TM9SF1 promotes bladder cancer cell growth and infiltration
Long Wei,Shi-Shuo Wang,Zhi-Guang Huang,Rong-Quan He,Jia-Yuan Luo,Bin Li,Ji-Wen Cheng,Kun-Jun Wu,Yu-Hong Zhou,Shi Liu,Sheng-Hua Li,Gang Chen
Abstract BACKGROUND Bladder cancer (BC) is the most common urological tumor.It has a high recurrence rate,displays tutor heterogeneity,and resists chemotherapy.Furthermore,the long-term survival rate of BC patients has remained unchanged for decades,which seriously affects the quality of patient survival.To improve the survival rate and prognosis of BC patients,it is necessary to explore the molecular mechanisms of BC development and progression and identify targets for treatment and intervention.Transmembrane 9 superfamily member 1 (TM9SF1),also known as MP70 and HMP70,is a member of a family of nine transmembrane superfamily proteins,which was first identified in 1997.TM9SF1 can be expressed in BC,but its biological function and mechanism in BC are not clear.AIM To investigate the biological function and mechanism of TM9SF1 in BC.METHODS Cells at 60%-80% confluence were transfected with lentiviral vectors for 48-72 h to achieve stable TM9SF1 overexpression or silencing in three BC cell lines (5637,T24,and UM-UC-3).The effect of TM9SF1 on the biological behavior of BC cells was then investigated through CCK8,wound-healing assay,transwell assay,and flow cytometry.RESULTS Overexpression of TM9SF1 increased the in vitro proliferation,migration,and invasion of BC cells by promoting the entry of BC cells into the G2/M phase.Silencing of TM9SF1 inhibited in vitro proliferation,migration,and invasion of BC cells and blocked BC cells in the G1 phase.CONCLUSION TM9SF1 may be an oncogene in BC.
Key Words: TM9SF1;Bladder cancer;Biological function;Cell function assay;Oncogene
lNTRODUCTlON
Bladder cancer (BC) is the most common urological tumor,ranking tenth among global malignant tumors and fourth among male malignant tumors.It has a high recurrence rate,displays tutor heterogeneity,and resists chemotherapy,imposing a huge cost burden on healthcare systems and affecting the prognosis and quality of survival in patients[1-4].Age-standardized prevalence rates show considerable variation across geographic regions and are expected to continue to rise over the next two decades[5].Several BC risk factors have been identified;in addition to geography and age,gender and exposure to a variety of carcinogens,of which smoking is the most prevalent,greatly influence the risk[6,7].Moreover,age-standardized mortality rates have begun to decline in developed countries but are on the rise in lowincome regions worldwide[8].
The most prominent symptom of BC is microscopic or gross hematuria.Seventy-five percent of bladder tumors are uroepithelial carcinomas confined to the mucosa,that is,non-muscle-invasive BC (NMIBC)[9].Muscle-invasive BC(MIBC) is BC that has invaded the deeper layers of the bladder wall or has metastasized[10-13].For patients with NMIBC,transurethral resection of the bladder tumor (TURBT) is the standard treatment,while radical cystectomy is indicated for patients with MIBC.To prevent BC recurrence or worsening,TURBT in selected patients is supplemented with an intravesical drip[10,14].
Despite some improvements in surgery and anesthesia techniques and the widespread adoption of perioperative chemotherapy,the long-term survival of BC patients has remained unchanged for decades[15].However,advanced molecular studies have greatly increased the understanding of disease biology.To find better treatments for this disease,we investigated transmembrane 9 superfamily member 1 (TM9SF1) to determine its biological function and mechanism in BC and to assess whether it could be a therapeutic target.
TM9SF1,also known as MP70 and HMP70,was first identified in 1997[16].TM9SF1 is localized on membranes and is an integral part of the membranes in which it is active,including autophagosome and lysosomal membranes and cytoplasmic vesicles.TM9SF1 is ubiquitously expressed in human tissues,widely expressed in yeast,plants,and mammals,and highly conserved[17].Studies have identifiedTM9SF1expression in BC,and through genome-wide microarray analyses of tissue sections,TM9SF1has been determined to be a common differentially expressed gene in BC[18].However,the biological function ofTM9SF1in BC cells is still unclear,and more in-depth exploration of its biological function is needed.
This study explored the effect ofTM9SF1on BC cells’ biological phenotype by constructing stable cell transfectants with overexpression or silencing ofTM9SF1.The biological relationship betweenTM9SF1and BCin vitrowas validated,with an aim to provide a new direction for and new way of thinking about targeted BC therapy.
MATERlALS AND METHODS
Cell culture
We purchased the uman BC cell line 5637,tool cells,and the human embryonic kidney cell line 293T from the cell bank at the Committee of Typical Culture Conservation at the Chinese Academy of Sciences as well as the human bladder transitional cell cancer cell line T24 from Guangzhou Genio Biotechnology Co.,Ltd.and the human bladder transitional cell cancer cell line UM-UC-3 from Wuhan Punose Life Technology Co.,Ltd.Cells were cultured in a 5% CO2incubator at 37 °C.
Stable transfectant construction for TM9SF1 overexpression
The lentivirus used for constructing the stable TM9SF1-overexpressing cell strain was provided by Hanheng Biologicals;its vector system consisted of pSPAX2,pMD2G,and a shuttle plasmid carrying the target genes.The three plasmids were co-transfected into the 293T packaging cellviaa transfection reagent.After lentiviral infection,quantitative real-time polymerase chain reaction (qRT-PCR),which is an experimental method for detecting RNA or DNA molecules and quantifying their content,was used to verify the efficiency ofTM9SF1overexpression in BC cells.
Stable transfectant construction for TM9SF1 silencing
Lentiviral vectors are gene therapy vectors that have been developed based on human immunodeficiency virus-1.TheTM9SF1silencing plasmid used for constructing short hairpin RNA (shRNA) was provided by GeneCopoeia.The vector used is psi-LVRU6H,which has BamHI (5') and EcoRI (3') cloning sites,is 7451 bp in length,is ampicillin resistant,and has a thaumatin screening marker.The lentiviral infection was followed by qRT-PCR to validate the silencing efficiency ofTM9SF1in BC cells.
Cell proliferation
Cell Counting Kit-8 (CCK8) was used to detect cell proliferation.WST-8 (2-[2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5(2,4-disulfophenyl])-2H-tetrazole monosodium salt],the main component of the kit,reacts with intracellular mitochondria to generate an orange formazan dye in proportion to the number of viable cells.This indirectly detects the number of viable cells and shows cell proliferation.The optical density (OD) value was measured at 450 nm using a microplate reader;the higher the OD value,the more the live cells.
Cell migration and invasion
The migration of the stably transfected strains,which demonstrated silenced or overexpressedTM9SF1,was verified by cell wound-healing and transwell assays.First,the cells were incubated until they spread over the bottom of the plate.Next,a 1000 μL pipette tip was used to trace a line across the cell plane along a straight ruler held perpendicular to the bottom of the plate.The culture was then continued by adding medium containing the appropriate percentage of fetal bovine serum (FBS),which is the natural medium in the cell culture (5637: 1%;T24: 1%;UM-UC-3: 4%).Starting at 0 h,scratches at the same location were photographed under an inverted fluorescence microscope at intervals of 12 h or 48 h.The area of the scratches was calculated,and the healing rate was determined using ImageJ software.
For the transwell migration and invasion assays,500 μL of complete medium was first added to the 24-well plates.Logarithmically growing 5637,T24,and UM-UC-3 BC cells were digested,centrifuged,and counted using cell resuspension with a culture medium containing 2% FBS.To measure invasion,100 μL of suspended cells was added on top of the solidified matrix gel in the upper chamber of each transwell and incubated for 24 h.After which,the chambers were removed,fixed,and stained with methanol and crystal violet.All photography was performed using a Leica DMi8 inverted fluorescence microscope and calculated using ImageJ software.
Cell cycle analysis
The original medium was aspirated,and fresh serum-free medium was added based on starvation treatment in the incubator for 12 h.Then,the serum-free medium was aspirated,and a complete medium containing 10% FBS and 1%double antibody was added.Incubation continued for 12 h to synchronize the cell status of the experimental and control groups.Finally,5 × 105cells were removed and processed using a cell cycle staining kit (Lianke Bio).A flow cytometer was used to detect the cells.
Statistical analysis
Student’sttest in SPSS 25.0 was employed to analyze the statistical significance of the experiments,andP< 0.05 was defined as statistically significant.
RESULTS
Effects of TM9SF1 overexpression on BC cell proliferation, migration, invasion, and cell cycle progression
Stable transfectant construction for TM9SF1 overexpression:The relative expression of theTM9SF1gene in BC cells transfected withTM9SF1-overexpressed lentiviral vector was detected by qRT-PCR in three cell lines (5637,T24,and UMUC-3) using cells transfected with the empty lentiviral vector as a control group.The expression ofTM9SF1was 27.4-fold higher in theTM9SF1overexpression group (OE group) of 5637 cells than in the empty vector group,3.5-fold higher inTM9SF1-overexpressing T24 cells,and 12.5-fold higher inTM9SF1-overexpressing UM-UC-3 cells.All these values were statistically significant (P< 0.05),indicating that stable transfectants overexpressingTM9SF1were successfully constructed in all three BC cell lines (Figure 1).
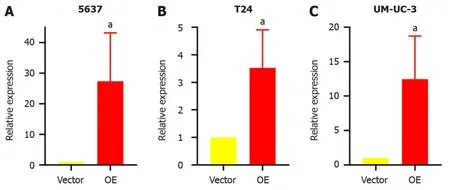
Figure 1 Relative expression of TM9SF1 in bladder cancer cells transfected with TM9SF1-overexpressing lentiviral vector and empty vector. A-C: Relative expression of TM9SF1 in 5637 (A),T24 (B),and UM-UC-3 (C) bladder cancer cells transfected with TM9SF1-overexpressing lentiviral vector and empty vector.aP < 0.05.OE: TM9SF1-overexpressing lentiviral vector.
TM9SF1 overexpression promotes BC cell proliferation in vitro:The cell activity was tested by a CCK8 assay.The results showed that compared with the control group transfected with the empty vector,the proliferation rate of BC cells in theTM9SF1overexpression group was significantly higher at 72 h in the 5637 cells,96 h in the T24 cells,and 48 h and 72 h in the UM-UC-3 cells (P< 0.05) (Figure 2).This finding indicates thatTM9SF1overexpression plays a role in promoting BC cell proliferationin vitro.

Figure 2 Effect of overexpression of TM9SF1 on proliferation of bladder cancer cells. A-C: Promoting effect of overexpression of TM9SF1 on proliferation of 5637 (A),T24 (B),and UM-UC-3 (C) cells at 24 h,48 h,72 h,and 96 h by CCK8 assay.aP < 0.05,bP < 0.01,cP < 0.001.OE: TM9SF1-overexpressing lentiviral vector.
TM9SF1 overexpression promotes BC cell migration in vitro:In this study,we used a wound-healing assay to detect the migration ability of cells.After calculating the change of scratch area,we found that in the 5637 cells,the wound healing rate of theTM9SF1overexpression group was significantly higher than that of the control group at 12 h,24 h,and 36 h (P< 0.01).The scratch healing rate of the cells in the T24 overexpression group was also significantly higher than that of the control group at 24 h and 36 h (P< 0.01).Although UM-UC-3 cells were less likely to migrate toward the scratches,the rate of scratch closure in the OE group was still slightly higher than that in the control group and was statistically significant at 96 h (P< 0.01) (Figure 3).

Figure 3 Effect of overexpression of TM9SF1 on migration of bladder cancer cells by scratch assay (50 ×). A-C: Scratches under a microscope and wound-healing rate of transfected 5637 (A) and T24 (B) cells at 0 h,12 h,24 h,36 h,and UM-UC-3 cells (C) at 0 h,48 h,and 96 h by wound-healing assay.bP <0.01.OE: TM9SF1-overexpressing lentiviral vector.
A transwell migration assay was performed to verify the changes in cell migration ability.After 24 h of incubation,the number of cells passing through the transwell membrane was counted.As shown in Figure 4,the number of cells passing through the membrane was significantly higher in the three BC cell lines withTM9SF1overexpression than in the control group (P< 0.05).
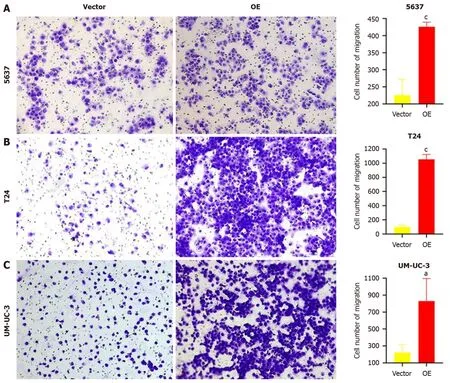
Figure 4 Effect of overexpression of TM9SF1 on cell migration by transwell cell migration assay (100 ×). A-C: After 24 h for cell culture,the number of migrating 5637 (A),T24 (B),and UM-UC-3 (C) cells in the TM9SF1 overexpression groups was more than that of the empty vector groups.aP < 0.05,cP <0.001.OE: TM9SF1-overexpressing lentiviral vector.
By combining the above two migration experiments,we speculate that the overexpression ofTM9SF1can significantly improves the migration ability of BC cells.
TM9SF1 overexpression promotes BC cell invasion in vitro:Matrigel matrix gel was used to mimic the extracellular matrix.The high-nutrient culture medium in the lower chamber of the transwell was separated from the low-nutrient culture medium in the upper chamber,which caused the BC cells to secrete hydrolases and move to pass through the filter membrane lined with matrix gel.This can be used to assess the invasive ability of the BC cells.After analyzing the fixed stained cells,we found that the number of cells in the OE group of the three BC cell lines was 1.3 times more than that in the empty vector group (P< 0.05),indicating that the overexpression ofTM9SF1has a significant effect in promoting the invasive ability of BC cells (Figure 5).

Figure 5 Effect of overexpression of TM9SF1 on invasion of bladder cancer cells by transwell invasion assay (100 ×). A-C: After 24 h for cell culture,the number of invasive 5637 (A),T24 (B),and UM-UC-3 (C) cells in the overexpression groups was significantly more than that of the empty vector groups.aP< 0.05,cP < 0.001.OE: TM9SF1-overexpressing lentiviral vector.
TM9SF1 overexpression promotes cell entry into the G2/M phase:Flow cytometry was used to investigate the effect ofTM9SF1overexpression by detecting the percentage of cells distributed in different phases of the cell cycle.The results showed that the proportion of cells distributed in the G1 phase in all three BC cell lines was smaller in the OE group than in the empty vector group,while the proportion of cells in the G2/M phase was higher in the OE group than in the empty vector group.However,for the 5637 and UM-UC-3 cell lines,the OE group had more cells in the S phase than in the empty vector group,while the opposite was true regarding the T24 cell line.Although the statistical results of 5637 cells in the S,G2/N,and G1 phases as well as the T24 cells in the G1,S,and G2/M phases were not significant,the changing trend combined with the statistical results of the UM-UC-3 cells in the G1,S,and G2/M phases suggests thatTM9SF1overexpression has a certain effect on BC cell cycle progression,reducing the proportion of cells in the G1 phase and promoting the entry of cells into the G2/M phase (Figure 6).
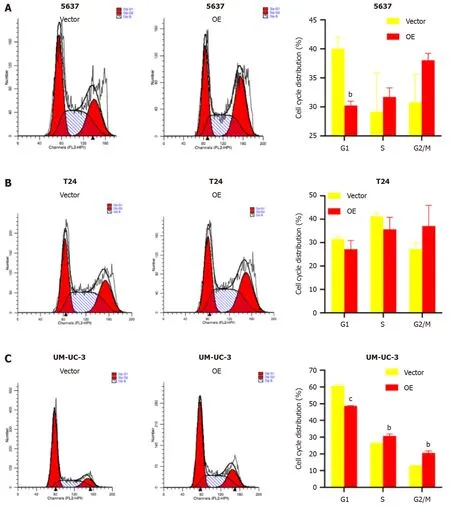
Figure 6 Effect of overexpression of TM9SF1 on bladder cancer cell cycle progression. A-C: Cell cycle results in (A) 5637,(B) T24,and (C) UM-UC-3 cells.bP < 0.01,cP < 0.001.OE: TM9SF1-overexpressing lentiviral vector.
Effects of TM9SF1 silencing on BC cell proliferation, migration, invasion, and cell cycle progression
Stable transfectant construction for TM9SF1 silencing:shRNA was used to constructTM9SF1-silenced stable transfectants in three cell lines: 5637,T24,and UM-UC-3.TheTM9SF1-silenced stable transfectants and the cells transfected with the empty lentiviral vector as a control group [short hairpin vector (shVector) control group] were used.qRT-PCR was used to detect theTM9SF1silencing efficiency in BC cell transfectants withTM9SF1silencing (shTM9SF1group).The quantitative results showed that the silencing efficiency was 88.09%,90.39%,and 92.04% in the 5637,UM-UC-3,and T24 cells,respectively.All these values were statistically significant (P< 0.01),suggesting that theTM9SF1-silencing stable transfectants were successfully constructed in all three BC cell lines (Figure 7).

Figure 7 Relative expression of TM9SF1 in bladder cancer cells transfected with TM9SF1-silencing lentiviral vector and empty vector. A-C:Relative expression of TM9SF1 in 5637 (A),T24 (B),and UM-UC-3 (C) bladder cancer cells transfected with TM9SF1-silencing lentiviral vector and empty vector.aP< 0.05,bP < 0.01,cP < 0.001.shVector: Short hairpin vector;shTM9SF1: Short hairpin TM9SF1.
TM9SF1 silencing inhibits BC cell proliferation in vitro:To test the effect of silencingTM9SF1on cell proliferation in BC cells,we examined cell viability using a CCK8 assay.Compared with the shVector control group transfected with the empty vector,the proliferation rate of BC cells in the shTM9SF1group was significantly reduced in the 5637 cells at 24 h,48 h,72 h,and 96 h;in the T24 cells at 48 h,72 h,and 96 h;and in the UM-UC-3 cells at 48 h (P< 0.05).This finding indicates that silencingTM9SF1has an inhibitory effect on BC cell proliferationin vitro(Figure 8).
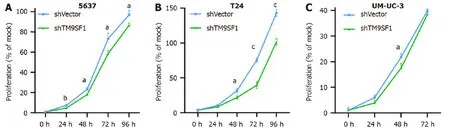
Figure 8 Effect of TM9SF1 knockdown on proliferation of bladder cancer cells. A-C: Inhibiting effect of TM9SF1 knockdown on proliferation of 5637(A),T24 (B),and UM-UC-3 (C) cells at 24 h,48 h,72 h,and 96 h by CCK8 assay.aP < 0.05,bP < 0.01,cP < 0.001.shVector: Short hairpin vector;shTM9SF1: Short hairpin TM9SF1.
TM9SF1 silencing inhibits BC cell migration in vitro:Cell scratch and transwell migration assays were used to detect the migration ability of the cells.Of the three BC cell lines,the 5637 and T24 cells were selected for the cell scratch assay(Figure 9).The closure rate of theTM9SF1-silenced 5637 cells was higher than that of the control group at 12 h and 24 h but was statistically significant only at 24 h (P< 0.01).The closure rate of theTM9SF1-silenced T24 cells compared to the empty control group was statistically significant at 12 h,24 h,and 36 h.Moreover,the T24 cells’ closure rate significantly decreased at 24 h and 36 h (P< 0.001).
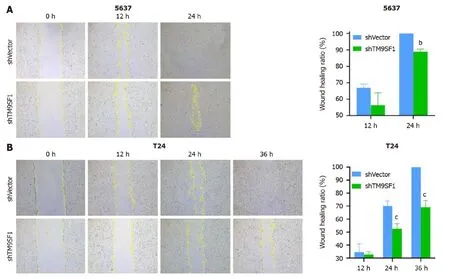
Figure 9 Effect of TM9SF1 knockdown on migration of bladder cancer cells by scratch assay (50 ×). A-C: Scratches under a microscope and wound-healing rate of 5637 (A) and T24 (B) at 0 h,12 h,24 h,and 36 h by wound-healing assay.bP < 0.01,cP < 0.001.shVector: Short hairpin vector;shTM9SF1:Short hairpin TM9SF1.
All three BC cell lines were subjected to transwell migration assays to validate the inhibitory effect ofTM9SF1silencing on the migratory ability of BC cells.After spreading the cells in the transwell chambers,they were incubated for 24 h.The chambers were then removed and washed with phosphate buffered solution (PBS),and the cells that passed through the filter membrane were fixed with methanol.The cells under the filter membrane were then stained purple with crystal violet (the excess dye was removed with PBS).Next,the chambers were air dried at room temperature.Once dried,images of the cells were capturedviaa microscope,and the cells were counted using ImageJ software.This information was statistically analyzed using SPSS and graphed using GraphPad Prism 8.0.As can be seen in Figure 10,the number of cells that passed through the filter membrane was significantly lower in all three BC cell strains than in the shVector group (P< 0.01).By comprehensively analyzing both assays,we can conclude that silencing the expression ofTM9SF1in BC cells can significantly reduce their migration ability.

Figure 10 Effect of TM9SF1 knockdown on cell migration by transwell cell migration assay (100 ×). A-C: After 24 h for cell culture,the number of migrating 5637 (A),T24 (B),and UM-UC-3 (C) cells in the TM9SF1 knockdown (short hairpin TM9SF1) groups was less than that of the control (short hairpin vector)groups.bP < 0.01,cP < 0.001.shVector: Short hairpin vector;shTM9SF1: Short hairpin TM9SF1.
TM9SF1 silencing inhibits BC cell invasion in vitro:Invasion occurs when malignant tumor cells enter adjacent host tissue by secreting proteins that digest the tissue cells’ extracellular matrix.Detecting a tumor’s invasion ability can help determine the rate of metastasis,as cell invasion is the first step: Tumor cells break through the basement membranein situ,infiltrate blood and lymphatic vessels,colonize other tissues,and proliferate.The transwell invasion assay mimics thein vivoenvironment of the human body and can therefore determine the invasive ability of tumor cells.
Therefore,in this study,BC cells were induced to secrete hydrolytic enzymes to pass through a layer of Matrigel matrix gel (which mimics the extracellular matrixin vivo) on a transwell filter membrane.After that,the BC cells that had passed through the matrix gel and reached the bottom of the membrane were washed,fixed,stained,dried,photographed,and counted to determine their invasive ability.
The number of 5637 cells in the shTM9SF1group withTM9SF1silencing was 1/1.42 of that in the shVector group.The number of T24 cells in theTM9SF1-silenced group was 1/2.46 of that in the control group.The number of UM-UC-3 cells in the shTM9SF1group was 1/2.6 of that in the shVector group.In all groups,Pvalue was less than 0.05.The results suggest that the invasion ability of BC cells is significantly reduced afterTM9SF1is silenced (Figure 11).

Figure 11 Effect of TM9SF1 knockdown on invasion of bladder cancer cells by transwell invasion assay (100 ×). A-C: After 24 h for cell culture,the number of invasive 5637 (A),T24 (B),and UM-UC-3 (C) cells in the TM9SF1 knockdown (short hairpin TM9SF1) groups was significantly lower than that of the control (short hairpin vector) groups.aP < 0.05,bP < 0.01.shVector: Short hairpin vector;shTM9SF1: Short hairpin TM9SF1.
TM9SF1 silencing blocks cells in the G1 phase:To investigate the effect of silencedTM9SF1on the BC cell cycle,the shTM9SF1cells collected from the 5637,T24,and UM-UC-3 cell lines were stained and detected by flow cytometry.The number of cells distributed in each phase of the cell cycle was analyzed and compared.In all three BC lines,the shTM9SF1group had a higher proportion of BC cells in the G1 phase than did the shVector group (P< 0.05).The number of 5637 cells in the S phase was lower in theTM9SF1-silenced group than in the shVector group,but the difference was not significant.However,the number of 5637 cells in the G2/M phase was significantly lower in the shTM9SF1group than in the shVector group.The number of T24 cells in the S phase in the shTM9SF1group was significantly lower than that in the shVector group (P< 0.001).However,silencingTM9SF1did not have much effect on T24 cells in the G2/M phase.The number of the shTM9SF1group’s UM-UC-3 cells in the S phase was significantly less than the same cells in the shVector group (P< 0.05),but there was no significant difference found in cells in the G2/M phase.These combined results suggest that silencingTM9SF1expression inhibits BC cell proliferation by arresting BC cells in the G1 phase and prolonging the time between mitosis completion and DNA replication (Figure 12).

Figure 12 Effect of TM9SF1 knockdown on bladder cancer cell cycle progression. A-C: Cell cycle results of TM9SF1 knockdown (short hairpin TM9SF1) and control (short hairpin vector) groups in (A) 5637,(B) T24,and (C) UMUC-3 cells.aP < 0.05,cP < 0.001.shVector: Short hairpin vector;shTM9SF1:Short hairpin TM9SF1.
DlSCUSSlON
In this study,we confirmed thatTM9SF1is a pro-carcinogenic gene in BC by overexpressing and silencing it in the 5637,T24,and UM-UC-3 cell lines using cellular function assays.TM9SF1is expressed not only in BC but also in esophageal squamous cell carcinoma and cervical cancer,and because of its pro-cancer role,it can aggravate poor prognoses in patients[18-20].The experimental results of the present study revealed that overexpression ofTM9SF1reduces the number of cells in the G1 phase and prompts them to enter the G2/M phase and start mitosis,thereby promoting BC cell proliferation.However,the silencing ofTM9SF1blocks cells in the G1 phase and prevents them from entering the DNA replication phase,thereby inhibiting BC cell proliferation.It is hypothesized thatTM9SF1may promote BC cell proliferation by mainly affecting the G1 phase of BC cells,which in turn promotes the development of BC.AlthoughTM9SF1was experimentally demonstrated to be an oncogene of BC in this study,Weiet alfoundTM9SF1to be a gastric cancer suppressor geneviaN6,2'-O-dimethyladenosine (m6Am) sequencing.TM9SF1is a target of phosphorylated C-terminal domain-interactingfactor 1 (PCIF1);PCIF1 can reduce TM9SF1 translation by m6Am modification,while TM9SF1 can reverse the effect of PCIF1 on the invasiveness of gastric cancer cells[21].This indicates thatTM9SF1has different functions and effects in different tumor types.
In other studies,TM9SF1has been shown to synergistically interact with the tumor marker gene estrogen receptorbinding fragment-associated antigen 9 (EBAG9) to regulate the epithelial-mesenchymal transition in cancer cellsviaan attenuated anti-tumor immune response,leading to immune escape,which results in tumor growth[22].Therefore,in addition to affecting the biological phenotype of BC and promoting BC development,TM9SF1can also affect an antitumor immune response to BC,the mechanism of which must be studied in future research.
Furthermore,it has been shown through a combination of suppression subtractive hybridization and transmembrane trapping techniques thatMyc-taggedTM9SF1is localized on the surface of transfected COS-7L cells[23].If the same technology can be used for BC,researching targeted therapy may go further.TM9SF1also plays a crucial role in autophagy.One study showed that its high expression can worsen the prognosis of patients with cervical cancer[24].From this perspective,TM9SF1may affect the occurrence and development of BC by regulating autophagy.However,this study was based on the results obtained fromin vitrocell experiments,and expression detection in BC tissues still needs to be improved.
This study was the first to attempt to construct a stable BC cell line to investigate the overexpression and silencing ofTM9SF1usingin vitroexperiments for the purpose of exploring the pro-cancer effect ofTM9SF1in BC.We verified that overexpressedTM9SF1enhances the growth,migration,and invasion of BC cells and promotes their entry into the G2/M phase of the cell cycle.This information not only provides a new target in developing treatments for BC but is also a source of hope for BC patients.
CONCLUSlON
TM9SF1is suspected to function as an oncogene in BC,as it has been shown to enhance the growth,migration,and invasion of BC cells while also promoting their entry into the G2/M phase of the cell cycle.Moreover,beyond its influence on the biological phenotype of BC and its role in advancing BC development,there is a need for further investigation into the mechanisms underlying these effects,specifically focusing onTM9SF1’s impact on autophagy and antitumor immune responses.Consequently,delving into genetic factor analysis and targeted therapies in the realm of cancer treatment holds promising prospects.
ACKNOWLEDGMENTS
Thanks to Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis for providing technical support.Thanks to China Undergraduate lnnovation and Entrepreneurship Training Program (202310598044) and Future Academic Star of Guangxi Medical University(WLXSZX23112) for the experimental platforms.
ARTlCLE HlGHLlGHTS
Research background
Bladder cancer (BC) is the most common urological tumor.It has a high recurrence rate,displays tutor heterogeneity,and resists chemotherapy.Furthermore,the long-term survival rate of BC patients has remained unchanged for decades,which seriously affects the quality of patient survival.To improve the survival rate and prognosis of BC patients,it is necessary to explore the molecular mechanisms of BC development and progression and identify targets for treatment and intervention.Transmembrane 9 superfamily member 1 (TM9SF1),also known as MP70 and HMP70,is a member of a family of nine transmembrane superfamily proteins that was first identified in 1997.TM9SF1can be expressed in BC,but its biological function and mechanism in BC are not clear.
Research motivation
Previous studies have identifiedTM9SF1expression in BC,and through genome-wide microarray analyses of tissue sections,TM9SF1has been found to be a commonly differentially expressed gene in BC.However,the biological function ofTM9SF1in BC cells remains unclear.To discover better treatment options for this disease,it is crucial to conduct a more in-depth exploration of its biological behavior.
Research objectives
This study explored the effect ofTM9SF1on BC cells’ biological phenotype by constructing stable cell transfectants with overexpression or silencing ofTM9SF1.The biological relationship betweenTM9SF1and BCin vitrowas validated,with an aim to provide a new way of thinking about targeted BC therapy.
Research methods
Cells at 60%-80% confluence were transfected with lentiviral vectors to achieve stable overexpression or silencing ofTM9SF1in three BC cell lines (5637,T24,and UM-UC-3).The effect ofTM9SF1on the biological behavior of BC cells was investigated through CCK8,wound-healing assay,transwell assay,and flow cytometry.
Research results
In this study,we confirmed thatTM9SF1is a pro-carcinogenic gene in BC by overexpressing and silencing it in the 5637,T24,and UM-UC-3 cell lines using cellular function assays.The experimental results of the present study revealed that overexpression ofTM9SF1reduces the number of cells in the G1 phase and prompts them to enter the G2/M phase and start mitosis,thereby promoting BC cell proliferation.Yet,the silencing ofTM9SF1blocks cells in the G1 phase and prevents them from entering the DNA replication phase,thereby inhibiting BC cell proliferation.It is hypothesized thatTM9SF1may promote BC cell proliferation mainly by affecting the cell cycle progression of BC cells,which in turn promotes BC development.
Research conclusions
This study was the first to attempt to construct a stable BC cell line to investigate the overexpression and silencing ofTM9SF1usingin vitroexperiments for the purpose of exploring the pro-cancer effect ofTM9SF1in BC.We verified that overexpression ofTM9SF1enhances the growth,migration,and invasion of BC cells and promotes their entry into the G2/M phase of the cell cycle.TM9SF1may be an oncogene in BC.
Research perspectives
In addition to influencing the biological phenotype of BC and promoting its development,TM9SF1may also impact the anti-tumor immune response in BC.Further research is needed to elucidate the mechanisms underlying this effect.Furthermore,genetic factor analysis and targeted therapy aimed at inhibiting carcinogenesis offer promising prospects.
FOOTNOTES
Author contributions:Wang SS,He RQ,Cheng JW,Li SH,and Chen G conceived and designed the study;Wei L,Wang SS,Luo JY,Li B,Wu KJ,Zhou YH,and Liu S performed the experiments,and acquired and analyzed the data;Wei L,Wang SS,and Huang ZG wrote the manuscript;He RQ,Luo JY,Li B,Cheng JW,Li SH,and Chen G revised and corrected the draft;all authors approved the final version of the article.
Supported byNational Natural Science Foundation of China,No.82260785.
lnstitutional review board statement:The study did not involve human or animal subjects.
lnstitutional animal care and use committee statement:The study did not involve animal experiments.
Conflict-of-interest statement:All authors declare no conflict of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Long Wei 0009-0008-6849-6838;Shi-Shuo Wang 0000-0003-4788-0562;Zhi-Guang Huang 0000-0003-4457-9491;Rong-Quan He 0000-0003-0479-0145;Jia-Yuan Luo 0000-0003-4712-5217;Bin Li 0009-0002-9889-1800;Ji-Wen Cheng 0000-0002-0900-760X;Kun-Jun Wu 0000-0002-1492-4533;Yu-Hong Zhou 0009-0008-2315-2097;Shi Liu 0009-0004-7692-6974;Sheng-Hua Li 0000-0002-0125-6345;Gang Chen 0000-0003-2402-2987.
S-Editor:Lin C
L-Editor:Wang TQ
P-Editor:Yuan YY
 World Journal of Clinical Oncology2024年2期
World Journal of Clinical Oncology2024年2期
- World Journal of Clinical Oncology的其它文章
- Unlocking the potential-vitamin D in prostate cancer prevention
- Updates on management of gliomas in the molecular age
- Deregulation of interferon-gamma receptor 1 expression and its implications for lung adenocarcinoma progression
- Elucidating the molecular basis of ATP-induced cell death in breast cancer: Construction of a robust prognostic model
- ldentification of immune cell-related prognostic genes characterized by a distinct microenvironment in hepatocellular carcinoma
- Population-based X-ray gastric cancer screening in Hiroshima prefecture,Japan
