oxidized state(+2) [3,27,28].The presence of the cobalt oxidation state may be due to exposure to air during the preparation process or during the transfer to the tube furnace for calcination,which is common in Cobased catalysts [4].
3.1.5.BET
The specific surface area and pore size of the material and its distribution are important parameters for judging the activity of the catalyst.The larger the specific surface area is, the more active sites are present, the better the effect of dispersion on the metal centers is, and the better the catalytic activity of the metal play catalyst is.As shown in Fig.6(a), the sample shows a typical IV isothermal curve at a relative pressure of 0.4-1.0, implying the presence of a mesoporous structure[29].In Fig.6(b),the pore size distribution is in the range of 0-100 nm,with 2-50 nm occupying 90% of the sites.This finding indicated that the material's pores were mostly mesopores, and a small amount of macropores was also present[30].
3.2.Catalytic performance study
3.2.1.AP catalyzed without mass fraction of catalyst
The catalyst content is among the important factors affecting the catalytic performance of the catalyst,as shown in Fig.7(a),the catalytic pyrolysis of AP was analyzed using different contents of C(CS)/g-C3N4/Co,where the addition amounts of 3 wt%,5 wt%,8 wt%,10 wt%,and 15 wt%reduced the AP pyrolysis peak by 90.5 K,105.8 K,107.1 K,108.4 K,and 106.4 K,respectively(Fig.7(b)).The ability to degrade the pyrolysis peak gradually increased with increasing addition; 15 wt% was weaker than 10 wt% C (CS)/g-C3N4/Co in reducing the pyrolysis peak of pure AP,presumably because of the decrease in catalytic activity due to agglomeration of metal centers when the addition amount was too much.The best catalytic performance was achieved when the addition amount was 10 wt%;10 wt% was chosen for the activation energy measurement and further investigation of the kinetic parameters.
3.2.2.AP catalyzed without mass fraction of catalyst
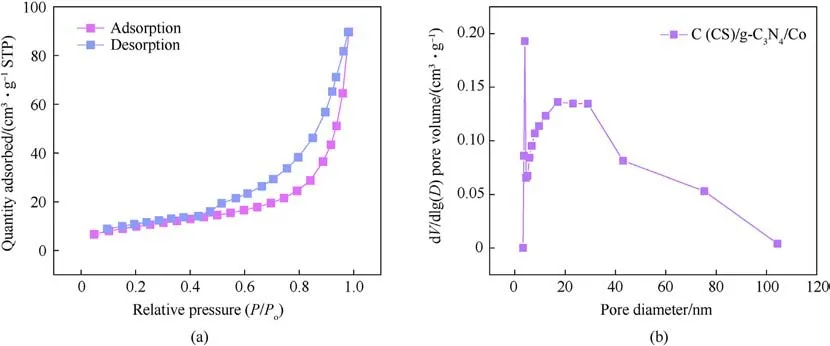
Fig.6.(a) N2 adsorption desorption of isotherms of C (CS)/g-C3N4/Co; (b) Pore size distributions of C (CS)/g-C3N4/Co.
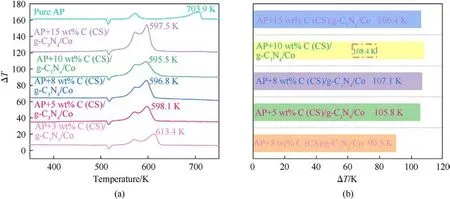
Fig.7.(a) DTA curves for different percentages of C (CS)/g-C3N4/Co catalyzed AP; (b) Catalytic capacity comparison chart.
To further investigate the thermal decomposition behavior during the thermal decomposition of AP, parameters such as activation energy or reaction rate can be probed.The kinetic parameters of the thermal decomposition of DTA were tested for pure AP and 90 wt% AP+10 wt% C (CS)/g-C3N4/Co at different ramp rates.The results are shown in Fig.8.As shown in Fig.8(a), the heatabsorbing peak of pure AP exists between 500 K and 525 K,which corresponds to the transformation of the crystalline phase[12].The two exothermic peaks correspond to the low-temperature decomposition peak (LTD) and the high temperature decomposition peak (HTD) [4].Compared with the pure AP, the hightemperature decomposition peaks of the AP system with the addition of the catalyst were reduced to a certain extent at different heating rates, as shown in Fig.8(c).Moreover, the pure AP system had two obvious exothermic peaks, whereas the two exothermic peaks of the AP system with the addition of the catalyst gradually merged into one exothermic peak as the heating rate increased.These results indicated that the catalyst could effectively reduce the high temperature decomposition peaks of the AP, and the thermal decomposition rate of AP was accelerated.The activation energy calculated by Kissinger method was 215.9 kJ/mol for pure AP(Figs.8(b)) and 171.9 kJ/mol for 90 wt% AP+10 wt% C (CS)/g-C3N4/Co (Fig.8(d)), and the reduced activation energy value was 44 kJ/mol.Constant k and the rate constant increased from 1.03×10-1to 29.1(Tables 1 and 2).Both the decrease in activation energy and the increase in rate constant indicated that the catalyst accelerated AP's thermal decomposition.
where β is the rate of temperature rise(K/min),Eais the activation energy(kJ/mol),Tpis the high temperature decomposition peak,R is the ideal gas constant (8.314 J/(mol·K)), and A is the prefactor.The reaction rate constant k (s-1) is obtained by the joint calculation of Eqs.(1) and (2), where T in Eq.(2) is 673.15 K.
3.2.3.Comparison of catalytic performance of several catalysts

Fig.8.(a) DTA curves for different heating rates of pure AP; (b) dependence of ln(β/Tp2) on 1000/Tp during AP decomposition; (c) DTA curves for different heating rates of AP + C(CS)/g-C3N4/Co; (d) dependence of ln(β/Tp2) on 1000/Tp during AP + C (CS)/g-C3N4/Co.

Table 1 Kinetic parameters of thermal decomposition of pure AP.
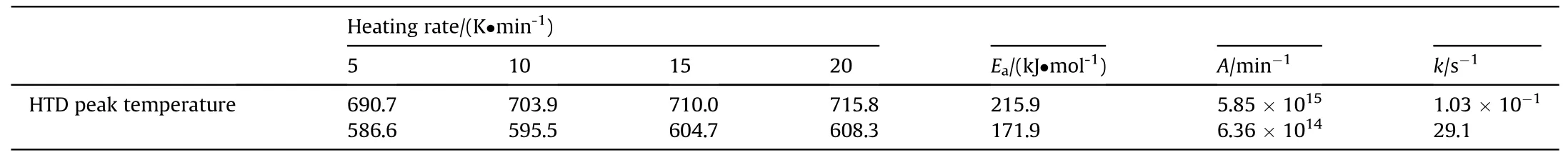
Table 2 Kinetic parameters of thermal decomposition of AP + C (CS)/g-C3N4/Co.
To investigate the existence of a certain coordination of this ternary system, a series of catalysts such as C (CS)/Co, g-C3N4/Co,and g-C3N4,were prepared.The thermal analysis results of 90 wt%AP+10 wt% C (CS)/Co, 90 wt% AP+10 wt% g-C3N4/Co, and 90 wt%AP+10 wt% g-C3N4at four ramp rates of 5 K/min,10 K/min,15 K/min, and 20 K/min are shown in Figs.9(a)-9(c).The peak temperatures of the heat absorption and low temperature decomposition peaks of the AP system with the addition of catalyst did not change significantly when compared with the thermal analysis curves of the four different heating rates of pure AP, thereby indicating that C (CS)/Co, g-C3N4/Co, g-C3N4did not have significant catalytic effects on the heat absorption and low temperature decomposition peaks.Fig.9(c) shows the comparison of the catalytic performance of three catalysts, namely, C (CS)/g-C3N4/Co, C(CS)/Co,and g-C3N4,for AP;they reduced the AP high temperature decomposition peak by 108.4 K, 98.1 K, and 53.1 K, respectively.Fig.9(d) shows the numerical differences between the three catalysts as follows.(i) g-C3N4does have a catalytic effect on AP as described in the literature[16].(ii)The catalytic effects of C(CS)/Co and g-C3N4/Co indicate that the presence of CS and graphitic phase carbon nitride has a dispersive effect on the metal center.(iii) The ternary system C (CS)/g-C3N4/Co is more effective than C (CS)/Co and g-C3N4/Co in reducing the AP pyrolysis peak by 10.3 K and 10.7 K, respectively, indicating that the metal center cobalt is the main catalyst,g-C3N4plays a secondary role,and CS has a stronger dispersion effect compared with g-C3N4.
The values of the high temperature decomposition peaks of the four catalysts, namely, C (CS)/g-C3N4/Co, g-C3N4, C (CS)/Co, and g-C3N4/Co,to reduce AP were summarized and the results are shown in Table 3.The catalytic results of similar catalysts in the literature were also compared.The comparison results show that C (CS)/g-C3N4/Co is promising for the catalysis of AP.

Fig.9.(a)DTA curves for different heating rates of AP+C(CS)/Co;(b)DTA curves for different heating rates of AP+g-C3N4/Co;(c)DTA curves for different heating rates of AP+g-C3N4; (d) numerical comparison.
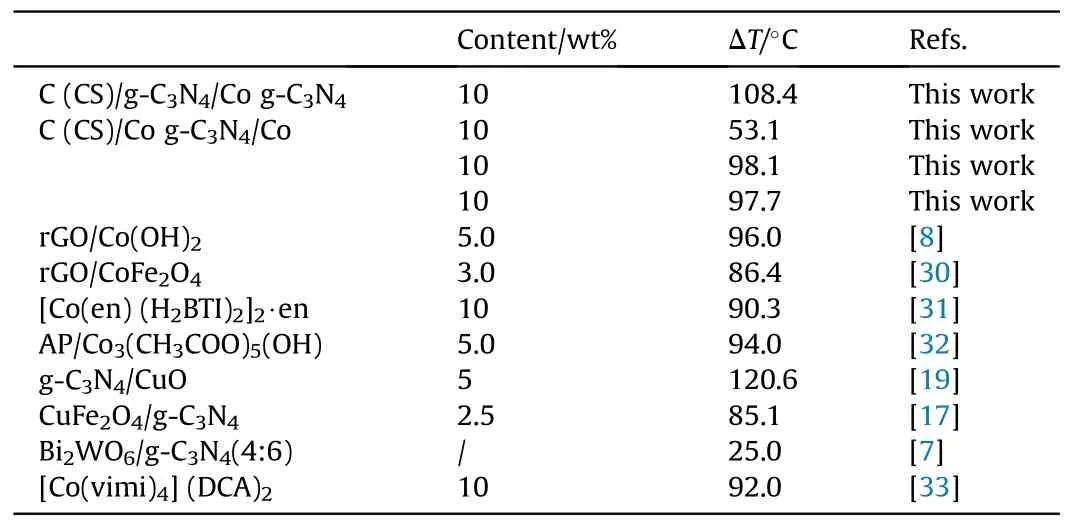
Table 3 Comparison of catalytic AP capacity with literature.
3.2.4.TG testing of several catalysts
The TG plot of pure AP,90 wt%AP+10 wt%(C(CS)/g-C3N4/Co,C(CS)/Co, g-C3N4/Co, g-C3N4), is shown in Fig.10(a).Two distinct inflection points exits in the TG curves of pure AP, indicating the existence of two weight loss phases in the range of 550-750 K.The first weight loss can be observed in the 550-650 K range,which is attributed to the low temperature thermal decomposition(LTD)of AP.The second weight loss is observed in the 650-750 K range,which corresponds to the main decomposition stage of AP,i.e.,high temperature thermal decomposition(HTD)[30].In the presence of g-C3N4, two more obvious weight loss phases remained in AP, but there was a large advancement in the temperature of mass loss(Fig.10(b)).The presence of three catalysts, C (CS)/g-C3N4/Co, C(CS)/Co, and g-C3N4/Co, made the two weight loss phases of pure AP overlap into one weight loss phase,which is consistent with the results of DTA.
4.Conclusions
C (CS)/g-C3N4/Co ternary system carbon aerogel was prepared by a simple preparation method.The large specific surface area of CS and g-C3N4effectively inhibited the aggregation of Co and effectively increased its catalytic AP activity,as shown by scanning and transmission characterization.When 10 wt% C (CS)/g-C3N4/Co was added, the high temperature decomposition peak of AP was reduced by 108.4 K, the activation energy was decreased from 215.9 to 171.9 kJ/mol for pure AP,and the rate constant of HTD of AP was increased from 1.03 × 10-1to 29.1.The catalyst had a good effect on the reduction of the high temperature decomposition peak of AP.The catalyst had good application prospects for the reduction of the high temperature decomposition peak of AP.
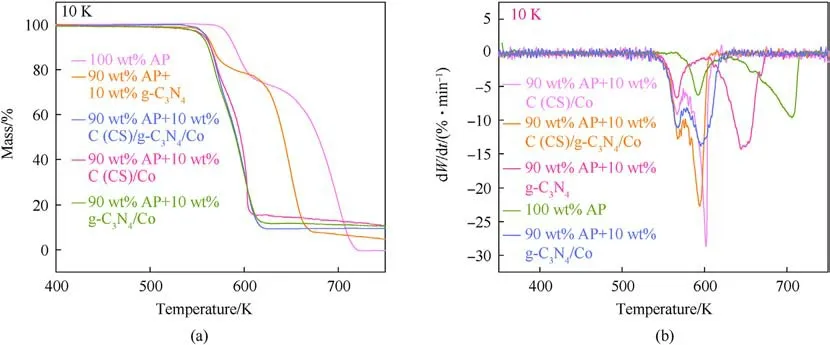
Fig.10.(a) TG curves of several types of catalysts; (b) DTG curves of several types of catalysts.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was carried out with the financial support received from the Natural Science Foundation of China (21875192),Outstanding Youth Science and Technology Talents Program of Sichuan (no.19JCQN0085), and Open Project of State Key Laboratory of Environment-friendly Energy Materials (Southwest University of Science and Technology, No.22fksy18).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.04.011.
- Defence Technology的其它文章
- Evolution of molecular structure of TATB under shock loading from transient Raman spectroscopic technique
- MTTSNet:Military time-sensitive targets stealth network via real-time mask generation
- Vulnerability assessment of UAV engine to laser based on improved shotline method
- Free-walking: Pedestrian inertial navigation based on dual footmounted IMU
- Investigation of hydroxyl-terminated polybutadiene propellant breaking characteristics and mechanism impacted by submerged cavitation water jet
- Estimation of surface geometry on combustion characteristics of AP/HTPB propellant under rapid depressurization

