Deregulation of interferon-gamma receptor 1 expression and its implications for lung adenocarcinoma progression
Angeles C Tecalco-Cruz,Karen H Medina-Abreu,Enrique Oropeza-Martínez,Jesus Zepeda-Cervantes,Aleida Vázquez-Macías,Marina Macías-Silva
Abstract Interferon-gamma (IFN-γ) plays a dual role in cancer;it is both a pro-and an antitumorigenic cytokine,depending on the type of cancer.The deregulation of the IFN-γ canonic pathway is associated with several disorders,including vulnerability to viral infections,inflammation,and cancer progression.In particular,the interplay between lung adenocarcinoma (LUAD) and viral infections appears to exist in association with the deregulation of IFN-γ signaling.In this mini-review,we investigated the status of the IFN-γ signaling pathway and the expression level of its components in LUAD.Interestingly,a reduction in IFNGR1 expression seems to be associated with LUAD progression,affecting defenses against viruses such as severe acute respiratory syndrome coronavirus 2.In addition,alterations in the expression of IFNGR1 may inhibit the antiproliferative action of IFN-γ signaling in LUAD.
Key Words: Interferon-gamma;IFNGR1;JAK1;Antiviral;Anti-tumor;Lung adenocarcinoma
lNTRODUCTlON
According to data provided by the Global Cancer Observatory and recently published analyses,in 2020,there were more than 9.9 million deaths by cancer worldwide.Lung cancer was the leading cause of death (18%) in both males and females,and lung adenocarcinoma (LUAD) was the histological type with the highest incidence in both men (39%) and women (57%)[1,2].Changes in lung cancer incidence patterns that reflect the increase in LUAD may be attributed to several risk factors,including cigarette smoking,exposure to environmental pollution,cooking oil fumes,indoor charcoal burning,and nonsmoker exposure[3,4].Studies of mechanistic insights at the molecular level in LUAD have shown the presence of alterations in the signaling pathways that drive its initiation and progression.The intracellular signaling disruptions collectively contribute to the aggressive phenotype,invasive nature,and metastatic propensity of LUAD[3,4].
The interferon-gamma (IFN-γ) signaling pathway is among the most deregulated signaling pathways in LUAD.IFN-γ is a cytokine that plays a pivotal role in immune responses,the orchestration of leukocyte trafficking,antiviral and antibacterial defense,and the modulation of cellular proliferation and apoptosis[5-8].IFN-γ signaling is thought to trigger antitumoral activities and has protumorigenic effects depending on the cancer context.Hence,IFN-γ can induce apoptosis in some cellular contexts,whereas in others,IFN-γ can induce the expression of programmed death ligand 1(PD-L1),favoring its binding to its receptor PD-1 on activated T cells and suppressing their cytotoxic effect[9].IFN-γinduced actions occur when it binds to its receptor complex (IFNGRs),promoting the activation of its canonical signaling pathway with the phosphorylation of signal transducer and activator of transcription 1 (STAT1),which acts as a transcription factor to mediate IFN-γ-dependent gene expression;therefore,IFNGR homeostasis is crucial for the signaling of this interferon[5,10,11].In this mini-review,we focus on describing and analyzing the relevance of IFN-γ receptor 1 (IFNGR1) in LUAD and its implications for IFN-γ signaling and the progression and complication of this cancer type.
CANONlCAL SlGNALlNG PATHWAY OF lFN-γ AND lTS CELLULAR RECEPTORS
IFN-γ signal is transduced through a heterotetrameric receptor complex comprising two IFNGR1 and two IFNGR2.This receptor complex induces antiviral,proapoptotic,and antiproliferation activitiesviathe JAK/STAT1 pathway[12].Janus kinase (JAK) 1 and JAK2 bind to the intracellular regions of IFNGR1 and IFNGR2,respectively.After IFN-γ is recognized by its receptors,JAKs are activatedviatransphosphorylation[13-16].JAKs phosphorylate IFNGR1,generating a docking site for STAT1 proteins.These are also phosphorylated by JAKs,forming P-STAT1 dimers that are translocated to the nucleus to regulate tissue-specific gene expression[17-20].
IFNGR1 and IFNGR2 are central to the signaling of IFN-γ.IFNGR1 recognizes and binds IFN-γ,whereas IFNGR2 interacts mainly with IFNGR1 and promotes intracellular signaling.The interaction between IFN-γ and IFNGR1:IFNGR2 induces JAK2 autophosphorylation,followed by JAK1 transphosphorylation by JAK2[21].IFNGR1 is phosphorylated in Y440 by activated JAK kinases,generating a docking site for the interaction of STAT1viaits Src-homology 2 domain[18].STAT1 is phosphorylated on Y701 by JAK2,promoting its homodimerization,and association with the gamma-activated site (GAS) element on the regulatory regions of IFN-γ-regulated genes to modulate their expression[21].Interferon regulatory factor 1 (IRF-1) is a primary IFN-γ target gene that encodes for a transcription factor that recognizes interferonsensitive response element elements,modulating the expression of a second cascade of IFN-γ target genes (Figure 1)[13].

Figure 1 Canonical signaling pathway of interferon-gamma. The heterotetrameric receptor complex for interferon-gamma (IFN-γ) comprises IFNGR1 and IFNGR2.JAK1 and JAK2 proteins are associated with intracellular domains of IFNGR1 and IFNGR2,respectively.The binding of IFN-γ to its receptor complex promotes the transphosphorylation of JAK1/2,the phosphorylation of IFNGR1,and the recruitment of STAT1,followed by the STAT1 phosphorylation by JAK proteins.Phosphorylated STAT1 translocates into the nucleus to modulate the transcription of genes containing gamma-activated sequence motifs in their promoter regions.IFN-γ: Interferon-gamma;GAS: Gamma-activated sequence;ISGs: Interferon-stiumlated genes.
IFNGR1 may exhibit moderate expression,while IFNGR2 has lower expression levels and depends on external stimuli for regulation[22].IFNGRs play a pivotal role in the immune response against viral diseases[23-27].For instance,IFNGR1-/-andIFNGR1-/-mouse models are viable but susceptible to several viral infections.Patients with mutations in these genes are also more susceptible to infection,particularly mycobacterial infections[28-32].Moreover,IFNGR1 appears to play an influential role in tumor growth since compromised tumor rejection has been reported in experiments with different models,such asIFNGR1-/-mice,using IFN-γ neutralizing antibodies or studies with dominant-negativeIFNGR1mutations[33-35].
In the context of cancer,tumor cells exhibit variations in the levels ofIFNGR1andIFNGR2.For example,deficiencies inIFNGR1andIFNGR2expression can occur in acute myeloid leukemia[36].The deficiencies,overexpression,and polymorphisms of IFNGR1/2 may collectively impact IFN-γ signaling,affecting immune responses to infectious diseases and the predisposition to cancer[37-39].In particular,research has indicated that variations inIFNGR1expression affect the response to IFN-γ in different temporal and spatial contexts[40].
EXPRESSlON OF IFNGR1/2 lS DEREGULATED lN SEVERAL TYPES OF CANCER
The altered expression and abundance of IFNGRs have been reported in diverse cancer contexts.Interestingly,the University of ALabama at Birmingham CANcer data analysis Portal (UALCAN) dataset indicates significant statistical changes in the expression ofIFNGR1and/orIFNGR2in the majority of cancer types (20/35;Table 1).The expression ofIFNGR1displays an increase (8 cancers),a decrease (9 cancers),or no change (3 cancers) with respect to normal tissue.Of these,the expression of bothIFNGR1/2receptors is upregulated (8 cancers) or downregulated (2 cancers) compared to normal tissue,butIFNGR1is downregulated andIFNGR2is upregulated in 4 cancers.WhereasIFNGR1can be up-or downregulated,IFNGR2is mainly upregulated in cancers compared to healthy tissue (15/20 upregulated,2/20 downregulated,and 3/20 no change).These data suggest that the deregulation ofIFNGR1can be differential toIFNGR2in a manner dependent on cancer type[41,42].

Table 1 The expression of IFNGR1 and IFNGR2 in several cancer types
In addition,some studies have demonstrated the relevance of changes in the expression ofIFNGRsin cancer.For example,IFNGR2upregulation by RUNX1 transcription factor is associated with growth,migration,and invasion,along with a poor prognosis of low-grade glioma[43].However,principally,the deregulation of IFNGR1 has been reported in some cancer types.
EXPRESSlON AND ABUNDANCE OF lFNGR1 ARE ALTERED lN SEVERAL CANCER TYPES
It has been reported that patients with breast cancer exhibiting elevated levels of IFN-γ and/or IFNGR1 may undergo tumor rejection,whereas those with intermediate levels may experience tumor recurrence[44].ReducedIFNGR1expression has also been observed in patients with mammary tumors,suggesting a decrease in IFN-γ signaling[45].In the context of ovarian cancer,patients whose tumors express high levels ofIFNGR1have a significantly better survival rate than those whose tumors have low levels[46].The loss of this IFN-γ receptor results in a poor prognosis for patientswhose cancer is more aggressive,and the benefit of treatment with IFN-γ is reduced or nonexistent[33].Thus,changes inIFNGR1expression appear to be particularly strongly related to the progression of specific cancer types.
Interestingly,polymorphisms in theIFNGR1promoter are correlated with susceptibility to diseases such as leishmaniasis,tuberculosis,leprosy,and hepatitis[47].Polymorphisms of this receptor are also associated with some cancer types;for example,polymorphisms inIFNGR1have been associated with gastric cancer[48] and rectal cancer[49].For example,IFNGR1rs3799488 polymorphism is associated with a risk of developing rectal cancer[50],whereas the presence of rs2234711 in theIFNGR1promoter is associated with an increased risk of developing colorectal cancer[51].Moreover,the risk of developing colon and rectal cancer is associated with polymorphisms in IFN-γ and its receptors,the influence of other genes related to inflammation,the use of nonsteroidal anti-inflammatory drugs,and smoking[50].Nevertheless,a longer overall survival has been observed in patients with variations in theIFNGR1promoter region(rs2234711,rs9376267) diagnosed with metastatic colorectal cancer under treatment with bevacizumab-based chemotherapy[52].
Furthermore,the polymorphisms onIFNGR1have been associated with early stage breast cancer with depression[53],chronic lymphocytic leukemia[54],classic infantile Kaposi’s sarcoma[55,56] and hepatocellular carcinoma[57].
In addition,IFNGR1 protein can be a target of posttranslational modifications[58].For example,IFNGR1 can be ubiquitinated by STUB1,an E3 ubiquitin-protein ligase that negatively regulates IFNGR1,thereby reducing IFN-γ sensing.STUB1 depletion increases IFNGR1 abundance and enhances IFN-γ response,promoting an IFN-γ-STAT1-IRF1 axis to induce the activation of genes associated with antigen processing and presentation[59].The abundance of IFNGR1 can also be regulated by palmitoylation,leading to accelerated lysosomal degradation of IFNGR1.The palmitoylated cysteine in IFNGR1 acts as a signal for its interaction with AP3D1,targeting it to lysosomes.Hence,whenAP3D1is downregulated,IFNGR1 levels are increased,and when IFNGR1 palmitoylation is pharmacologically inhibited,IFNGR1 is stabilized.Moreover,high optineurin protein levels have been positively associated with the survival of melanoma patients,but the loss of optineurin facilitates IFNGR1 binding to AP3D1 and increases AP3D1-mediated IFNGR1 lysosomal sorting and degradation[60].Additionally,MUC1-C is a protein with transmembrane domains that protects epithelial niches;however,its prolonged activation can promote oncogenesis and the epithelial-mesenchymal transition(EMT) of castration-resistant prostate cancer cells.Interestingly,MUC1 associates with expression ofIFNGR1,STAT1andIRF1.Moreover,it has been reported that the downregulation of MUC1 leads to the activation of FBXW—an E3 ubiquitinprotein ligase—for IFNGR1 degradationviathe ubiquitin-proteasome system[61].Phosphorylation is another posttranslational modification that can alter the stability of IFNGR1.For example,glycogen synthase kinase 3 beta (GSK3β)phosphorylates IFNGR1,which protects this receptor from proteasomal degradation by ubiquitin,increasing its stability and promoting IFN-γ signaling and IFN-γ-induced inflammation;therefore,GSK3β has been proposed as an anticancer target[58,62].Specifically,high levels of phosphorylated GSK3β are detected in LUAD;accordingly,the inactivation of GSK3β may be useful as a pharmacological treatment for this cancer type[63].Glycosylation is another posttranslational modification of IFNGR1,which has been proposed as a signal necessary for IFN-γ signaling,but also as a signal associated with the stability of the receptor[64].
PRlNClPAL MOLECULAR lMPLlCATlONS OF IFNGR1 DEREGULATlON lN CANCER
The deregulation of IFNGR1 can affect IFN-γ-induced molecular mechanisms and cellular responses.Hence,IFN-γ has been reported to induce downregulation of IFNGR1 in myeloid cells,reducing their response to IFN-γ inflammatory stimuli and promoting anti-inflammatory effects.In addition,reducedIFNGR1expression decreases the antiproliferative effects induced by IFN-γ,suggesting that the downregulation of IFNGR1 may diminish sensitivity to IFN-γ in myeloid and nonlymphoid cells[49].Moreover,cancer cells can express IFN-γ-induced PD-L1 to bind to its receptor PD-1 on T cells,promoting resistance to the host immune system.Consequently,the downregulation ofIFNGR1may result in reducedPD-L1/CD274gene expression;this is associated with resistance to treatment against PDL1/PD1 in melanoma and colorectal cancer[9].
IFNGR1expression is commonly downregulated in human colorectal cancers and mouse intestinal adenoma models.Particularly,colorectal cancer patients have a more prolonged median survival when they express higher IFNGR1 levels,suggesting that IFN-γ signaling is critical for maintaining a tumor-inhibitory microenvironment in the context of colon cancer[60,65].Genes involved in inflammation,such asHif1a,and genes encoding matrix metalloproteinases,such asMMP3,MMP7,andMMP9,are inhibited by IFN-γ under normal conditions.The expression of these genes is enhanced by the absence ofIFNGR1in murine models of human familial adenomatous polyposis,which is regarded as a premalignant lesion for colon cancer.In contrast,tumor suppressor genes,such asCdx2,Cdhr2,andCdhr5,are negatively regulated byIFNGR1downregulation,leading to the M1 phenotype in tumor-associated M2 macrophages[66].Therefore,alteredIFNGR1expression is associated with dysregulated IFN-γ signaling,which may enhance tumor progression.IFNγ inhibits β-catenin activity and induces apoptosis in colon cancer cells.However,IFNGR1deficiency affects IFN-γ signaling,increasing the invasiveness of intestinal tumors with the development of anemia in mice,considering that IFNγ regulates hematopoiesis[49].
Aside from IFNGR1’s function as a transmembrane receptor,it has been discovered that the IFNGR1 receptor can translocate to the nucleus in uterine cancer cells treated with IFN-γ,whereas the IFNGR2 subunit is not endocytosed or transported to the cell nucleus.IFNGR1 does not have a DNA-binding domain,so its association with STAT1 allows it to bind to GAS elements located in the promoter region of IFN-γ-activated genes,such as IRF-1 and indolamine dioxygenase (IDO),increasing their expression[67,68] .Thus,the activity of IFNGR1 as a transmembrane receptor and as a nuclear protein may be affected by its deregulation.
Despite the dysregulation ofIFNGR1in several cancer types,particularly in LUAD,the alterations of this receptor have been related to cancer progression and complications such as viral infections.This suggests the multifunctional implications of IFN-γ signaling in the context of lung cancer,as discussed below.
lFN-γ SlGNALlNG lS DEREGULATED lN LUAD
IFN-γ signaling is central to the gene expression that participates in the remodeling of LUAD’s tumor microenvironment.For instance,in one study,seven IFN-γ response genes (CD74,CSF2RB,PTPN6,MT2A,NMI,LATS2,andPFKP) were identified and proposed as valuable prognostic predictors of LUAD[69].Nevertheless,it has been demonstrated that IFNγ can activate distinct signaling pathways dependent on its levels.Hence,low levels of IFN-γ activate the intercellular adhesion molecule-1 (ICAM1)-PI3K-AKT-Notch1 axis,promoting cancer stem-like properties.By contrast,the JAK1-STAT1-caspase pathway is activated by high levels of IFN-γ,inducing apoptosis in lung cancer cells[70].Furthermore,the activation of the JAK2-STAT1 and PI3K-AKT pathways in LUAD cells treated with IFN-γ has been shown.This is important since an antiproliferative action has been associated with the IFN-γ/JAK2-STAT1 axis,whereas PI3K-AKT signaling is associated with protumor activity.Hence,a reduction in PD-L1 expression was observed in response to PI3K inhibition,enhancing the IFN-γ-dependent antiproliferative signaling.Therefore,the antitumoral activity triggered by IFN-γ may be enhanced by blocking PI3K signaling.Thus,in response to IFN-γ in LUAD,crosstalk between the JAK2-STAT1 and PI3K-AKT pathways is generated,suggesting PI3K as a target for therapeutic treatment of LUAD[71].Moreover,IFN-γ enhances the expression of JMJD3,a histone demethylase that reduces H3K27 trimethylation on theZEB1gene promoter,resulting inZEB1transcription.IFN-γ-inducedZEB1expression is associated with the EMT,migration,and metastasis of LUAD cellsin vivo.The reduction ofZEB1inhibits EMT,migration,and metastasis,but does not affect the expression ofSTAT1andIRF1or the antitumor effects of the IFN-γ-STAT1-IRF1 axis.This study suggests that the negative regulation ofZEB1may inhibit the protumoral activities of IFN-γ signaling,favoring its antitumoral activities[72].
EXPLORlNG lFNGR1 EXPRESSlON AND OTHER lFN-γ CANONlCAL SlGNALlNG ELEMENTS lN LUAD
The Selamat dataset from Oncomine,and the Cancer Genome Atlas from UALCAN,indicate thatIFNGR1gene expression is significantly reduced in the tumors of patients with LUAD compared to healthy lung tissue[42] (Figure 2A).By contrast,IFNAR1(receptor 1 for IFN-α) expression shows no significant changes in LUAD patients compared to healthy lung tissue (Figure 2B).The gene expression ofJAK1andJAK2is also downregulated in the tumors of patients with LUAD compared to normal lung tissues (Figure 2C and D).These data suggest that the IFN-γ signaling pathway is desensitized in LUAD because of the downregulation of its components—IFNGR1,JAK1,andJAK2—whereas IFN-α/β signaling may remain active.

Figure 2 IFNGR1 and JAK1/2 are downregulated in lung adenocarcinoma. IFNGR1, IFNAR1, JAK1,and JAK2 expression in human lung adenocarcinoma (LUAD) tumors compared to normal tissue tumors.A-D: Selamat Lung dataset;E: The Cancer Genome Atlas from the University of ALabama at Birmingham CANcer data analysis Portal.Expression of IFNGR1 in LUAD based on the patient´s smoking habits.Reformed smoker 1 (< 5 years);Reformed smoker 2 (> 15 years).Results are considered significant (P < 0.05) in all cases,with the exception of IFNAR1 (NS: Not significant).
Interestingly,IFNGR1expression is significantly lower in smokers’ LUAD tumors than in those of nonsmokers and reformed smokers (Figure 2E),but the expression ofJAK1andJAK2is similar in the tumors of patients with LUAD who have different smoking habits.These data indicate that the reduction inIFNGR1in LUAD patients with smoking habits affects IFN-γ signaling.
Emerging evidence supports the idea that the tumor microenvironment affects LUAD progression and clinical outcome[73].IFN-γ within the tumor microenvironment can positively regulate immune checkpoint molecules,such as IDO1[74].Moreover,inactivation ofIFNGR1affects a small subset of lung cancer and prevents response to IFNg[75].All data suggest thatIFNGR1expression may be a useful biomarker for LUAD;nevertheless,additional studies are required to define its prognosis value and its possible cooperative correlation with JAK1/2 expression as a biomarker.
Because IFN-γ is central to immune responses,a higher susceptibility to viral infections and an elevated risk of cancer have been associated withIFNGR1deficiency[76,77].IFN-γ signaling deregulationvia IFNGR1downregulation is associated with lung cancer progression and seems to have an influence on susceptibility to viral infections (e.g.,infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)],as discussed in the following sections.
lNTERPLAY BETWEEN lFN-γ SlGNALlNG AND SARS-COV-2 lN LUAD
Relationship between IFN-γ signaling, LUAD and SARS-CoV-2 infection
The role that IFN-γ signaling plays in viral respiratory infection and lung cancer may depend on the expression status ofIFNGR1.For example,a relationship between lung cancer and infections with SARS-CoV-2 seems to exist.SARS-CoV-2 is responsible for coronavirus disease 2019 (COVID-19),which has caused millions of deaths worldwide[78].COVID-19 symptoms range from those similar to the common cold to more severe manifestations,such as lung injury,damaged alveoli,acute respiratory failure,acute respiratory distress syndrome,and injury to other organs[79].The SARS-CoV-2 spike (S) glycoprotein can bind to angiotensin-converting enzyme 2 (ACE-2),promoting the virus’s entry into cells.HigherACE-2expression translates to higher receptors for SARS-CoV-2,favoring infection.Moreover,the same SARSCoV-2 virus leads to the upregulation ofACE-2[79].Some comorbidities,such as chronic obstructive lung disease,diabetes,and hypertension,have also been associated with an increase in the expression ofACE-2[78].Similarly,patients with lung cancer display high levels ofACE-2[80];therefore,lung cancer poses a higher risk of SARS-CoV-2 infection and its complications[79].Researchers have thus proposed that the SARS-CoV-2 receptorACE-2is upregulated in lung cancer cells,increasing the risk of COVID-19[81].
ACE-2is an interferon-stimulated gene[82] with several binding sites for transcription factor STAT in its promoter region[80].Histone modifiers,such as HAT1,HDAC2,and KDM5B,may regulateACE-2expression[78].Moreover,the deregulation ofACE-2expression may be associated with a reduction in DNA methylation[81].IFNs can induce a novel truncatedACE-2isoform[83].The mechanism for ACE-2 regulation is critical since it is upregulated in lung cancer patients who have a lower survival rate[84].Furthermore,LUAD patients are reportedly more susceptible to SARS-CoV-2 infection than lung squamous cell carcinoma patients[85].
In addition,the lung tumor microenvironment may promote the invasion of viruses,thereby increasing the severity of COVID-19[86].Smokers are at a higher risk of severe complications and have higher mortality rates associated with COVID-19 than nonsmokers[87].Interestingly,Selamat lung datasets from Oncomine indicate a reduction inIFNGR1andJAK1/2but an increase inACE-2expression in LUAD tumors compared to healthy counterparts (Figure 3A).IFNGR2andDNAJA3(a modulator of IFN-γ signaling) are upregulated,whereas the expression ofIFN-γ,which encodes IFN-γ,shows no significant change in either condition.Thus,the reduction ofIFNGR1expression in LUAD affects IFN-γ signaling,whereas the expression of the SARS-CoV-2 receptorACE-2increases,which may also exacerbate susceptibility to COVID-19.Nevertheless,the downregulation ofIFNGR1may also facilitate several viral infections in cancer contexts.
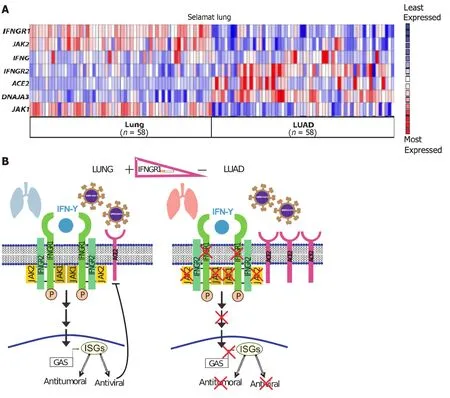
Figure 3 lnterplay between antitumoral and antiviral activities induced by interferon-gamma in the pulmonary context. A: Heat map of interferon-gamma (IFN-γ) signaling components and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor,angiotensin-converting enzyme 2(ACE2),in lung adenocarcinoma (LUAD).IFNG, IFNGR2, IFNGR1, JAK1, JAK2,and DNAJA3 expression in human LUAD tumors and normal tissue.Selamat Lung datasets;B: Proposed model: There is a functional IFN-γ signaling pathway for controlling cell proliferation and viral infections in normal conditions.In LUAD,there is an increase in the ACE2 expression,facilitating the SARS-CoV-2 infection.Moreover,the expression of IFN-γ signaling components is reduced,decreasing the antiproliferation and antiviral defenses.IFN-γ: Interferon-gamma;GAS: Gamma-activated sequence;ISGs: Interferon-stimulated genes.
Risk of LUAD in recovered SARS-CoV-2 patients
Viral infections can also promote a higher incidence of lung cancer.For example,infection with immunosuppressive pathogens,including human immunodeficiency virus,reduces the number of CD4+T cells,which is associated with a higher incidence of lung cancer[88].Moreover,an association with LUAD has been proposed for patients who have recovered from SARS-CoV-2 infection.Upon infection with SARS-CoV-2,the cellular landscape is primarily inflammatory.Viral RNA may be detected by pattern recognition receptors found in endosomes,such as TLR3 and TLR7[89].Furthermore,the recognition of SARS-CoV-2 mRNA by the cytosolic receptors,retinoic acid-inducible gene I and melanoma differentiation-associated gene 5,leads to the activation of NF-kB,which,in turn,can translocate to the cell nucleus and trigger the transcription of mRNAs encoding for proinflammatory cytokines,such as IL-1β,IL6,and TNF-α[90,91].IFN-γ is another essential cytokine with antiviral properties that plays a crucial role during viral infections[92].However,the inflammatory response is sometimes prolonged,resulting in tissue damage[93].The production of antiinflammatory molecules [IL10 and growth factors,e.g.,vascular endothelial growth factor (VEGF)] is induced as a means of controlling inflammation and limiting tissue damage;however,these anti-inflammatory factors can promote a tumoral microenvironment[94].In addition,hypoxia occurs during SARS-CoV-2 infection owing to interstitial and alveolar edema caused by increased permeability in the lung capillaries[95].Under hypoxic conditions,hypoxia-inducible factor (HIF-1)is activated.HIF-1 comprises two subunits—HIF-1α and HIF-1β.To compensate for these hypoxic conditions,HIF-1a can activate the synthesis of other genes,including hematopoietic hormone,VEGF,enzymes involved in glycolysis,and glucose carrier proteins[96].Nonetheless,HIF-1α also promotes the vascularization of solid tumors,including lung tumors[97].The expression status ofIFNGR1in recovered SARS-CoV-2 patients remains to be clarified,which may help elucidate the relationship among IFNGR1,LUAD,and viral infections.
DlSCUSSlON
IFN-γ is a cytokine that fulfills a dual function in cancer.In some cancer types,IFN-γ prevents tumor development.However,IFN-γ is also known to promote metastasis,thereby evading the immune system.The mechanisms responsible for this duality remain elusive;however,some molecular mechanisms implicated in LUAD have been proposed,including changes in IFN-γ concentration and the simultaneous crosstalk between pro-and antitumoral pathways activated by IFN-γ.Interestingly,the information contained in public databases indicates that the expression of type I IFN-α/β receptor (IFNRA1) is not significantly affected compared to the expression ofIFNGR1,which is lower in the tumors of LUAD patients than in healthy tissues.Furthermore,the expression of JAK1/2 is affected in LUAD.Interestingly,LUAD patients with a smoking habit have lowerIFNGR1expression,indicating that IFN-γ signaling is affected,altering its antiviral,antiproliferative,and proapoptotic actions.These data suggest that the IFNGR1 signaling is altered in lung cancer patients and more affected in LUAD patients who have a smoking habit.
Moreover,IFN-γ is known for its antiviral properties.The entry of SARS-CoV-2 into the cells is favored by the upregulation ofACE-2,whereas IFN-γ signaling pathways and its antiviral activities are reduced in LUAD.This may explain the greater susceptibility to SARS-CoV-2 among lung cancer patients,particularly LUAD patients.The reduction of IFN-γ signaling also implies a decrease in IFN-γ-dependent antitumoral actions,promoting lung cancer progression.Thus,IFNγ administration or high levels of endogenous IFN-γ may have no effect on lung cancer cells due to a lack ofIFNGR1,and downstream pathway components (JAK1/2),limiting its antitumoral and antiviral functions in these cells.These IFN-γ signaling elements may be restored to enhance protection against SARS-CoV-2 and regulate cancer progression.
The reduction of IFNGR1 in LUAD may also influence pathways that increase the risk of SARS-CoV-2 infection or other infections that may be more aggressive in LUAD patients than in healthy individuals (Figure 3B).Interestingly,patients who have contracted COVID-19 may be at higher risk for lung cancer,considering the inflammation conditions during infection.Importantly,IFN-γ,HIF-1,and the simple fact of a cigarette smoking habit increase ACE-2 levels.This is undesirable in the context of SARS-CoV-2 infection,as the upregulation ofACE-2has been associated with lung cancer[82,84,98].Further studies on the molecular mechanisms that control the expression and function of IFNGR1 in LUAD and other cancer types are warranted.
CONCLUSlON
In conclusion,the expression ofIFNGR1andJAK1/2is affected in LUAD.IFNGR1 is the first component in IFN-γ signaling.Consequently,a decrease in IFNGR1 inhibits the antitumoral and antiviral actions of IFN-γ.Therefore,patients with LUAD display lower IFNGR1 levels that promote cancer progression;this seems to be associated with several complications,including a greater risk for infections (such as COVID-19) and,ultimately,poor outcomes.Novel therapies restoringIFNGR1expression could be used as new approaches for LUAD in personalized medicine.
FOOTNOTES
Author contributions:Tecalco-Cruz AC planned and participated in the research,organization of this article,and wrote the manuscript;Medina-Abreu KH,Oropeza-Martínez E,Zepeda-Cervantes J,Vázquez-Macías A,and Macías-Silva M participated in the research and wrote some parts of the manuscript;Tecalco-Cruz AC,Medina-Abreu KH,and Oropeza-Martínez E prepared some figures for this article;All authors revised the final manuscript.
Conflict-of-interest statement:All the authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the
original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Mexico
ORClD number:Angeles C Tecalco-Cruz 0000-0001-9199-3834;Jesus Zepeda-Cervantes 0000-0003-2069-2027.
S-Editor:Liu JH
L-Editor:A
P-Editor:Zhang XD
 World Journal of Clinical Oncology2024年2期
World Journal of Clinical Oncology2024年2期
- World Journal of Clinical Oncology的其它文章
- Unlocking the potential-vitamin D in prostate cancer prevention
- Updates on management of gliomas in the molecular age
- Elucidating the molecular basis of ATP-induced cell death in breast cancer: Construction of a robust prognostic model
- ldentification of immune cell-related prognostic genes characterized by a distinct microenvironment in hepatocellular carcinoma
- Population-based X-ray gastric cancer screening in Hiroshima prefecture,Japan
- Endoscopic resection for calcifying fibrous tumors of the gastrointestinal tract
