Limonin inhibits the stemness of cancer stem-like cells derived from colorectal carcinoma cells potentially via blocking STAT3 signaling
Wei-Feng Zhang,Cheng-Wei Ruan,Jun-Bo Wu,Guo-Liang Wu,Xiao-Gan Wang,Hong-Jin Chen
Abstract BACKGROUND Limonin is one of the most abundant active ingredients of Tetradium ruticarpum.It exerts antitumor effects on several kinds of cancer cells.However,whether limonin exerts antitumor effects on colorectal cancer (CRC) cells and cancer stemlike cells (CSCs),a subpopulation responsible for a poor prognosis,is unclear.AIM To evaluate the effects of limonin on CSCs derived from CRC cells.METHODS CSCs were collected by culturing CRC cells in serum-free medium.The cytotoxicity of limonin against CSCs and parental cells (PCs) was determined by cholecystokinin octapeptide-8 assay.The effects of limonin on stemness were detected by measuring stemness hallmarks and sphere formation ability.RESULTS As expected,limonin exerted inhibitory effects on CRC cell behaviors,including cell proliferation,migration,invasion,colony formation and tumor formation in soft agar.A relatively low concentration of limonin decreased the expression stemness hallmarks,including Nanog and β-catenin,the proportion of aldehyde dehydrogenase 1-positive CSCs,and the sphere formation rate,indicating that limonin inhibits stemness without presenting cytotoxicity.Additionally,limonin treatment inhibited invasion and tumor formation in soft agar and in nude mice.Moreover,limonin treatment significantly inhibited the phosphorylation of STAT3 at Y705 but not S727 and did not affect total STAT3 expression.Inhibition of Nanog and β-catenin expression and sphere formation by limonin was obviously reversed by pretreatment with 2 μmol/L colievlin.CONCLUSION Taken together,these results indicate that limonin is a promising compound that targets CSCs and could be used to combat CRC recurrence and metastasis.
Key Words: Limonin;Colorectal cancer;STAT3 signaling;Cancer stem-like cells;STAT3;Aldehyde dehydrogenase 1
lNTRODUCTlON
Colorectal cancer (CRC) is recognized worldwide as one of the most common malignancies and frequently leads to cancer-related death.Currently,numerous treatment strategies for aggressive CRC have been developed;however,promising and novel strategies still need to be developed[1,2].Accumulating evidence demonstrates that the presence of a small subpopulation of cancer cell-derived parental cells (PCs) termed cancer stem-like cells (CSCs) contributes to tumor recurrence and metastasis.The presence of CSCs is considered the main cause of therapy resistance in several kinds of cancers,including CRC[3].CSCs are characterized by diverse cancer-initiating properties,including self-renewal capacity and metastatic potential[4,5].Several putative markers,such as Nanog[6] and β-catenin[7],were reported to be highly expressed in CSCs derived from CRC and correlated with high-risk cases in CRC patients.Targeting CSCs to overcome their promoting effects on chemoresistance or radioresistance has been a recent research focus.
Limonin,a triterpenoid compound,is one of the most abundant molecules inTetradium ruticarpum[8].It has several bioactivities,including anti-schistosomal,anti-malarial,antiviral,anti-inflammatory,antioxidant and cholesterollowering activities[9-13].Limonin was also found to exert antitumor effects.Chenet al[8] reported that limonin inhibits angiogenesis and metastasis of human breast cancer.It has also been reported that limonin increases the radiosensitivity of nasopharyngeal carcinoma[14].Importantly,limonin was reported to be involved in the regulation of the stemness of CSCs.A previous report showed that limonin inhibits the protein level of P-glycoprotein in human colon and leukemia cell lines,which display a high proportion of CSCs[15].In nasopharyngeal carcinoma cells,limonin inhibits stemness and malignant behaviors by inhibiting cell cycle distribution[14].However,the roles of limonin in CSCs in CRC cell progression have never been disclosed.
STAT3 is a transcription factor that exerts multiple important biological roles in the processes of normal and transformed cells[16].Moreover,STAT3 is considered to exert an essential role in maintaining hallmark genes that are critical for the stem cell phenotype,including aldehyde dehydrogenase (ALDH)[17].STAT3 activation was also reported to be critical in regulating the maintenance of the stemness of CSCs.
In hepatocellular cancer,STAT3 activation promotes stemness by inducing epithelial mesenchymal transition progression[18].In HER2-negative breast cancer,STAT3 activation was also observed to be correlated with CSC properties[19].Hence,in this study,we explored potential of utilizing limonin to inhibit CSCs among CRC cells by regulating STAT3 activation.
MATERlALS AND METHODS
Cell culture and CSC enrichment
The human CRC cell lines,HCT116 and SW480,were kept in liquid nitrogen and maintained in DMEM with addition of 10% fetal bovine serum (FBS),100 μ/mL of penicillin,100 μg/mL of streptomycin.Cell culture was kept in 5% CO2incubator at 37 ℃ and medium was replaced every 2-3 d.For enriching CSCs,cells were cultured in DMEM/Ham Nutrient Mixture F-12 (F-12) (at ratio of 1:1) supplemented with epidermal growth factor (EGF,20 ng/mL),basic Fibroblast Growth Factor basic (bFGF,10 ng/mL),and 2% B27 for 14-21 d.
Cell viability analysis
The 5 × 103cells were seeded in each well on a 96-well plate and allowed to attach overnight.To detect the cell viability,10 μL of cholecystokinin octapeptide-8 was added into each well for 2 h extra incubation,followed by an absorbance measurement at 450 nm using a Multiskan spectrum microplate reader (Thermo Electron Corporation,Waltham,MA,United States).All assays were performed in triplicate and independently repeated three times.
To perform limonin (Sigma-Aldrich,Cat.No.: L9647,St.Louis,MO,United States) treatment,10-60 μmol/L of limonin was supplemented and after 24-h incubation,cells were employed for following assays.
Cell cycle distribution assay
The 1 × 106cells were collected and washed using ice-cold PBS for three times.Cells were suspended in 300 μL of ice-cold PBS and fixed with 700 μL of ice-cold 99.5% ethanol overnight.After three careful washed using ice-cold PBS,cells were stained with 50 μg/mL propidium iodide (PI,Sigma-Aldrich,St.Louis,MO,United States) and analyzed by Navios flow cytometers (Beckman Coulter,Brea,CA,United States).
Migration and invasion
The 24-well transwell chemotaxis chamber technique (Millipore,Billerica,MA,United States) was employed to detect migration capacity.DMEM/F12 medium with addition of 10% FBS was placed in the low chamber room.CSC spheres were trypsinized and single cell suspension were collected.Then,1 × 104cells were suspended in Serum-free medium with addition of 2% B 27,10 ng/mL EGF,and 20 ng/mL bFGF and seeded into the upper chamber (pore size,8 μm).The chamber was placed in incubator for 24 h incubation at 37 °C.24 h later,the chamber was obtained and the cells on the upper surface of chamber were wiped away using a cotton swab.Cells were fixed using 4% paraformaldehyde for 15 min at 37 °C and then stained with 0.05% crystal violet for 30 min at room temperature followed by 3 washes with ice-cold PBS.The number of cells that have migrated to the lower surface of the chamber were counted in 5 randomly selected fields using a X71 (U-RFL-T) fluorescence microscope (Olympus,Melville,NY).To detect the invasion capacity,same amount of cells were seeded into chamber pre-coated using Matrigel for 48-h incubation.Same procedure was performed to detect the cells on the lower membrane of chambers.Each assay was performed in triplicate wells.
Tumor formation on soft agar
The 5 × 103cells were suspended in serum-free medium with addition of 2% B 27,10 ng/mL EGF and 20 ng/mL bFGF.Cells were dissolved in 0.3% low-melting agarose (Sigma-Aldrich,St.Louis,MO,United States) and were allowed to grow for 14 days and colonies were identified as having > 50 μm in diameter.
Western blot
To obtain total protein,100 μL of lysis buffer containing 1% NP-40,100 mmol/L Tris-HCL pH 7.5,150 mmol/L NaCL,5 mmol/L EDTA was added into 1 × 106cells and maintained on ice for 30 min.Then,sample was centrifuged at 12000 g,4℃,for 10 min.Supernatant containing total protein was collected and measured using Pierce BCA protein assay kit(Thermo Scientific,Waltham,MA,United States).20 μg of the total protein was fractionated in an 8% SDS-PAGE gel and transferred to a Nitrocellulose membrane,which were then blocked using PBS containing 0.25% Tween 20 (PBS-T),5%skim milk for 1 h at room temperature.Then,membrane was incubated with primary antibodies described as followed for 1 h at room temperature: Anti-Nanog antibody (cat.No.: ab109250),anti-β-catenin antibody (cat.No.: ab32572),anti-βactin antibody (cat.No.: ab8226),anti-STAT3 antibody (cat.No.: ab68153),anti-phosphorylated STAT3 (Y705) antibody(cat.No.: ab278669),anti-phosphorylated STAT3 (S727) antibody (cat.No.: ab32143),anti-Histone H2A antibody (cat.No.:177308).All antibodies were diluted in 1:1000 using PBS-T.After three washes with PBS-T,membranes were incubated with the corresponding secondary antibodies goat anti-rabbit IgG antibody diluted in 1:5000 (cat.No.: ab7090) for 2 h at room temperature.Obtained membranes were detected using SuperSignal Wester Femto Maximum Sensitivity Substrate(Thermo Scientific,Waltham,MA,United States).Blots were imaged and analyzed with Quantity One (Bio-Rad Laboratories,Hercules,CA,United States).
ALDH1 staining
To flow cytometric analysis of ALDH1,cells were re-suspended and incubated with ALDH1-FITC antibody (Millipore,Billerica,MA,United States),according to the manufacturer’s instructions for 30 min on ice,washed with PBS three times,and fixed with 4% paraformaldehyde for later analysis using BD FACS Canto II,BD Biosciences,San Jose,CA,United States.
Nude mice experiment
All the animal experiments were conducted according to the Animal ethics committee in Affiliated Hospital of Nanjing University of Traditional Chinese Medicine (No.2019 DW-13-02).12 Four-week-old female BALB/c nude mice were purchased from Nanjing JunKe Laboratory Animal Technology Co.Ltd,(Nanjing,China).All animals were raised in the SPF animal facilities (temperature 21-25 °C;humidity 50%-60%;well-ventilated cages) kept on 12 h/12 h light-dark cycle.Food and water were available ad libitum.Mock group of CRC model was established by injecting 2 × 105CSCs derived from HCT116 cells,into the skin of each nude mouse (6 nude mice).Treated group was established by injecting 2× 105CSCs pretreated with limonin for 48 h.We observed the growth of the tumors,and the tumor size was measured at day 10,15,20,25 and 30.One month after the injection,the subcutaneous tumors of nude mice were excised.
Hematoxylin and eosin staining
After all rats were sacrificed,tumor samples were fixed in 10% formalin for 24 h and then were dehydrated by washing in ascending grades of ethanol.Samples were then sectioned and embedded in paraffin wax for hematoxylin and eosin(H&E) staining.The stained sections were observed for morphological changes.Images were taken under 200 ×magnification at five random fields of view under a X71 (U-RFL-T) fluorescence microscope (Olympus,Melville,NY).
Statistical analysis
A standard two-tailed unpaired student t test was used to calculate differences between samples.One-way analysis of variance was used to determine statistically significant differences from the mean in the xenograft study.
RESULTS
Limonin exerts cytotoxicity to human CRC cells
It has been reported that limonin exert broad and effective anticancer activity,including liver cancer[20],intestinal cancer[21],and breast cancer[22].To evaluate whether limonin exerts cytotoxicity against CRC cells,10-60 μmol/L of limonin was cultured with HCT116 or SW480 cells for 24 h.As it is shown in Figure 1A,HCT116 showed 30% Inhibitory Concentration (IC30) value of 38.9 ± 1.5 μmol/L and half inhibitory concentration (IC50) value of 58.4 ± 2.1 μmol/L;SW480 showed IC30value of 42.3 ± 2.1 μmol/L and IC50value of 63.2 ± 1.7 μmol/L.Then,20 or 40 μmol/L of limonin was employed for 24-h incubation and HCT116 or SW480 cell cycle distribution were analyzed by performing PI staining followed by flow cytometry assay.Expectedly,40 μmol/L of limonin,but not 20 μmol/L of limonin treatment,increased proportion of G1/G0phase (Figure 1B).
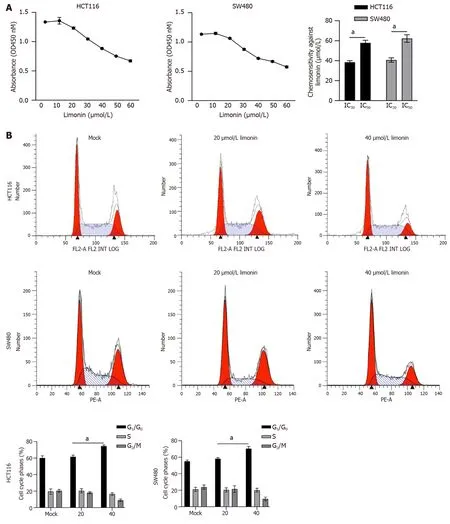
Figure 1 Limonin inhibits cell viability and cell cycle distribution in colorectal cancer cells. A: After being cultured with 10-60 μmol/L limonin for 24 h,cell viability was measured by performing cholecystokinin octapeptide-8 and IC30 or IC50 was calculated;B: After being cultured with 20 or 40 μmol/L limonin for 24 h,cell cycle distribution was measured.aP < 0.05 vs Mock group.
To further confirm its anti-tumor effects on other malignant behaviors,we accessed its effects on migration and invasion.As presented in Figure 2A,40 μmol/L of limonin treatment significantly decreased transferred cells to lower membrane in transwell without or with Matrigel,demonstrating that limonin decreased cell migration and invasion in colorectal cells.Moreover,after 10-14 d incubation with limonin,formed colonies and tumors > 50 μm in diameter decreased obviously (Figure 2B).
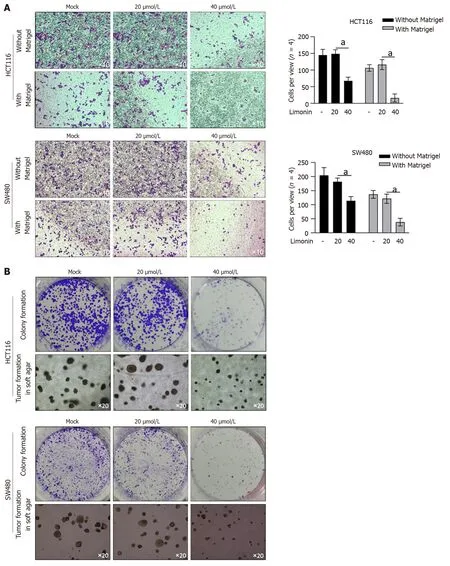
Figure 2 Limonin inhibits malignancies in colorectal cancer cells. A: After being cultured with 20 or 40 μmol/L limonin for 24 h without Matrigel,or for 48 h with Matrigel,cells transferred to lower membrane were fixed,stained and counted.aP < 0.05 vs Mock group;B: After being cultured with 20 or 40 μmol/L limonin for 14 d,colonies on plates or soft agar were fixed and stained.
Limonin inhibits stemness of CSCs derived from CRC cells
To evaluate the effects of limonin on stemness of CSCs enriched from CRC cells,including HCT116 and SW480,we added limonin ranged from 10-60 μmol/L for 96-h incubation.It is observed that limonin dissociated spheroids obviously(Figure 3A).Notably,20 μmol/L of limonin completely dissociated spheroids,which presented no detectable cytotoxicity to PCs of HCT116 and SW480 (Figure 1A).By detecting stemness hallmarkers,including Nanog and β-catenin,compared to PCs,CSCs derived from HCT116 or SW480 presented obvious higher levels of Nanog and β-catenin,which was obviously reversed by addition of 20 μmol/L of limonin (Figure 3B).Moreover,we then also detected proportion of ALDH1 in CSCs after limonin treatment.As illustrated in Figure 3C,CSCs in both HCT116 and SW480 presented higher proportion of ALDH1 positive population,and expectedly,addition of limonin decreased ALDH1-positive subpopulation.

Figure 3 Limonin inhibits stemness in cancer stem-like cells derived from colorectal cancer cells. A: After being culture with 10-60 μmol/L limonin for 10 d in serum-free medium,spheroid formation was observed;B: 20 μmol/L limonin was employed for treatment,and stemness hallmarkers,including Nanog and β-catenin were measured by western blot;C: Aldehyde dehydrogenase 1-positive proportion was measured by flow cytometry.PC: Parental cells;CSCs: Cancer stem-like cells.
Limonin decreased malignancies of CSCs from CRC cells
To further confirm,whether the anti-stemness effects of limonin also affects malignancies in CSCs derived from HCT116 and SW480,we treated CSCs with 20 μmol/L of limonin for 24 h.Moreover,10 and 20 μmol/L of limonin significantly decreased invasion (Figure 4A) and tumor formation in soft agar (Figure 4B),indicating its effects on malignant behaviors.
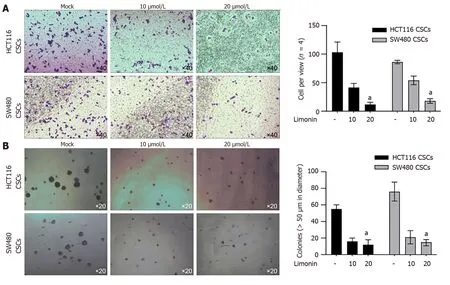
Figure 4 Limonin inhibits malignancies in cancer stem-like cells derived from colorectal cancer cells. A: Cell invasion capacity was measured by performing transwell assay after being cultured with 10 or 20 μmol/L limonin;B: After limonin treatment,tumor formation in soft agar was performed.aP < 0.05 vs Mock group.CSCs: Cancer stem-like cells.
We then transplanted CSCs pre-treated with limonin for 48 h into nude mice for extra 30-d maintenance.As presented in Figure 5A,limonin pre-treatment significantly decreased tumor formation.By detecting H&E staining,limonin pretreatment slightly affects pathological structure (Figure 5B),and obviously decreased positive proportion of Ki67,indicating that inhibition of stemness decreased tumor formation in nude mice (Figure 5C).Taken together,these results indicate that,limonin might inhibited stemness and subsequently decreased malignant behaviors,including invasion and tumor formation.

Figure 5 Limonin pre-treatment inhibits tumor formation in nude mice. A: Cancer stem-like cells pretreated using 20 μmol/L limonin or not were seeded in nude mice and be maintained for 30 d.From day 10,tumor size was measured every five days.aP < 0.05 vs Mock group;B: Hematoxylin and eosin staining was performed to detect physiological morphology;C: Ki67 was stained.
Limonin targeted and inhibited phosphorylation of STAT3
We then explored the potential mechanism contributing to limonin-resulted roles on the stemness and malignancies of CSCs derived from CRC cells.By considering that STAT3 signaling is mostly engaged in CSC progression[23],we evaluated the expression and location of phosphorylated STAT3 (p-STAT3) in CSCs and PCs derived from HCT116 or SW480,and found that the expression of p-STAT3 (Y705) in the cytoplasmic and nuclei fractions was significantly increased in CSCs compared to PCs subpopulation,which is reversed by limonin treatment (Figure 6A).Notably,the expression of p-STAT3 (S727) and total STAT3 protein levels were unaffected.To further confirm whether affected STAT3 signaling is responsible for stemness,we employed a STAT3 activator,colievlin (2 μmol/L),for CSC pretreatment.As presented in Figure 6B and C,inhibition of sphere formation induced by limonin was obviously reversed by pretreatment of 2 μmol/L of colievlin.These results indicate that,limonin inhibits stemness,at least partially,viainhibiting STAT3 signaling.
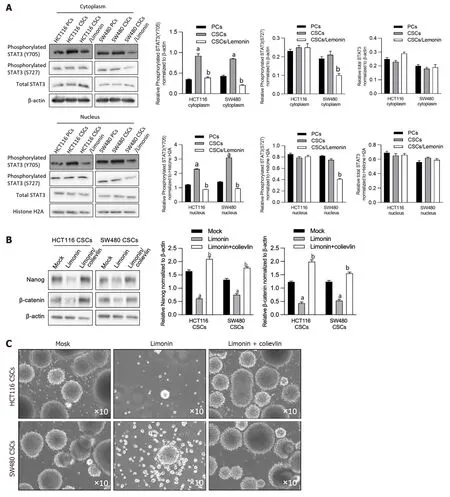
Figure 6 Limonin inhibits STAT3 signaling. A: After being cultured using limonin,total STAT3,phosphorylated STAT3 (Y705) or STAT3 (S727),in cytoplasmic or nucleus fraction,were measured by performing western blot;B: Stemness hallmarkers,including Nanog and β-catenin,were detected by western blot after Limonin treatment with or without 2.5 μmol/L colievlin pretreatment;C: Sphere formation was observed after Limonin treatment with or without 2.5 μmol/L colievlin pretreatment.aP < 0.05 vs parental cells group;bP < 0.05 vs cancer stem-like cells group.PCs: Parental cells;CSCs: Cancer stem-like cells.
DlSCUSSlON
Recently,natural products have received increasing attention as novel therapeutics for a wide range of cancers because they have a wide range of anticancer effects and,unlike existing chemoagents,have few side effects.Limonin was reported to have anticancer effects in various studies[8,14,15].We selected limonin as a novel therapeutic candidate,which may target the CSCs of CRC cells,as these compounds have not yet been studied to prevent recurrence or metastasis induced by the existence of CSCs.
Our results indicated that limonin exerts antitumor effects on malignant behaviors in CRC cells and inhibits the stemness of CSCs derived from CRC cells.Notably,a relatively low concentration of limonin,which fails to exert cytotoxicity,inhibits the stemness of CSCs,demonstrating that limonin regulates CSCs and PCs in different manners.The presence of limonin promotes the dissociation of CSC spheres and decreases the protein levels of Nanog and β-catenin and the percentage of the ALDH1-positive cells without obviously affecting cell viability and cell cycle distribution.Cell proliferation is a critical tumor-promoting process in CRC cells and is considered a leading cause of poor prognosis.Our results indicate that limonin could specifically target the stemness of CSCs without affecting other malignant behaviors and may be considered a specific stemness inhibitor in CRC.
Accumulating evidence suggests tumor-initiating effects of STAT3 activation in several kinds of cancers,including breast cancer[24],lung cancer[25],and CRC[26].Zhanget al[27] reported that activated STAT3 increased the number of breast cancer stem cells,which was reversed by microRNA-7.In lung cancer,activation of interleukin-6/JAK/STAT3 signaling could enhance lung cancer initiation by promoting the CSC subpopulation[28].Additionally,in CRC,STAT3 activation was also reported to be tightly correlated with tumor initiation and progression by regulating the abundance of CSCs[26].These results demonstrated that STAT3 is critical in regulating the stemness of CSCs.Consistently,in our results,limonin treatment significantly decreased STAT3 activation by inhibiting its phosphorylation,which is correlated with the decreased stemness of CSCs.The addition of 2.5 μmol/L colivelin,a STAT3 activator,significantly reversed the inhibitory role of limonin on the stemness of CSCs derived from CRC cells.This indicates that limonin may,at least partially,inhibit the stemness of CSCs by inhibiting STAT3 activation.In this study,we failed to evaluate the effect of high concentrations of limonin on CSCs,which should be investigated in future studies.
STAT3 phosphorylation is closely related to tumor growth,invasion and migration in CRC[29].Limonin treatment obviously suppressed metastasis and tumor growth mediated by CSCs,which is consistent with previous findings.However,we failed to detect the effect of limonin on cell proliferation after 24 h of treatment.We hypothesized that in CSCs,relatively short-term treatment inhibits STAT3 activation and leads to loss of stemness instead of cell proliferation capacity.In further investigations,it is worth revealing the exact effects of long-term and short-term limonin treatment.
CONCLUSlON
In conclusion,we demonstrated that limonin acts as an antitumor agent by targeting both CSCs and PCs among CRC cells.Furthermore,limonin inhibits STAT3 activation and inhibits the stemness of CSCs derived from CRC cells.Taken together,these findings highlight that limonin could be used as a promising therapeutic agent to CSCs in CRC.
ARTlCLE HlGHLlGHTS
Research background
Colorectal cancer (CRC) is one of the most common types of cancer globally.In the last few decades,research efforts have been directed towards understanding the cellular and genetic mechanisms underlying the development and progression of CRC.This has led to the identification and study of a specific subset of cells within colorectal tumors,known as cancer stem cells or cancer stem-like cells (CSCs).The concept of CSCs originated in the mid-20thcentury but was not widely accepted until the late 1990s.The idea behind CSCs is that a small proportion of cancer cells within a tumor have stem cell-like properties,including the ability to self-renew and differentiate,which contribute to tumor initiation,progression,metastasis,and recurrence.They are also often associated with resistance to traditional cancer therapies.
Research motivation
Several studies have suggested that limonin,a natural compound found in citrus fruits,can exert anti-tumor effects.These effects appear to be due to multiple mechanisms,including the induction of apoptosis (programmed cell death),the inhibition of cancer cell proliferation,and the suppression of metastasis.Based on this,we then investigated whether it exerts suppressing effects on CSCs.
Research objectives
In this research,we evaluated the effects of limonin,a natural compound which is one of the most abundant active ingredients of Tetradium ruticarpum on stemness of CSCs derived from colorectal carcinoma cells.We also investigated the potential mechanism of limonin on stemness of CSCs.
Research methods
We performed cellular experiment to verify the suppressing effects of limonin on CSCs and performed animal experiment to verify its effectsin vivo.
Research results
Limonin was found to have suppressive effects on CRC cell activities,which included cell growth,movement,invasion,colony growth and tumor development in soft agar.Even at relatively low concentrations,limonin reduced the expression of stemness markers such as Nanog and β-catenin,decreased the percentage of aldehyde dehydrogenase 1-positive CSCs,and lowered the rate of sphere formation,showing that limonin hinders stemness without cytotoxicity.Furthermore,the treatment of limonin reduced invasion and tumor development in soft agar and in nude mice.Also,limonin treatment notably inhibited the phosphorylation of STAT3 at Y705 but not S727 and had no effect on total STAT3 expression.The limonin-induced reduction in Nanog and β-catenin expression and sphere formation was noticeably offset by a pretreatment with 2 μmol/L colievlin.
Research conclusions
In summary,the findings suggest that limonin holds potential as a compound targeting CSCs,and might be utilized for tackling the recurrence and metastasis of CRC.
Research perspectives
Can limonin to be used as a chemotherpeutic sensitizor?
FOOTNOTES
Author contributions:Zhang WF and Chen HJ are responsible for this project and designed experiments;Zhang WF,Ruan CW and Wu JB are responsible for performing experiments;Wu GL and Wang XG are responsible for data collection and data analysis;Zhang WF and Chen HJ are responsible for manuscript writing;All authors were aware of this manuscript.
lnstitutional animal care and use committee statement:All the animal experiments were conducted according to the Animal ethics committee in Affiliated Hospital of Nanjing University of Traditional Chinese Medicine,No.2019 DW-13-02.All mouse models were housed in the SPF animal facilities (temperature 21-25 °C;humidity 50%-60%;well-ventilated cages).
Conflict-of-interest statement:No conflicts of interest was declared.
Data sharing statement:All data generated or analysed during this study are included in this published article.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines,and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Guo-Liang Wu 0000-0003-1225-7287;Hong-Jin Chen 0000-0003-2222-3582.
S-Editor:Fan JR
L-Editor:A
P-Editor:Yu HG
 World Journal of Clinical Oncology2024年2期
World Journal of Clinical Oncology2024年2期
- World Journal of Clinical Oncology的其它文章
- Unlocking the potential-vitamin D in prostate cancer prevention
- Updates on management of gliomas in the molecular age
- Deregulation of interferon-gamma receptor 1 expression and its implications for lung adenocarcinoma progression
- Elucidating the molecular basis of ATP-induced cell death in breast cancer: Construction of a robust prognostic model
- ldentification of immune cell-related prognostic genes characterized by a distinct microenvironment in hepatocellular carcinoma
- Population-based X-ray gastric cancer screening in Hiroshima prefecture,Japan
