Effect of particle size of single-crystalline hierarchical ZSM-5 on its surface mass transfer in n-heptane catalytic cracking
Xiaoxue Zhang, Shuman Xu, Jing Hao, Xiaojin Xie, Fengqiu Chen,2, Dangguo Cheng,2,
1 College of Chemical and Biological Engineering, Zhejiang Provincial Key Laboratory of Advanced Chemical Engineering Manufacture Technology, Zhejiang University,Hangzhou 310027, China
2 Institute of Zhejiang University-Quzhou, Quzhou 324000, China
Keywords: Single-crystalline Hierarchical ZSM-5 Particle size Surface barriers n-Heptane catalytic cracking
ABSTRACT Single-crystalline hierarchical ZSM-5 zeolites with different particle sizes(namely 100,140,and 200 nm)were successfully prepared by adjusting the amount of tetrapropylammonium hydroxide (TPAOH), and investigated in n-heptane catalytic cracking reaction. Diffusional measurements by zero-length column(ZLC)method showed that the apparent diffusivities of n-heptane decreased with the reduction of particle size,indicating the existence of surface barriers.Moreover,with the decrease of particle size,the additional diffusion path length increased, which meant the influence of surface barriers became more apparent.Despite the change of surface barriers,the intracrystalline diffusion still dominated the overall diffusion. Catalytic performance showed that the zeolite with smaller particle size had better stability.
1. Introduction
With the rapid development of integrated industry chains,domestic market demand for light olefins [1], such as ethylene and propylene, has been increasing, and the imbalance between supply and demand of light olefins has been standing out year by year. It is reported that the production of light olefins [2] mainly includes four processes,namely catalytic cracking,steam cracking,methanol to olefins and alkane dehydrogenation. Among them,naphtha catalytic cracking is a promising production route due to its lower reaction temperature [3,4]. With remarkable properties such as large specific surface area, excellent shape selectivity,adjustable acidity and high hydrothermal stability, traditional microporous ZSM-5 zeolite has been widely used in catalytic cracking reactions [5]. However, the application of ZSM-5 zeolite is seriously plagued by diffusion limitation because of its intrinsic micropores (typically less than 2 nm) restrict the accessibility of active sites,lead to carbon deposition and even deactivation of catalyst, especially when bulky molecules are involved [6,7].
It has been considered that intracrystalline diffusion plays a dominant role in the mass transfer of zeolites.Therefore,researchers introduced mesopores [8,9] and/or macropores [10] into the microporous zeolite structure to construct hierarchical ZSM-5 zeolite [11,12] or prepared nanosized ZSM-5 zeolite [13] in order to eliminate intracrystalline diffusion limitation. Hierarchical and nanosized zeolites are widely used in different fields such as catalytic cracking, alkylation and isomerization reaction [14,15].However,in recent years,many studies have found that in addition to intracrystalline diffusion,the adsorption and diffusion process of probe molecules on zeolite particle surface [16] and micropore mismatch or orientation difference between sub-crystal surfaces of polycrystalline materials also produce diffusion limitations,i.e.surface barriers [17] and grain boundary resistance [18–21],respectively.
With the development of characterization techniques, Kargeret al.[22–26]observed intuitively the existence of surface barriers using interference microscopy (IFM) and infrared microscopy(IRM).Researches further found that the particle size[27]and surface properties [28–30] such as pore blockage, pore narrowing could alter surface barriers. The role of surface barriers becomes increasing significance, especially when particle sizes reduce to nanoscale. Chenet al. [31] measured the diffusivity of methanol on SAPO-34 using the oscillating microbalance reactor.They found that SAPO-34 with the smaller particle size had the smaller diffusivity because of the existence of surface barriers. Teixeiraet al.[32] systematically measured apparent diffusivities which varied over 3 orders of magnitude in cyclohexane-silicalite-1 (particle size ranging from 35 nm to 3 μm) system by ZLC method. It was believed that surface barriers had a great impact on diffusion in small particle size zeolites. Lercheret al. [27] compared the transport of aromatic compounds in small and large MFI particles by the frequency response method.These researches showed that the diffusion could be controlled by surface effects for the small particles.
However, the above researches are only aimed at microporous zeolites,and there is little research focusing on the surface barriers in hierarchical zeolites[17,20].Yeet al.[33]compared the catalytic activity of polycrystalline hierarchical Pt/Beta zeolite with different particle sizes, and found that Pt/Beta zeolite with larger particle size had poor catalytic activity due to the extended diffusion path.The diffusion properties of polycrystalline zeolites are coupled with the influence of many factors, including surface barriers,intracrystalline diffusion and grain boundary resistance, and it is difficult to eliminate the influence of grain boundaries of polycrystalline zeolites.
In this work,we attempt to prepare a series of single-crystalline hierarchical ZSM-5 zeolites, in order to eliminate the influence of grain boundary resistance. On this basis single-crystalline hierarchical ZSM-5 with different particle sizes were successfully prepared by adjusting the amount of TPAOH. The apparent diffusivities (Deff) of all samples were measured by ZLC method.Based on the dual-resistance model (DRM),Deffis composed by intracrystalline diffusion and surface barriers. Therefore, the surface permeability (α) is introduced to realize the decoupling of intracrystalline diffusion resistance and surface barriers, and then the effect of particle sizes on surface mass transfer is explored.Finally,the prepared zeolites were applied in the catalytic cracking reaction ofn-heptane, the relationship between surface barriers and catalytic activity was discussed.
2. Materials and Methods
2.1. Catalyst synthesis
Synthesis of single-crystalline hierarchical ZSM-5 zeolites:Zeolites were synthesized as follows[34].First,tetrapropylammonium hydroxide (TPAOH, Sinopharm Chemical Reagent, 25% (mass) in aqueous solution) was dispersed in DI water, an appropriate amount of tetraethyl orthosilicate (TEOS, Sinopharm Chemical Reagent, AR) was dropwise added into the solution, stirring at room temperature for 3–6 h until TEOS was hydrolyzed completely(without oil–water separation)to obtain solution 1.Sodium aluminate solid powder (NaAlO2, Aladdin) and sodium hydroxide solid(NaOH, Sinopharm Chemical Reagent, AR) were dissolved into deionized water and dissolved by ultrasonic to get a clarified solution 2. After that, solution 2 was slowly added into solution 1, followed by adding the L-lysine (C6H14N2O2, Aladdin, 97%) as mesoporous template, stirring for 2 h until mixed evenly. The molar composition of precursor solution was 1.0 SiO2: 0.003 Al2O3: 0.015 Na2O:xTPAOH (x =0.450, 0.475, 0.500): 0.4 Llysine: 9 H2O. The zeolite was prepared by hydrothermal crystallization in a Teflon-lined stainless-steel autoclave, which was placed in a conventional oven, initially crystallized at 90 °C for 2 d and further continued at 170 °C for 2 days. The solid sample was filtered,washed to neutral with DI water and ethanol for several times, dried overnight in oven at 60 °C, and then calcined in the tubular furnace at 550 °C for 6 h. In order to obtain H-type ZSM-5 zeolites,the calcined solid sample was ion-exchanged with a solution of 1 mol∙L-1ammonium chloride (NH4Cl, Sinopharm Chemical Reagent, >99%) at 80 °C, stirring for 6 h. After washing,centrifugation and drying, the zeolite sample was calcined again in tubular furnace under the condition of 550 °C for 3 h. The samples synthesized with TPAOH/SiO2ratios of 0.450, 0.475, 0.500 were labeled as Z5-200, Z5-140 and Z5-100, respectively.
Synthesis of single-crystalline microporous ZSM-5 zeolite: the molar composition of precursor solution was 1.0 SiO2: 0.003 Al2O3:0.015 Na2O:0.450 TPAOH:9 H2O,and other operations were kept the same as above. The obtained sample was named as Z5-Micro and used for cmparison.
2.2. Catalyst characterization
X-ray diffraction (XRD) patterns were collected by the Phillips X’Pert3 Powder-17005730 diffractometer(Panalytical B.V.,Netherlands) equipped with monochromated Cu Kα radiation (40 kV,40 mA) in the 2θ range from 5° to 50°. Scanning electron microscopy (SEM) images were observed by the SU-8010 microscope(Hitachi Limited, Japan) at 15 kV. Transmission electron microscopy (TEM) images were observed by the HT-7700 Exalens at 120 kV(Hitachi Limited,Japan),equipped with X-ray energy spectrometer (4Be-98Cf). N2adsorption–desorption curves of the samples were measured by ASAP2020 analyzer (Micromeritics, USA).
Temperature-programmed desorption of ammonia (NH3-TPD)was measured by AutoChemⅡ 2920 (Micromeritics, USA),equipped with the thermal conductivity detector (TCD) used to detect and record the signal.The Brønsted and Lewis acidic properties of the samples were obtained from pyridine-adsorbed infrared(Py-IR)spectra using Nicolet iS50 Fourier Transform infrared spectrometer (Thermo Fisher Scientific’s, USA). The scanning range is 4000–650 cm-1, the resolution is 2 cm-1, and 32 imaging scans are performed.
2.3. Diffusion performance test of catalyst
The zero-length column technique [35] developed by Eic and Ruthven was used to determine the reciprocal of the apparent diffusion time(Deff/R2)ofn-heptane in the zeolites.Prior to the experiments,the samples were activated in pure He flow(10 ml∙min-1)at 200 °C for 12 h. Helium through the bubbling carried the probe molecule(n-heptane)into the sample chamber during adsorption.After 30 min, the adsorption ofn-heptane on the sample reached equilibrium. Then the gas flow was switched rapidly to purge the samples with pure He (60 ml∙min-1). In the meantime, open the chromatographic workstation to record the transient effluent concentration measured by a flame ionization detector (FID).
Under the experimental assumption of ZLC method, the diffusion of probe molecule in catalyst during desorption satisfies the following equation:
wherecandc0are the transient and initial effluent concentrations of the probe molecule (here isn-heptane, mol∙m-3);Deffis the apparent diffusivity (m2∙s-1);Ris the radius of the particle (m). βnin Eq. (1) can be obtained from the following equation:
where βnis eigenvalues of the diffusion equation;Lis dimensionless parameters;Fis the volumetric flow rate of carrier gas(m3∙s-1);Kis the dimensionless Henry law constant;VSis the volume of sample(m3).
In long time region, Eq. (1) can be simplified as:
Among them,Deff/R2can be obtained by mathematically solving the slope of ln (c/c0)vs. time.Deffis the result of the coupling of intracrystalline diffusion and surface mass transfer, which can be described by:
where α is the surface permeability(describing the surface barriers,m∙s-1);lis half of the length of the one-dimensional flat model(m);Dois the intracrystalline diffusivity (m2∙s-1). The equivalent length of spherical sample can be determined by [36]:
whereRis the radius of the spherical sample(m).In order to calculate α,the following assumptions are made:(1)As for zeolites with same topological structure and similar pore structure, their intracrystalline diffusivities are the same [37]. (2) To make further analysis,we assume that the surface barriers of Z5-200 are negligible. Although this assumption may not be based entirely on facts,but we hope to obtain comparative results of relative diffusion coefficients between different samples based on this assumption, and the magnitudes of the data results are also in good agreement with those reported in the literature[38].Due to the existence of surface barriers,the overall diffusion path will prolong,the additional diffusion path lengths(σ)can be used to describe the surface barriers.σ can be calculated by the following equation [36].
where τeffis the apparent diffusion time (s).
2.4. Catalytic tests
The catalytic cracking reaction was carried out in a fixed-bed reactor with inner diameter of 8 mm. Prior to the experiment,the catalyst sample was pressed and sieved to 0.250–0.425 mm,followed by mixing it with silica sand(mass ratio=1/2).The reaction trial conditions are as follows:n-heptane (GC,Aladdin)as the model reactant and steam (molar ratio = 1/2) were carried out by N2which volumetric flowrate is 10 ml∙min-1into reactor, separately.The reaction temperature was 600°C and the weight hourly space velocity(WHSV)of reactant was 3 h-1.The products analysis was carried out on GC-9790 gas chromatograph (FULI, China),equipped with KB-Al2O3/Na2SO4capillary column (50 m × 0.32 nm) and FID.
The turnover frequency (TOF) based on the per Brønsted acid site which is the active sites of the catalytic cracking ofnheptane is used to describe the activity of the catalyst.
where TOF is turnover frequency (s-1);XAis the conversion rate ofn-heptane (%);FAis the molar flowrate ofn-heptane (×10–3mol∙s-1);CBis the Brønsted acid amount (×10–3mol∙g-1);Mcatalystis the mass of catalyst (g).
3. Results and Discussion
3.1. Structure, morphology features and texture of catalyst
Fig. 1 is X-ray diffraction (XRD) patterns of Z5-Micro, Z5-200,Z5-140, and Z5-100. The results indicates that all the synthesized samples have five diffraction peaks at 2θ = 7.9°, 8.8°, 23.1°, 23.8°and 24.3°, which are characteristic peak of ZSM-5 zeolites with typical MFI-type framework structure(JCPDS 44-0003)[39].These confirm that all the samples are ZSM-5 zeolites.
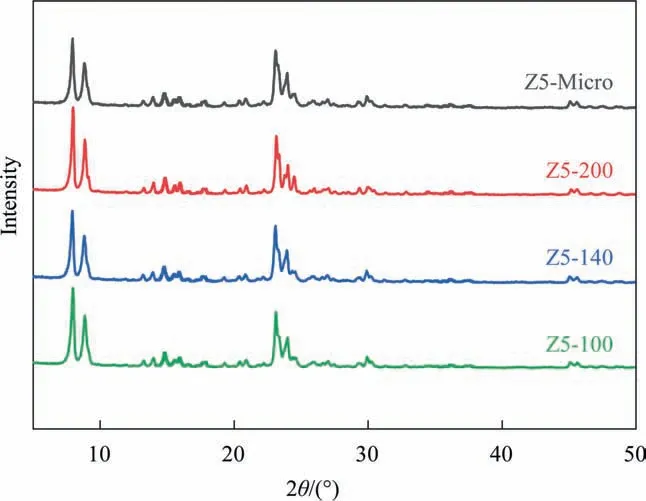
Fig. 1. XRD patterns of Z5-Micro, Z5-200, Z5-140, Z5-100.
As can be seen from SEM images(Fig.2),Z5-Micro exhibits regular cubic morphology with particle size ofca. 230 nm and no intracrystalline mesopores are observed. Comparing Z5-Micro and Z5-200, it demonstrates that the addition of L-lysine has an impact on the morphology of zeolite. Z5-200, Z5-140 and Z5-100 exhibit a relatively regular sphere in shape and coarse external surface.However,there are some differences in particle size.With the TPAOH/SiO2ratio increasing from 0.45 to 0.5, the particle size of Z5-200, Z5-140, Z5-100 decrease in turn accordingly. Typically, a higher concentration of organic structure directing agent will enhance the supersaturation degree of solution through enhancing the solubility of silicate ions and aluminate ions,which will accelerate the nucleation rate of the crystals [40]. With the identical addition of raw materials,more crystal nucleus will inevitably lead to the reduction of the particle size,which will promote the nanocrystallization of zeolite [41,42].

Fig.2. SEM images of(a)Z5-Micro,(c)Z5-200,(e)Z5-140,(g)Z5-100;inset:corresponding particle size distributions.TEM images of(b)Z5-Micro,(d)Z5-200,(f)Z5-140,(h)Z5-100; inset: corresponding FFT patterns.
The continuous orientation lattice fringe of four samples can be clearly seen from high-magnification TEM images (Fig. 2), indicating that the samples should be single crystal rather than stacked small particles [34]. Fourier transform diffraction (FFT) patterns show that all the samples are regular diffraction dot matrix,further confirming the single-crystalline structure of ZSM-5 zeolites.Therefore, the influence of grain boundary resistance on diffusion can be ruled out [20,33]. In addition, the low-magnification TEM images show the contrast of light and dark in Z5-200, Z5-140 and Z5-100, proving the coexistence of both micropores and intracrystalline mesopores [34], which can be further proved by physical adsorption.
Fig. 3 show N2adsorption–desorption isotherms and pore size distributions of samples respectively. Z5-Micro displays a typical type I isotherm according to the IUPAC classification,and the pores size is mainly around 2 nm, indicating that it is microporous zeolite. The isotherms of Z5-200, Z5-140 and Z5-100 are type IV, and H4-type hysteresis loops can be observed significantly, implying the presence of mesopore. At the same time, the intracrystalline mesopore diameters of Z5-200, Z5-140 and Z5-100 are centered at about 2.5 nm. Thus, these facts prove that the induction of Llysine in the synthesis affects the formation of mesopores and it can be used as a mesoporous template.
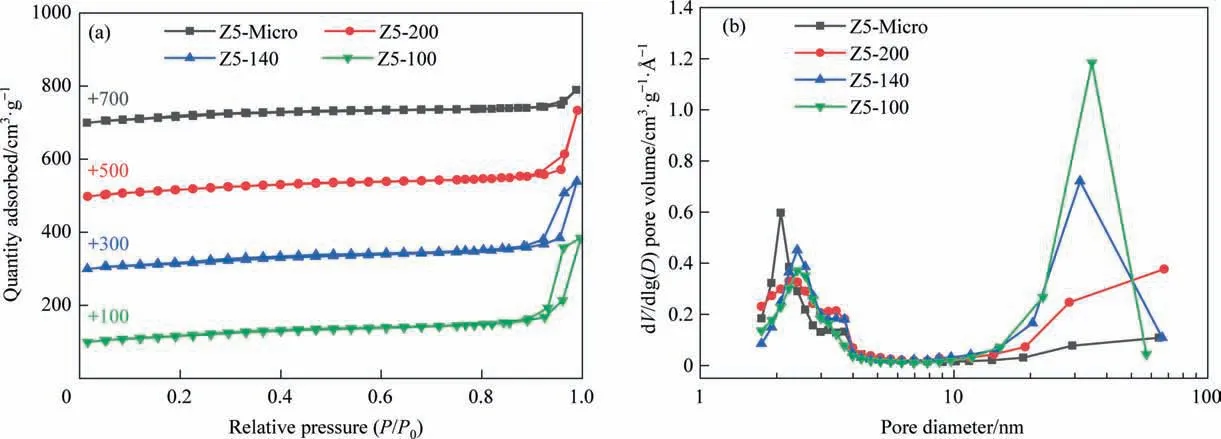
Fig. 3. (a) N2 adsorption–desorption isotherms and (b) pore size distributions of single-crystalline ZSM-5 zeolites (1Å = 0.1 nm).
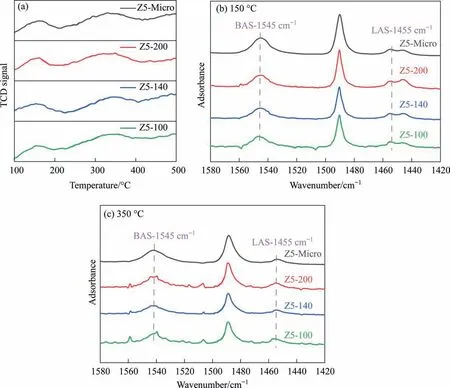
Fig. 4. (a) NH3-TPD curves, (b) 150 °C Py-IR spectra and (c) 350 °C Py-IR spectra of ZSM-5 zeolites.
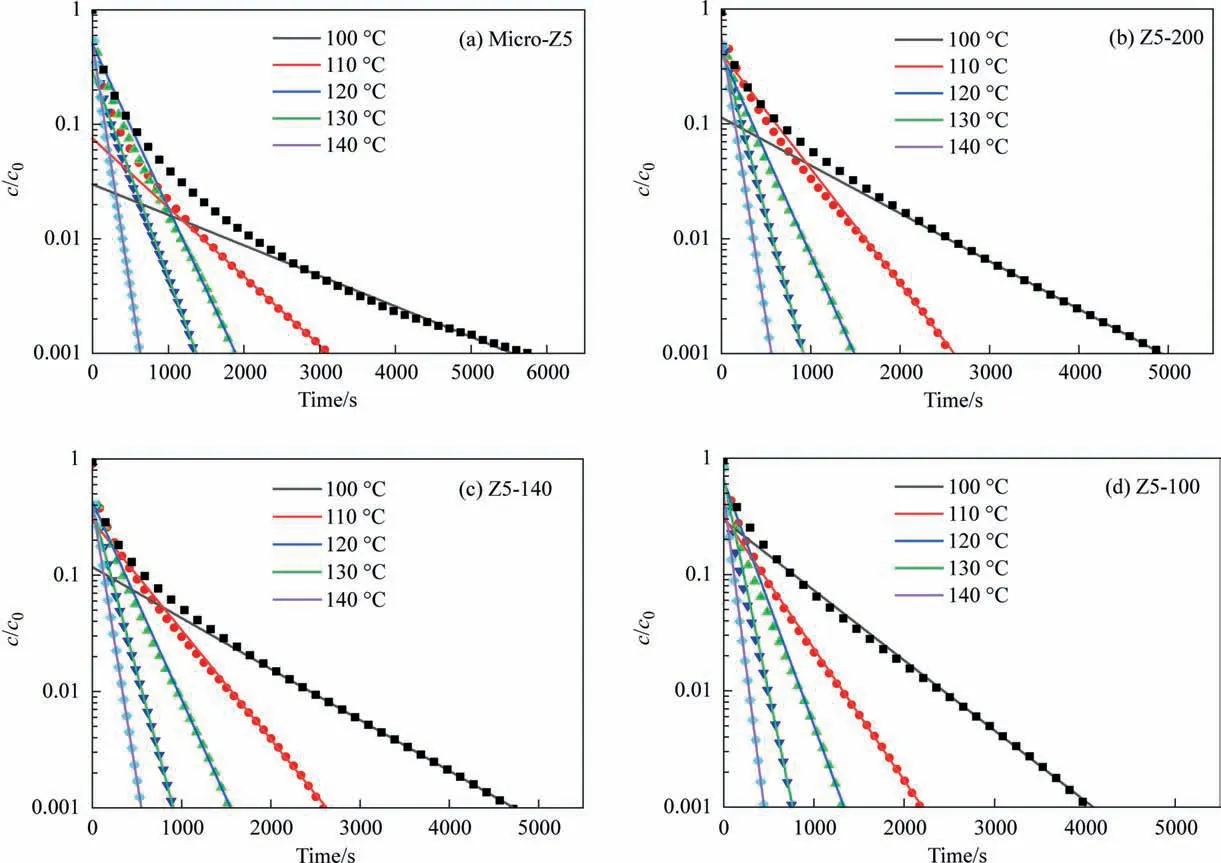
Fig.5. Desorption curves of n-heptane on(a)Z5-Micro,(b)Z5-200,(c)Z5-140,(d)Z5-100 measured by ZLC method;points represent experimental data,and the straight lines are obtained by fitting.
Table 1 lists the specific surface area and pore structure parameters of all samples.Comparing Z5-Micro,VMesoof Z5-200 increases significantly,further proving that L-lysine will promote the formation of mesopores. It is noticed thatSExtof Z5-200, Z5-140 and Z5-100 are basically unchanged. However,SBETandSMicrodecline,which may be due to the reduction of zeolite particle size and the disappearance of micropores near the mesopore surface.VMesohas increased to some extent from Z5-200, Z5-140 to Z5-100, this is because the reduction of the zeolite particle size, which do not affect the internal structure of the zeolites.Consequently,the three samples have similar internal meso-microscale pore structure.The research by Gaoet al. [36] and Remiet al. [37] showes that intracrystalline diffusivity is influenced by the crystal structure but not by the crystal size, thus we can consider that the three single-crystalline hierarchical ZSM-5 zeolites synthesized have the same intracrystalline diffusion properties.
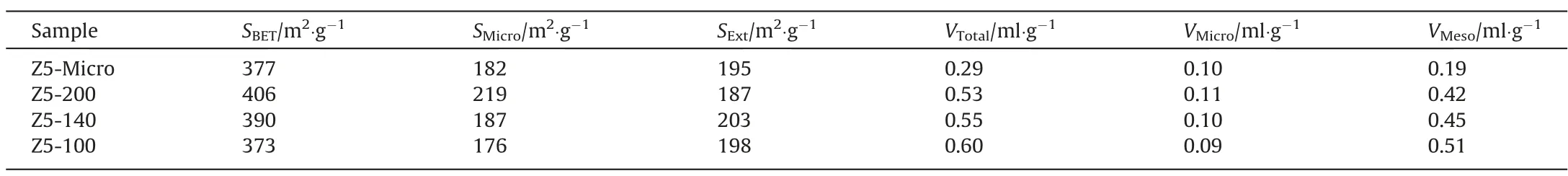
Table 1Specific surface area and pore structure parameters
3.2. Characterization of acid properties of catalyst
The acidic properties are shown in Fig.4(a)and the relevant calculation results are listed in Table 2.All samples have two obvious desorption peaks in NH3-TPD curves, the low temperature one about 155 °C and the high temperature one around 330 °C corresponds to the weak and the strong acid site of the zeolite respectively. Compared with that of Z5-Micro, the total acid amount of Z5-200 decreased by 14%. The total acid amount of Z5-200, Z5-140 and Z5-100 samples is 0.146, 0.138 and 0.135 mmol∙g-1,respectively. Although there is a slight difference in total acid amount, the three samples have almost same BAS amount(150 °C), and the difference in acid content is insignificant.
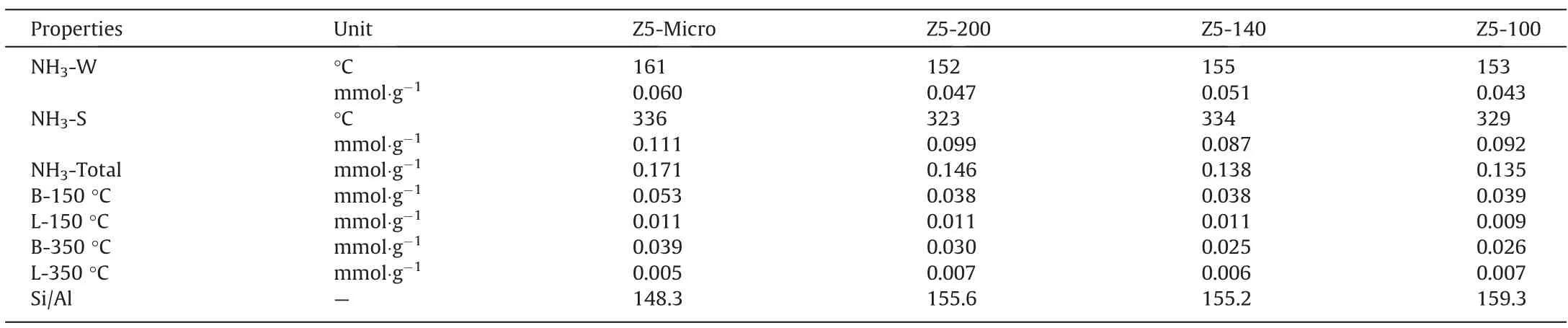
Table 2Calculation results of ZSM-5 by NH3-TPD, Py-IR and ICP
Fig.4(b)and(c)show the Py-IR spectra of all samples at 150°C and 350 °C, respectively. The characteristic absorption bands at 1455 cm-1and 1545 cm-1correspond to the Lewis acid sites(LAS) and Brønsted acid sites (BAS) of zeolite respectively. The BAS amount of Z5-Micro is obviously higher than that of other samples, that is because the coordination between L-lysine and Al ions [43]. The BAS amount of Z5-200, Z5-140, Z5-100 samples are 0.038, 0.038 and 0.039 individually at 150 °C, which are basically invariant, demonstrating that the levels of TPAOH will not change the amount of BAS.
Considering the characterization results of XRD,N2adsorption–desorption,SEM,TEM,NH3-TPD and Py-IR,it can be proved that we have successfully synthesized three single-crystalline hierarchical ZSM-5 with similar external morphology and internal pore structure, but different particle sizes. The synthesized samples have high crystallinity and narrow particle size distribution, which can be used for the next experimental comparison.
3.3. Diffusion performance evaluation of catalysts
ZLC method was adopted to measure the apparent diffusivities of probe molecule (i.e.,n-heptane) on ZSM-5 zeolites in order to reveal the influence of zeolites particle size on surface barriers.As shown in Fig. 5, after introducing mesopores in zeolites, the time for reducing relative concentration (c/c0) from 0.01 to 0.001 is shortened significantly. Compared with Z5-140 and Z5-100, it takes longer time forc/c0ofn-heptane on Z5-200 to decrease from 0.01 to 0.001,because of Z5-200 with a larger particle size has the longer diffusion path and the time required forn-heptane desorption is also longer.
Deff/R2ofn-heptane on Z5-Micro is smaller than other samples(Table 3),implying that mesopores can improve mass transfer.Deffofn-heptane on Z5-200 is greater than that on Z5-140 and Z5-100(Table 4).The mass transfer rate in pore of Z5-200,Z5-140,Z5-100 with identical porous structure is similar. Single-crystalline materials have no grain boundary resistance. The apparent diffusivities of the three samples should be similar,approximately equal to the intracrystalline diffusivity,but the actual results differ greatly.The abnormal phenomenon indicates the existence of surface barriers[32]. Surface barriers is affected by particle size and its influence on small particle zeolite is significant and cannot be ignored in the diffusion process.
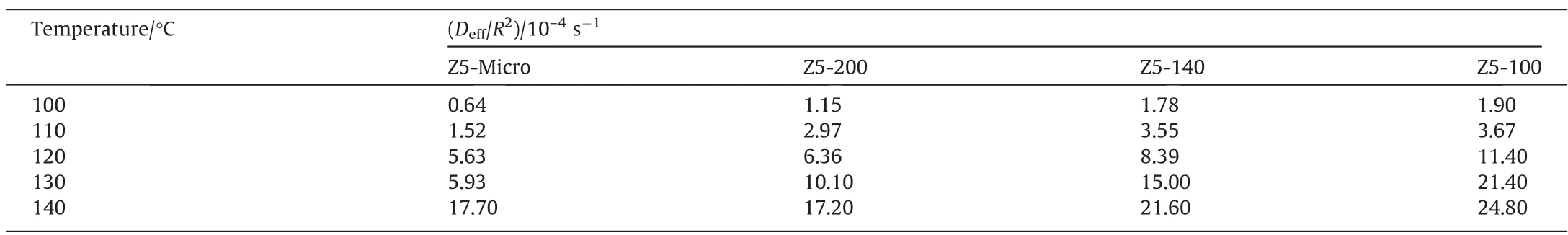
Table 3The reciprocal of the apparent diffusion time (Deff/R2) of n-heptane on single-crystalline ZSM-5 at different temperatures
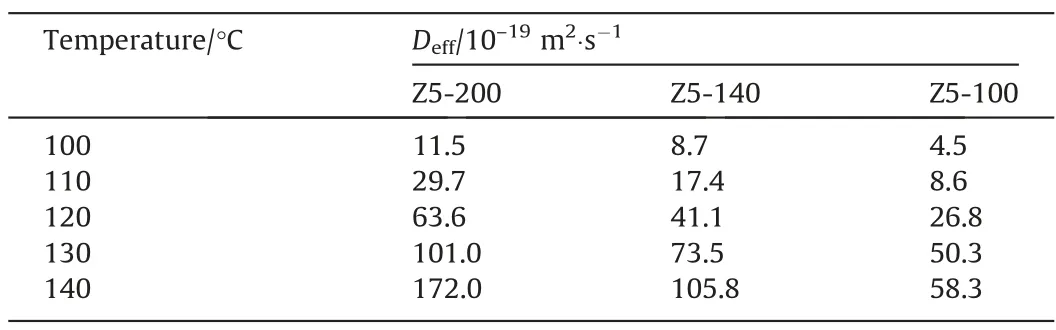
Table 4Apparent diffusivities of n-heptane on single-crystalline hierarchical ZSM-5 at different temperatures
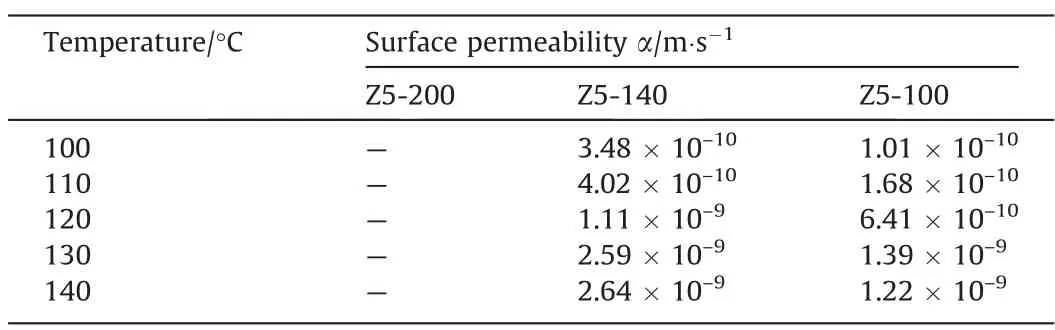
Table 5Surface permeabilities of single-crystalline hierarchical ZSM-5 zeolites
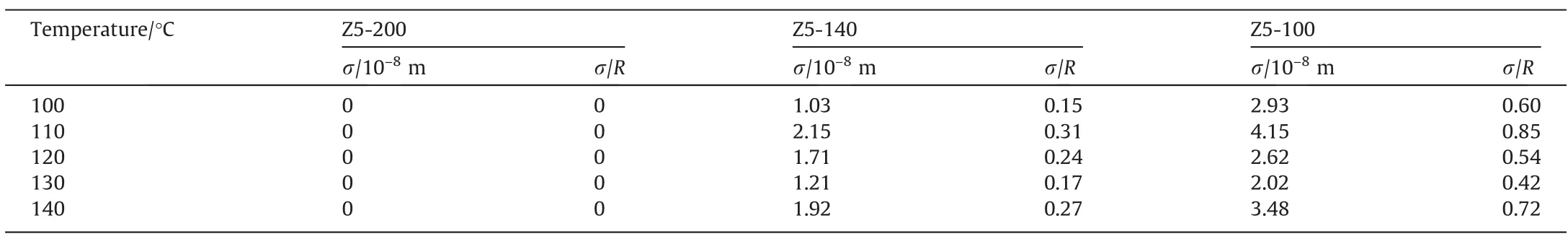
Table 6Additional diffusion path lengths (σ) of n-heptane in single-crystalline hierarchical ZSM-5 zeolites
In order to further explore the effect of particle size on the surface mass transfer of zeolites,the surface permeabilities and additional diffusion path lengths caused by surface barriers is calculated. The results are summarized in Tables 5 and 6 respectively. The calculated α based on this assumption is close to the value reported in the literature, which proves the rationality of the assumption [36,44].
With the decrease of particle size, the surface permeability decreases,which may be due to the extension of the diffusion path on the surface caused by factors such as surface pore blockages,surface pore narrowing and kinetic desorption limitation of small particle size zeolites [32]. BecauseDeffcouples the effect of intracrystalline diffusion and surface mass transfer [36,46,47], it is better to use σ to measure the relative value of surface barriers.The effect of surface barriers on diffusion process can be directly determined by σ/R(dimensionless parameter). When the temperature is in the range of 100–140°C,the dimensionless characteristic diffusion length of Z5-140 is between 0.15–0.31 and Z5–100 is between 0.42–0.85. This proves that the surface barriers have a more significant effect on the diffusion ofn-heptane in singlecrystalline hierarchical ZSM-5 with a small particle size. To sum up, as shown in Fig. 6, when the surface permeability of zeolite with bigger particle size is assumed to be infinite, its additional characteristic diffusion length is zero, thus ignores the influence of surface barriers for the moment. With the decrease of particle size, the additional characteristic diffusion length increases, indicating the proportion of surface mass transfer in diffusion increases.
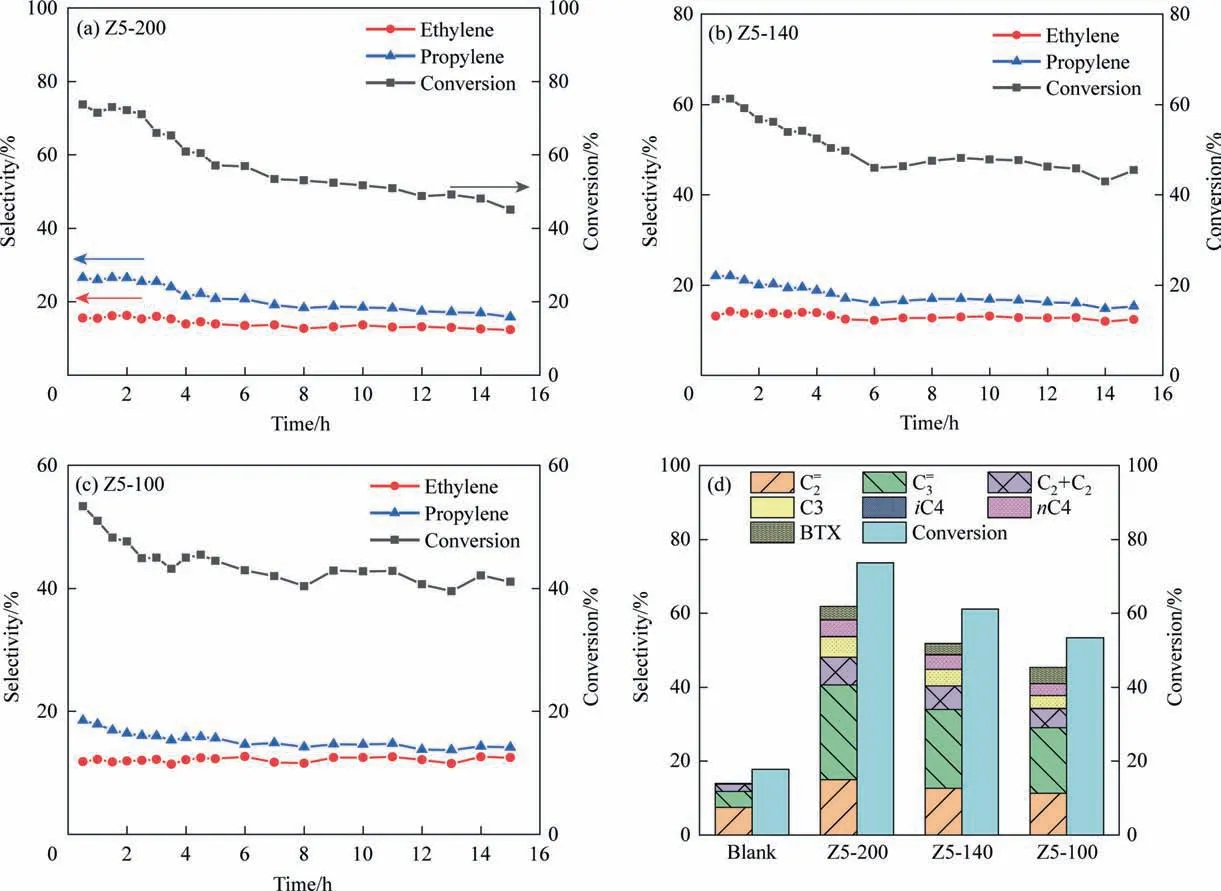
Fig. 7. The stability evaluation of (a) Z5-200, (b) Z5-140 and (c) Z5-100 at 600 °C; (d) Conversion of n-heptane and product distribution of the samples at 600 °C, t = 1 h,WHSV = 3 h-1.
3.4. Catalyst performance evaluation
It can be found that the initial conversion ofn-heptane and the selectivity of ethylene and propylene of Z5-200 sample are the best(Fig.7).This is mainly due to the larger surface permeability of Z5-200,and reactant molecules can reach the active site more quickly through the surface, so its initial conversion rate is higher. After reaction for 15 h, the conversion ofn-heptane on Z5-200, Z5-140 and Z5-100 decreased by 38.9%,25.5%and 23.0%,because the high exposure of active site. However, at this time, there is not much difference between the conversion ofn-heptane and the selectivity of ethylene and propylene.The catalytic stability of Z5-100 is best,because as increasing reaction time, coke compounds first deposit on the active sites in the outer layer,and the diffusion path of reactant molecules reaching the inner layer active site is extended,resulting in the reduction of activity [48,49]. Total diffusion path(including intracrystalline and additional diffusion path length,R+σ) of small particle size zeolite is short. Although the proportion of surface mass transfer in the overall diffusion is increased,intracrystalline diffusion still dominates, so the catalytic stability of small particle size zeolite is higher.
The TOF was calculated according to the amount of BAS (Py-H+(150 °C)), in order to eliminate the impact of slight differences in BAS amount (Fig. 8). The apparent reaction activation energy (Ea)of Z5-Micro is lower 12–23 kJ∙mol-1than that of hierarchical zeolites, suggesting the existence of intracrystalline diffusion limitation.Eaincreases in turn with the decrease of particle size,indicating that smaller particle size zeolite is less affected by diffusion and closer to the intrinsic diffusion. It should be pointed out that the apparent reaction activation energy calculated by this method is coupled with the influence of surface mass transfer and intracrystalline diffusion.
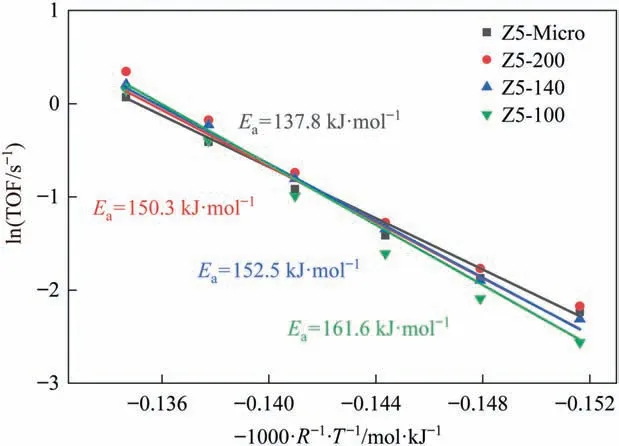
Fig. 8. Arrhenius plots of TOF per BAS for the ZSM-5 samples, WHSV = 100 h-1.
4. Conclusions
The single-crystalline hierarchical ZSM-5 with different particle sizes(100,140,200 nm)was successfully synthesized in this work.The apparent diffusivities and surface permeability ofn-heptane of three ZSM-5 samples are compared and analyzed to explore the effect of particle size on surface mass transfer and catalytic performance. The results show that when the particle size decreases from 200 to 100 nm, the apparent diffusivity decreases by more than one order of magnitude,which proves the existence of surface barriers. As the decrease of particle size, the additional diffusion path length increases, the proportion of surface mass transfer in diffusion as well. But intracrystalline diffusion still predominates in the overall diffusion. Therefore, single-crystalline hierarchical ZSM-5 with smaller particle size has better catalytic stability. The above factors should be comprehensively considered in the industrial production process.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The support from the National Natural Science Foundation of China (22278353) is greatly appreciated.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
