Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
Yuling Xie, Qilin Gu, Qian Jiang, Zhaoxiang Zhong,2,, Weihong Xing,
1 National Engineering Research Center for Special Separation Membrane, State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemical Engineering,Nanjing Tech University, Nanjing 210009, China
2 School of Environmental Science and Engineering, Nanjing Tech University, Nanjing 211816, China
Keywords: SiC membrane Sintering additive Reaction sintering Microstructure Oil-in-water emulsions
ABSTRACT Sodium-contained compounds are promising sintering additives for the low-temperature preparation of reaction bonded SiC membranes. Although sodium-based sintering additives in various original states were attempted, their effects on microstructure and surface properties have rarely been studied. In this work,three types of sodium-based additives,including solid-state NaA zeolite residue (NaA)and liquidstate dodecylbenzene sulfonate (SDBS) and water glass (WG), were separately adopted to prepare SiC membranes,and the microstructure,surface characteristics and filtration performance of these SiC membranes were comparatively studied. Results showed that the SiC membranes prepared with liquid-state SDBS and WG(S-SDBS and S-WG)showed lower open porosity yet higher bending strength compared to those prepared with solid-state NaA(S-NaA).The observed differences in bending strength were further interpreted by analyzing the reaction process of each sintering additive and the composition of the bonding phase in the reaction bonded SiC membranes. Meanwhile, the microstructural differentiation was correlated to the original state of the additives.In addition,their surface characteristics and filtration performance for oil-in-water emulsion were examined and correlated to the membrane microstructure.The S-NaA samples showed higher hydrophilicity, lower surface roughness (1.80 μm) and higher rejection ratio (99.99%) in O/W emulsion separation than those of S-WG and S-SDBS. This can be attributed to the smaller mean pore size and higher open porosity, resulting from the originally solid-state NaA additives. Therefore, this work revealed the comprehensive effects of original state of sintering additives on the prepared SiC membranes,which could be helpful for the application-oriented fabrication by choosing additives in suitable state.
1. Introduction
The treatment of oily wastewater,particularly those containing stable emulsified oil, has long been a focus in the field of water treatment[1–3].Due to the obvious advantages of low energy consumption, non-pollution and high separation efficiency,the membrane separation technology has been regarded as a vital pathway for oily wastewater treatment [4–6]. Porous ceramic membranes(such as Al2O3,ZrO2and SiC)with high mechanical strength,chemical resistance and outstanding hydrophilic properties have been developed as a competitive material for membrane separation technology [7–11].
Porous silicon carbide (SiC) ceramic membranes own superior temperature stability,chemical resistance and strong electronegativity and hydrophilicity [12], and thus have gained increasing attention in oily wastewater treatment.Low-temperature sintering of porous SiC ceramic membrane is of great economic and scientific significance. Recently, reactive sintering processes have been developed for preparing SiC membrane[13–15],which can greatly reduce the sintering temperature of SiC compared with the traditional recrystallization method in an inert atmosphere [16,17].Reactive sintering is achieved by adding sintering additives, that can melt and react with the SiO2formed by the surface oxidation of SiC at low temperatures to form a new bonding phase between the SiC particles,thus enabling SiC membranes to be prepared at a lower temperature [18–20]. Materials like kaolin clay [21], quartz sand [22], and metal oxides such as Al2O3[23], CaO [24], Y2O3[25], and ZrO2[26] have been commonly used as sintering additives for the preparation of reaction bonded SiC membranes.
Different types of sintering additives have been attempted to prepare porous SiC membranes at lowered temperatures,with various bonding phases such as mullite [23,27,28], cordierite [29,30]and sodium silicates [31]. Among them, sodium salts as extra flux components can be used to effectively reduce the sintering temperature of porous ceramics [32]. By adding Na-based sintering additives, SiC porous ceramics have been prepared at lower temperatures. For example, macroporous SiC supports with coarse SiC particles(D50=100 μm)can be prepared at 1150°C when SDBS and ZrO2were introduced [33], in which SDBS decomposed into Na2O which reacted with SiO2and ZrO2to form connecting necks.The bending strength of SiC supports gradually increased with the sintering temperature in the range of 1000–1250°C.Alternatively,NaA zeolite residue composed of Na, Al, Si and O elements were used for the preparation of SiC membranes and supports at 1000 °C and 1200 °C [34,35]. Moreover, the sintering temperature of SiC membranes can be reduced to 600°C by loading water glass and zirconia. When SiC powder was mixed with the combined additives, a new phase sodium zirconium silicate (Na2ZrSiO5)was obtained [36]. It was also observed that the bending strength greatly increased with the sintering temperature from 600 °C to 900 °C while remained almost unchanged above 900 °C.
Therefore,sodium-contained compounds such as sodium dodecylbenzene sulfonate (SDBS), water glass (WG) and NaA are promising sintering additives for low-temperature preparation of SiC membranes. Previous works on reaction bonded SiC membranes mainly focused on the effect of sintering temperature and content of each sodium-based sintering additive on the microstructure and mechanical strength, in order to achieve the low temperature preparation, while the existed differences between these SiC membranes in microstructure and properties have rarely been studied. The microstructure of ceramic membrane can also be influenced by the initial state of sintering additives. For example, some researchers have introduced watersoluble nitrates in the form of solutions to act as liquid-state sintering additives [37,38]. During the calcination process, the nitrates would transform corresponding oxides, which are much smaller than the commercial oxide powder.Thesein situgenerated small particles can be well dispersed in SiC powder, which could promote the sintering process.This stimulates our concern on differentiating the microstructural evolution in reaction bonded SiC membranes prepared with varied types of sintering additives.Although sintering additives in liquid-state (e.g.SDBS and WG)and solid-state (e.g.metal oxide) have been widely utilized to reduce the processing temperature of ceramic materials[33,34,36], the influence of their original state on the microstructure of the resulted ceramics, to our best known, has never been detailed.
In this work, solid-state NaA zeolite residue, and liquid-state SDBS and WG as a family of sodium-based additives were separately utilized to prepare reaction bonded SiC membranes. Different from the previous reports [33,34,36], this work focused on a comparable study on these SiC membranes, and as an example,the effect of original state of sintering additives on the microstructure of SiC membranes were emphasized. To understand the observed differences, the reaction sintering process was studied by the microstructure at different sintering temperature, and the roles of sintering additives were demonstrated by looking into the bonding phases.The surface characteristics and separation performance for the synthetic O/W emulsion at a low transmembrane pressure (0.2 bar, 1 bar = 0.1 MPa) were further evaluated.
2. Experimental
2.1. Raw materials
Green α-SiC powder with an average particle size of about 5 μm(Nantong Yuda Silicon Carbide Co., LTD, China.) was used as the raw material. Liquid-state sodium dodecylbenzene sulfonate(SDBS, purity ≥99.5%, Lingfeng Chemical Reagent Co., LTD, China)and water glass (WG, industrial grade, Shanghai Chuiheng Industrial Co., LTD, China) and solid-state NaA residue (Jiangsu Jiutian High-tech Co., LTD, China) were selected as the sodium-based sintering additives. Among them, the NaA residue (Jiangsu Jiutian High-tech Co.,LTD.)was collected from the waste liquid in the production line of the NaA zeolite membrane. Zirconia nanoparticles(ZrO2, 99%, Shanghai Macklin Biochemical Technology Co., LTD,China)were used as the source of zirconium and polyvinyl alcohol(PVA,Mw= 74800, Sinopharm Group Chemical Reagent Co., LTD,China)was used as the binder.Lubricant oil(Zhejiang Shell Chemical Petroleum Co., LTD, China) was the oil phase, and Span 80(1/10 mass of the oil phase) was added as the surfactant.
2.2. Preparation of SiC membranes
Three types of SiC membranes were prepared by using SiC powder(D50=5.0 μm)and different sintering additives(solid NaA,and liquid-state WG and SDBS). The recipe and preparation procedure of SiC membranes were adapted according to the previous works[33,34,36]. Similarly, when liquid-state SDBS and WG were used,10% (mass) of ZrO2nanoparticles were added, while previous works using SDBS as the additive were for the preparation of microporous supports with coarse SiC powder[26,33,39].The composition of the mixtures is shown in Table 1. In brief, SiC powder and the corresponding sintering additives were fully ball-milled for 2 h at a speed of 200 r∙min-1. Then, 5% (mass) PVA aqueous solution (8% (mass)) was added to the solid mixture to granulate the particles. Next, the mixtures were molded into discs (φ30 mm× 2 mm)and strips (50 mm× 6 mm× 4 mm)under an axial pressure of 8 MPa. After that, these green bodies were sintered in air with a ramping rate of 2°C∙min-1and held at different temperatures(900–1100°C)for 2 h.Once the sintering process was completed, the samples were gradually cooled to room temperature.

Table 1Starting material composition of different samples
2.3. Membrane characterizations
The phase composition of the membranes was characterized by using X-ray diffractometer(XRD,MiniFlex 600,Rigaku,Japan).The micromorphology and the mapping analysis of the samples were observed by using a scanning electron microscope (SEM, S-4800 Hitachi, Japan). Thermogravimetric analyzer (TG-DSC, Netzsch STA449F3,Germany)was used for the thermogravimetric analysis of the powder.The contact angle of water(3 μl deionized water)or oil droplets(3 μl lubricant oil)on the membrane surface was measured with a contact angle device (SCA20, Dataphysics, Germany).The SiC membrane surface roughness was analyzed by a highprecision surface profiler (VK1000, Keyence, Japan).
The bending strength of ceramic membranes was measured by a universal material testing machine (DR028G-1000, China)through the three-point flexural strength method.The open porosity of porous SiC ceramics was determined by the Archimedes drainage method. The specific method is described in the Supplementary Material. The mean pore size and pore size distribution of porous SiC ceramic membranes were measured by the bubble pressure method with a pore size analyzer (IPORE-1500AEX-Clam, PMI, America).
2.4. O/W emulsions separation
A stable oil-in-water emulsion with a concentration of 500 mg∙L–1was prepared by stirring the lubricating oil and Span 80 in water at 2000 r∙min-1for 15 min by using a high-shear emulsification machine (FM300, Fluko, China). No obvious demulsification or stratification was observed after the emulsion was stationary at room temperature for 72 h. The size distribution of oil droplets in O/W emulsion was measured using a dynamic light scattering(DLS,ZS-90,Malvern,UK).The size distribution of the oil droplets was shown in Fig.S1.The concentration of the oil in the O/W emulsion was converted from the absorbance determined by using a UV–visible spectrophotometer (UV-2600i, Shimadzu,Japan).
The filtration experiments were conducted by using a self-made cross flow setup (Fig. S2). The pure water flux and the permeate flux during the separation of O/W emulsion(F,L∙m-2∙h-1)and permeance(Q, L∙m-2∙h-1∙bar-1) were calculated by using Eqs.(1) and(2):
whereV(L) is the volume change of the permeating solution at a fixed time Δt(h), ΔP(MPa) is the transmembrane pressure, andA(m2) is the effective membrane area.
The oil rejection ratio(R)of the membrane was calculated using Eq. (3):
whereCfandCpare the oil concentrations (mg∙L–1) of the feed and filtrate solution, respectively.
3. Results and Discussion
3.1. Effects of sintering additives
Fig.1 shows the effect of sintering additives on the open porosity and bending strength of porous SiC ceramic membranes prepared at 1000 °C. As can be seen, the results of S-WG and S-NaA are similar to those reported in previous work [34,36]. With the content of sintering additives increasing from 6% (mass) to 14%(mass), the open porosity of S-NaA decreased slightly from 48%to 47%,while those of S-SDBS and S-WG dropped significantly from 44% to 36%. The bending strength of all types of ceramics membrane gradually increased with the content of sintering additives(Fig. 1(b)). Comparatively, the SiC membranes prepared with NaA residue showed the highest open porosity and the lowest bending strength, while those prepared with WG and SDBS showed a relatively close value in both open porosity and bending strength.The results can be explained by the Rice formula[40],where the bending strength of porous ceramics is negatively correlated to the open porosity.
The changes in mean pore size and pure water permeance with the content of sintering additives are shown in Fig. 1(c) and (d).The mean pore size of S-NaA and S-SDBS was relatively stabilized at 0.50 μm and 0.65 μm, while that of S-WG increased from 0.65 μm to 0.8 μm when the content above 10% (mass) (Fig. 1(c)).In Fig.1(d),it can be observed that the pure water permeance of all ceramic membranes showed a gradual increase followed by a decline with the increasing content of sintering additives. The SiC membranes showed a highest pure water permeance of 4266°L∙m-2∙h-1∙MPa-1, 4234° L∙m-2∙h-1∙MPa-1and 4993° L∙m-2∙h-1∙MPa-1when the content of SDBS, NaA, and WG were 8% (mass),12% (mass), and 12% (mass), respectively. The optimized contents of additives NaA and WG were in an agreement with those previously reported [34,36]. The increase in water permeance can be attributed to the formation and enrichment of neck connections,resulting in the stretching of the pores in SiC membranes. According to the H-P equation [41], the pure water permeance of the membrane can be influenced by both pore size and porosity.However, once the content of sintering additives was above a critical value, it occupies more space between particles, leading to increased densification between particles and decreased open porosity. As a result, the reduction in open porosity result in a notable reduction water permeance When the content of NaA was increased from 12% (mass) to 14% (mass), there observed a slight variation of porosity yet an obvious decreased pore size,and the pure water flux of S-NaA was thus dropped. Therefore,the highest pure water flux was observed at 12% (mass) of NaA additives.
We assume that such a difference in pore structure was related to the original state of the sintering additives.The pore structure of SiC membranes prepared using solid-state S-NaA was formed by the accumulation of SiC together with NaA particles during the molding and sintering process. On the contrary, the liquid-state WG and SDBS could uniformly distributed in SiC matrix and easily migrate towards the connection between the SiC particles due to their good fluidity. In the sintering process, the grains grew at the neck through the dissolution-reprecipitation mechanism[42,43].The gradual migration of liquid phase to the neck resulted in relatively large pores.Besides,SDBS would decompose with the increase in temperature, thereby leaving additional pores [44].Namely, SDBS to some degree served as a pore-forming agent.Therefore, the pore size of these two membranes prepared with liquid-state sintering additives (i.e., SDBS and WG) were larger than that prepared using solid-state NaA additives.
To verify the assumption,representative surface SEM images of the porous ceramic membranes with different contents of sintering additive are shown in Fig. 2. The trace of viscous flow state can be witnessed in both surface and cross-sectional SEM images (Figs. 2 and S3) of samples S-SDBS and S-WG, suggesting the liquid-phase sintering feature. Notably, a significant densification phenomenon was observed in S-SDBS when the content of sintering additives was increased to 14% (mass). In contrast, the samples S-NaA remained the rather porous microstructure. The observed differences in microstructure can explain the remarkable decrease in open porosity of S-SDBS and S-WG (Fig. 1(a)), where the enriched molten liquid phase derived from SDBS and WG gradually occupied these open pores.On the contrary,the sintering additives NaA produced a relatively rigid microstructure, and thus stabilized the open porosity at a relatively high level.
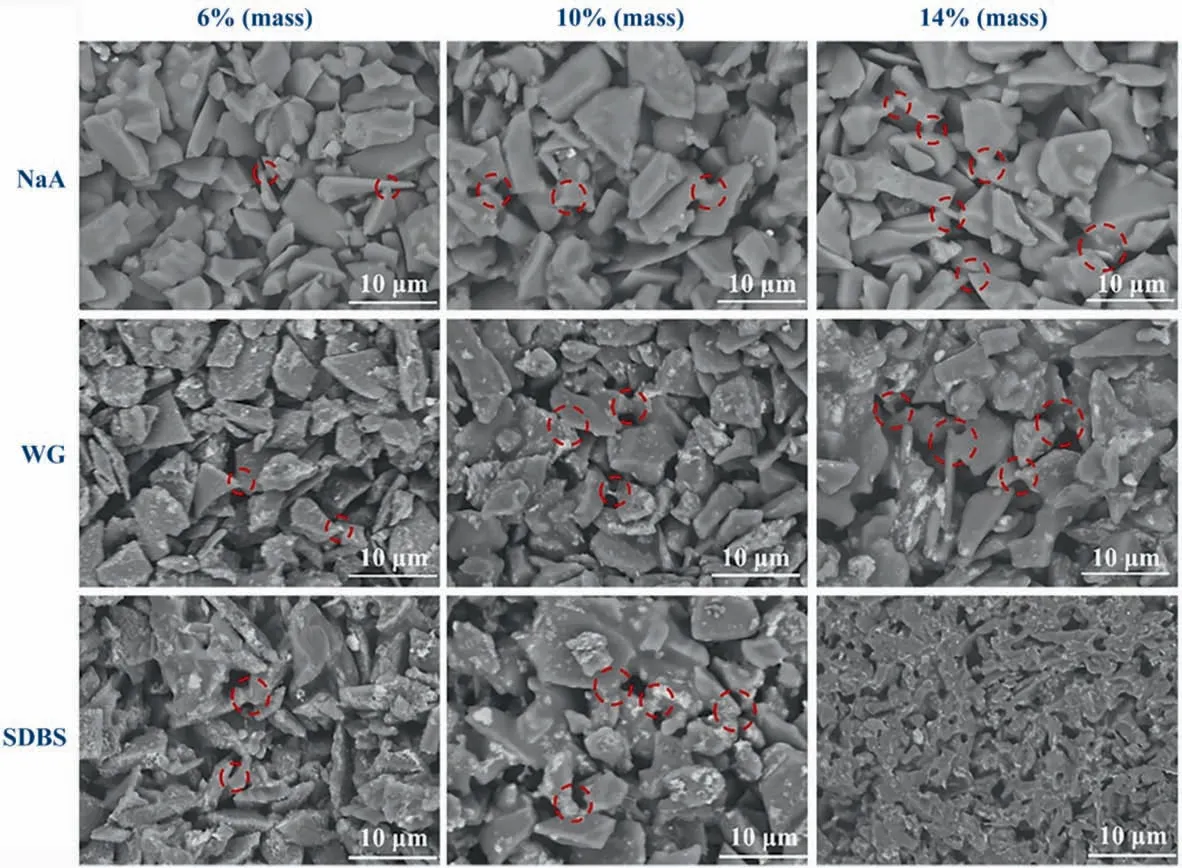
Fig. 2. Surface SEM images of porous SiC membranes prepared at 1000 °C using different additives with different contents.
In addition to the population, the phase composition of the bonding phase would significantly affect the physical and surface chemical characteristics of reaction-bonded SiC membranes [45].XRD patterns of SiC membranes, namely, S-WG (12% (mass)WG + 10% (mass) ZrO2+ 78% (mass) SiC), S-SDBS (8% (mass)SDBS + 10% (mass) ZrO2+ 82% (mass) SiC) and S-NaA (12% (mass)NaA+ 88%(mass) SiC)prepared at 1000 °C are presented in Fig. 3.In addition to the characteristic peaks of SiC and SiO2produced by the surface oxidation in air, the signature of nepheline (NaAlSiO4)was observed in the XRD pattern of S-NaA, and both sodium zirconium silicate(SZS)and zirconium silicate(ZrSiO4)phase were co-existed in S-SDBS and S-WG. Given that the intrinsically high hardness of sodium zirconium silicate[46,47],could have thus also contributed to the high bending strength of S-WG.
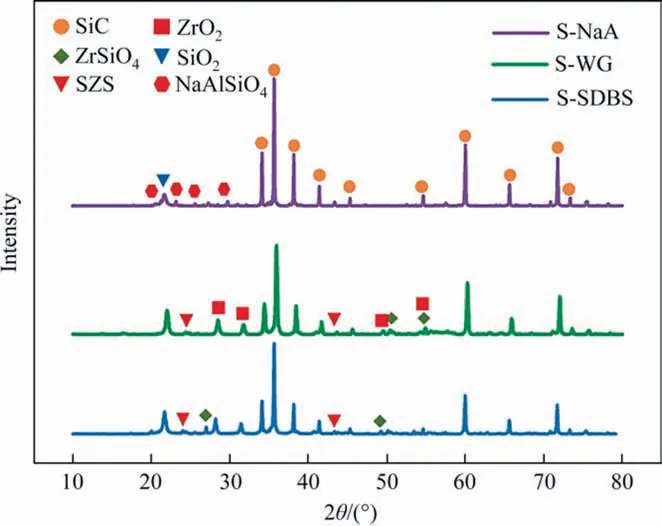
Fig. 3. XRD patterns of different types of SiC membranes prepared at 1000 °C.
3.2. Microstructural evolution
To understand the observed differences in porous microstructure, the effect of sintering temperature on the properties of these SiC ceramic membrane was further examined, where the contents of SDBS,WG and NaA were thus fixed at 8%(mass),12%(mass)and 12% (mass), respectively. Fig. 4 depicts the mean pore size, open porosity and bending strength of the different membranes prepared at different sintering temperatures. As the temperature increased from 900 °C to 1100 °C, the mean pore size of the SNaA changed negligibly(Fig.4(a)).As can be seen in Fig.2,the morphology of S-NaA remained almost the same,and only a small population of connecting necks was formed even at 1000 °C, mainly due to the poor viscous fluidity of NaA residue. On the contrary,the mean pore size of S-WG and S-SDBS increased gradually with the increasing sintering temperature and decreased slightly after reaching the peak value. In the liquid phase sintering process, the increase in temperature promoted the migration of liquid-state sintering additives to the neck,and the pores were thus stretched,resulting in the enlargement of pore size. However, a higher temperature would also lead to densification and thus reduce the average pore size.
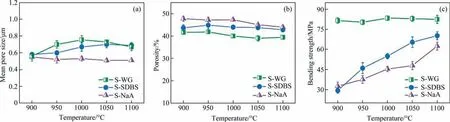
Fig.4. (a)Mean pore size,(b)porosity and(c)bending strength of SiC membranes prepared at different temperatures using solid-state NaA and liquid-state SDBS and WG as the additives.
When the temperature increased from 900 °C to 1100 °C, the open porosity of all types of SiC membranes slightly decreased and always kept the order of S-NaA > S-SDBS > S-WG (Fig. 4(b)).The bending strength of the S-SDBS and S-NaA membranes was improved from 30 MPa and 32 MPa to 70 MPa and 65 MPa while that of S-WG membranes was kept at about 80 MPa (Fig. 4(c)).The increased temperature promoted the surface oxidation of SiC to form SiO2and enabled the sintering additives that dispersed in SiC powders matrix migrate to the connecting points and react with the SiO2,so that the formation of connecting necks was stimulated. The increased population of the bonding phase resulted in the improved bending strength and reduced open porosity to a certain extent. An interesting phenomenon was that the bending strength of S-WG only fluctuated slightly in the studied temperature range. According to the previous work [36], SiC membranes can be prepared at 600°C when WG was introduced.It is therefore deduced that WG as a liquid sintering additive has been completely accumulated at the connections between SiC particles at 900 °C, and there was no significant change in bending strength with the further increase in temperature.
Further, the thermal behaviors of three types of green bodies were analyzed, and SiC + ZrO2mixture was included for comparison (Fig. 5(a)). It can be observed that, the SiC + ZrO2mixture did not show any obvious mass rising below 1000 °C, while the green bodies that contain Na-based additives presented a notable rise in mass. Especially, when WG was used as the sintering additive, the oxidation weight gain temperature was obviously decreased to 626°C.This can be explained by the viscous flow feature of WG, which can be more easily dispersed and evenly decorated on SiC particles (Fig. 5(b)). The decorated WG would change to liquid state at high temperature and then wrapped on the SiC particles, which can accelerate the mass transport of oxygen on its surface at high temperature [48], thereby enabling the passive oxidation of SiC at a relatively lower temperature.Therefore, the bending strength of S-WG was significantly higher and showed no obvious changes with the sintering temperature(Fig. 4(c)), and its bending strength was up to 80 MPa at 900 °C.In the green body of S-SDBS, SiC particles were well wrapped by SDBS (Fig. 5(d)), and the mass started to quickly increase above 866 °C. Correspondingly, the bending strength improved more quickly afterwards (Fig. 4(c)). For S-NaA samples, the mass rising was initialized at 792°C.Although the incorporation of solid oxides can also promote the passive oxidation process,their initial contact with the SiC particles were poor than WG and SDBS (Fig. 5).
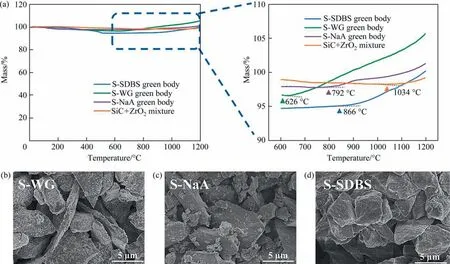
Fig. 5. (a)TGA curves of green bodies and the powder mixture of SiC + ZrO2, and SEM images of green bodies of (b) S-WG, (c) S-NaA and (d) S-SDBS.
The pore structure was largely formed by the stacking of SiC particles,while the neck connection was generated byin-situreaction between sintering additives(or their derivatives)and the SiO2generated by SiC surface oxidation. Therefore, the phase composition of each sintering additive after calcination at different temperatures were studied (Fig. 6). NaA residue started the transformation to Nepheline(NaAlSiO4)at 700°C and the transformation was completed at 800 °C [49] (Fig. 6(a)). For WG, it was mainly composed of Na2Si2O5at 600°C.When WG and ZrO2coexisted, the peak of sodium zirconium silicate (SZS) was firstly detected at 900 °C, and the intensity increased significantly at 1100 °C, suggesting that the reaction between silica, zirconia and sodium oxide was promoted (Fig. 6(b)). At 600 °C, the sintering additive SDBS decomposed to Na2O and Na2SO4. When SDBS was sintered together with ZrO2above 700°C,only the diffraction peak of ZrO2can be detected due to the complete decomposition of SDBS(Fig. 6(c)).
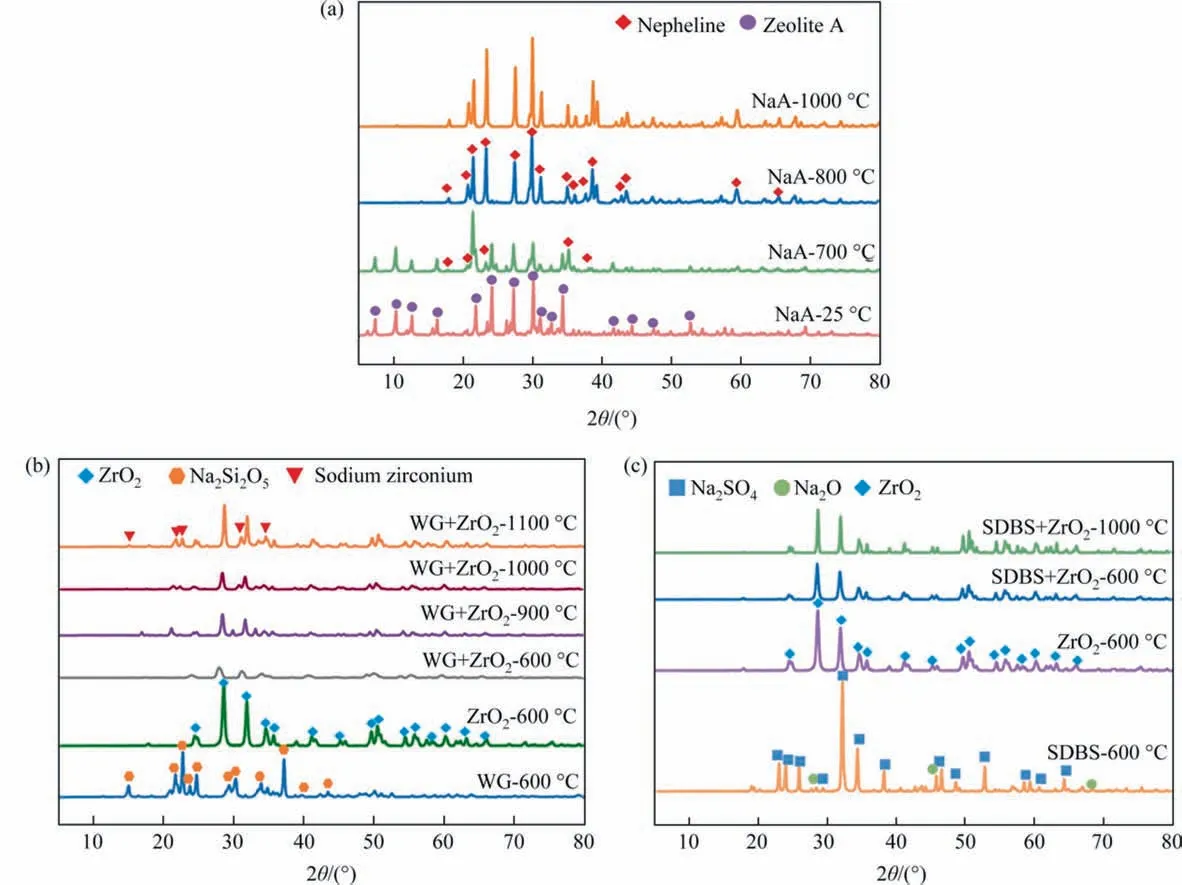
Fig. 6. XRD patterns of various sodium-based sintering additives after calcination at various temperature for 2 h: (a) NaA, (b) WG + ZrO2 and (c) SDBS + ZrO2.
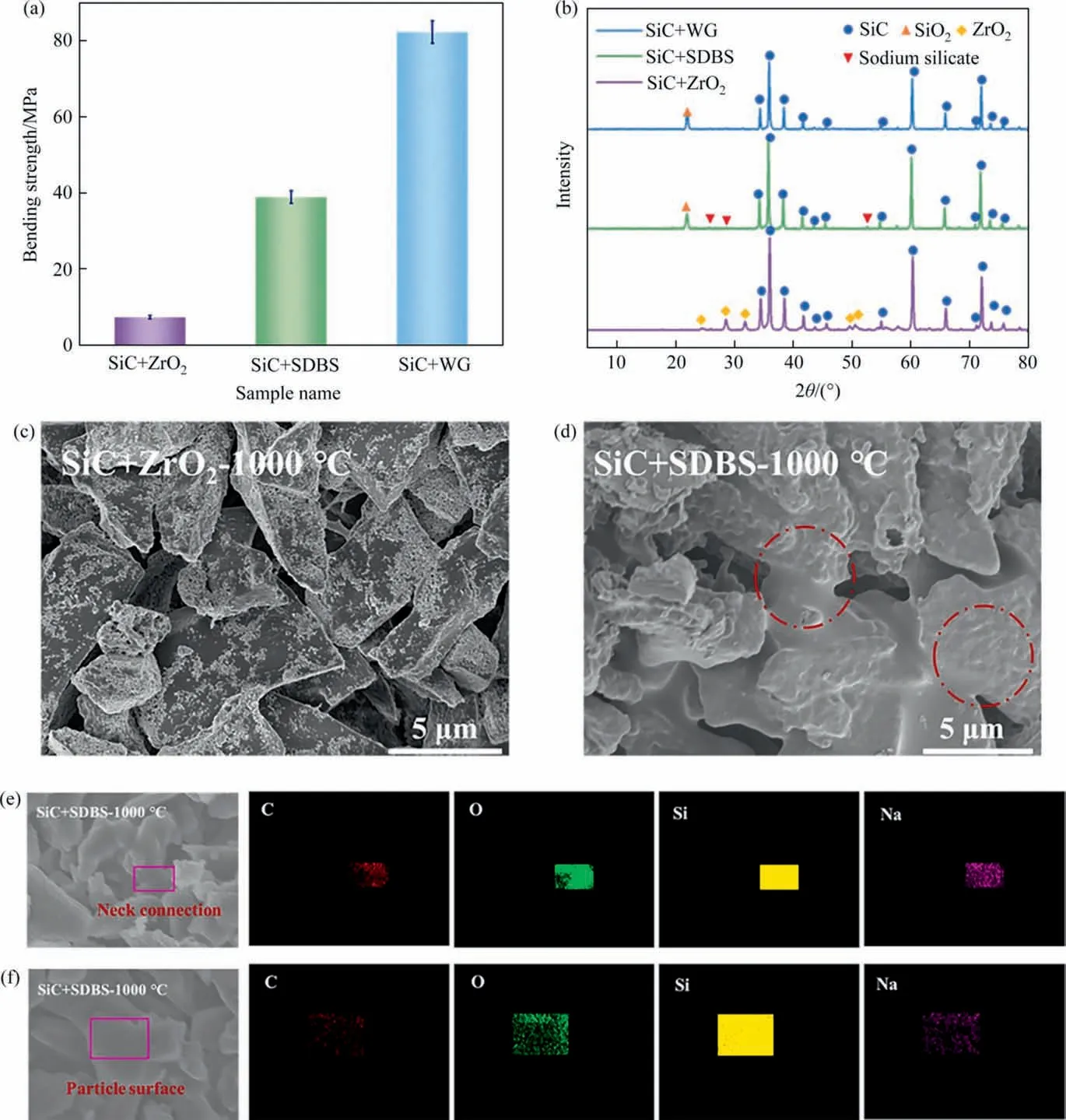
Fig.7. (a)Bending strength;(b)XRD patterns;(c)and(d)SEM images of SiC membranes prepared with different additives at 1000°C.Elemental distribution of SiC membrane prepared using SDBS as sintering additive at 1000 °C on different positions: (e) neck connection and (f) particle surface.
According to the XRD patterns in Fig. 3 and Fig. 6, it can be deduced that NaA residue decomposed to form NaAlSiO4at high temperature, which would partially react with silica to form NaAlSi3O8. NaAlSiO4, NaAlSi3O8and SiO2all served as the bonding phase between SiC particles in S-NaA. For S-SDBS and S-WG, the pore structure was formed by the reaction between SiO2,ZrO2and Na2O that generated by the decomposition of sintering additives at high temperature to form sodium zirconium silicate (SZS).
3.3. Roles of Na-based sintering additives
Control experiments were purposely designed to further clarify the roles of Na-based additives. In the absence of Na-based additives (i.e., only SiC and ZrO2), as shown in Fig. 7(a), the bending strength of the SiC membranes prepared at 1000 °C was only 7.3 MPa.While the bending strength of the samples prepared with the same sintering procedure were significantly improved to 39 MPa and 82 MPa, once the sintering additive SDBS and WG was introduced, respectively. In the XRD patterns (Fig. 7(b)), the characteristic peak of SiO2was not detected in the samples prepared without Na-based additives but can be observed evidently in others. From the SEM images shown in Fig. 7(c) and (d), we can see that the profile of each particle was clear, and no obvious connecting necks can be observed when no Na-based additives were added. Once Na-based additives were introduced, neck connections were obviously emerged, and the surface of these particles become smooth, suggesting the liquid-phase sintering process. The above results clearly demonstrated that the Nabased additives were the essential for low-temperature sintering rather than ZrO2.
The above conclusion was further evidenced by the elemental distribution in various areas of reaction bonded SiC membranes.As shown in Fig. 7(e) and (f), Na element was mainly gathered in the neck, which verified that Na-based additives promoted the migration to the neck at high temperature, then crystallized and grew at the joint, thereby improving the mechanical strength of SiC membranes.
The passive oxidation of SiC occurs at high temperature and/or high oxygen partial pressure,i.e.,SiC+1.5 O2(g)→SiO2+CO(g)[50].The formation of SiO2enabled the reactive sintering to be realized at a lower temperature.Fig.8 illustrates the microstructure evolution of SiC membranes prepared without and with different types of additives.
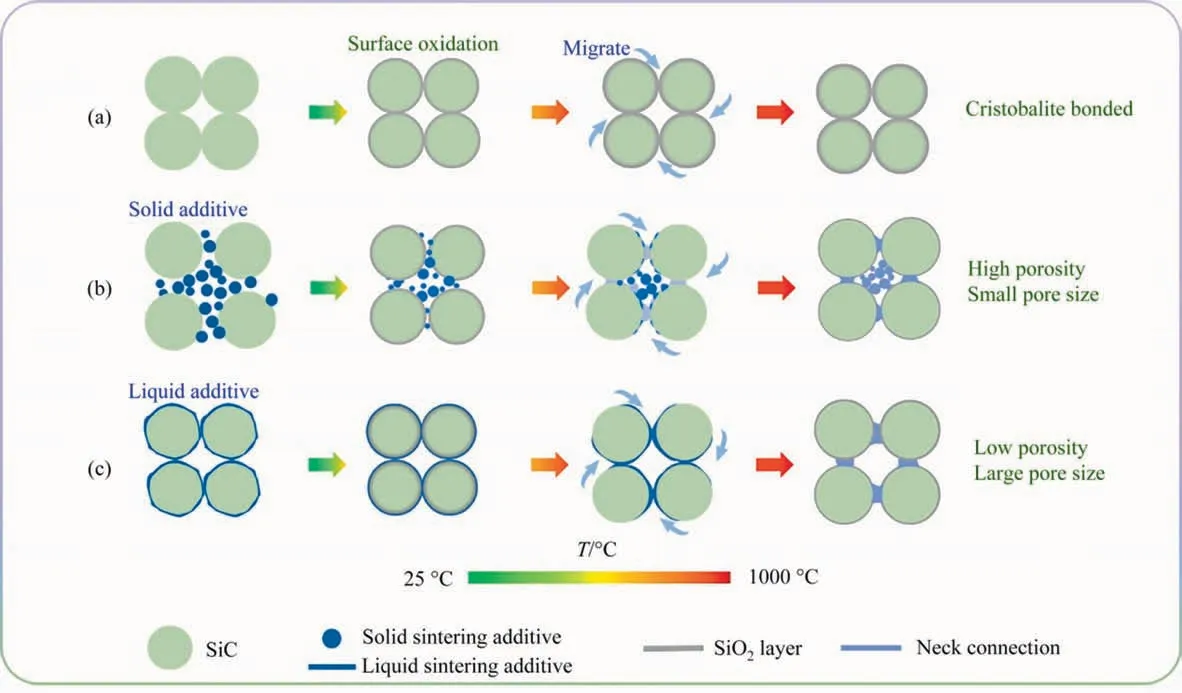
Fig.8. Schematic illustration of the sintering mechanism of reaction bonded SiC membranes(a)oxidation bonding,and reaction bonding enabled by(b)originally solid-state additive and (c) originally liquid-state additive.
In the absence of sintering additives,the necks between the SiC particles are composed solely by the SiO2generated by oxidation on the SiC particle surface (Fig. 8(a)). When the solid additives(i.e.,NaA)are introduced,they would locate among the SiC matrix,and the distance between these SiC particles would be enlarged,as shown in Fig. 8(b). With the increasing temperature, the additives would decompose into both the liquid and solid intermediates.The latter would react with the SiO2on the surface of SiC particles to generate a new phase, thereby forming necks between the SiC particles.At the same time,the accumulation of the solid intermediates generates some small pores in the SiC matrix, resulting in the higher porosity and smaller pore size of SiC membranes.
On the contrary when liquid-phase additives were introduced into the SiC matrix, they would promote the molding process and increase the density of green bodies (Fig. 8(c)). With the increase in sintering temperature,the surface oxidation of SiC particles would be accelerated. In this period, the liquid additives wrapped on the surface of SiC particles can not only increase the dissolving and/or diffusing kinetics of oxygen molecules and oxygen ions [51], but also can effectively improve the surface diffusion, thereby accelerating its migration to the connecting points between SiC particles, thickening the necks, and subsequently enlarging the pores.
3.4. Surface characteristics
Fig.9(a)presents the dynamic water contact angle of the representative SiC membranes prepared with different additives.As can be seen, these three membranes exhibited hydrophilic features.The formed silica layer on the membrane surface can interact with the water in the air to generate hydroxyl groups[52],giving rise to the rapid penetration of water droplet within 0.13 s.Among them,S-NaA with the maximum porosity (47%) showed the highest hydrophilicity, with the water contact angle reducing from 18° to 10° in 0.02 s and completely wetting the SiC membrane surface in 0.04 s. In relation to the different microstructures, it can therefore be concluded that the open porosity contributed more to the wettability than the pore size, and the membranes with larger open porosity showed smaller water contact angle and higher water transport ability.
It can be seen from Fig. 9(b) that the higher the porosity, the smaller the surface roughness of the SiC membrane. Moreover,when the pore size of the membrane is larger, the surface of the membrane is relatively smooth [53]. In other words, both S-NaA with higher porosity and S-WG with larger pore size have a smaller surface roughness. The surface roughness of S-SDBS was the largest, resulting in the increase of the contact area between the membrane surface and oil droplets, so the underwater oil contact angle of S-SDBS was slightly smaller than that of the other two membranes(Fig.9(c)).However,the underwater oil contact angles of all three membranes were over 150°, suggesting the superoleophobic feature underwater and great potential in oily wastewater treatment.
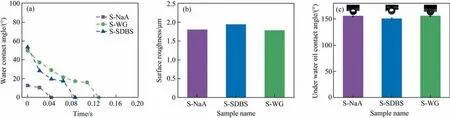
Fig. 9. Surface characteristics of SiC membranes prepared with the optimized content of additives (S-NaA:12% (mass), S-WG:12% (mass), S-SDBS:8% (mass))at 1000 °C: (a)dynamic water contact angle, (b) surface roughness and (c) underwater oil contact angle.
3.5. O/W emulsion separation
Membrane-based O/W emulsion separation is typically conducted in cross-flow filtration model, where energy input is required to generate the transmembrane pressure across the membrane and the cross-flow on the membrane surface[54].The filtration process conducted at a relatively low transmembrane pressure could reduce the energy consumption and operation cost. Also, as oil droplets are elastic particles, they can be compressed into the surface pores of the membrane under high pressure. Hence, low pressure filtration has been accepted as an effective method to reduce membrane pollution and enhance oil–water separation efficiency.Given the superior hydrophilic and underwater oleophobic feature of these SiC membranes (Fig. 9(a) and (c), the O/W emulsion separation experiments were carried out with the initial concentration, transmembrane pressure and cross-flow velocity of 500 mg∙L–1, 0.02 MPa and 0.5 m∙s-1, respectively.
The permeate fluxversusfiltration time was plotted in Fig. 10(a). It can be seen that all membranes showed a rapid decrease in permeate flux during the first 10 min,due to the relatively high viscosity of lubricant oil(125 mPa∙s,25°C).The stabilized flux after 30 min and rejection ratio of different membranes are summarized in Fig. 10(b)and (c) and Table S1. The three membranes showed a comparable stable permeate flux of 42–46 L∙m-2∙h-1,while the oil concentration in the filtrate largely depended on the membranes.
The removal efficiency of oil–water emulsion is influenced by both the pore structure and the surface characteristics of SiC membranes. When the pore size of SiC membranes is smaller, the oil phase in O/W emulsions can be better retained through the pore size sieving effect. Therefore, S-NaA with the smallest pore size has the highest rejection ratio and oil concentration in the filtrate was 0.05 mg∙L–1, while S-WG with the largest pore size has the lowest rejection ratio, with an oil concentration of 8.00 mg∙L–1in the filtrate. Encouragingly, the oil content in all the permeate at a TMP of 0.02 MPa can meet the discharge standard (<10 mg∙L–1,Fig.10(c)).In addition,the SiC membrane is superoleophobic under water, so that water in the O/W emulsion can pass through the SiC membrane more easily. As a result, S-NaA with the most hydrophilic and oleophobic properties showed the highest oil–water separation performance.The appearance of the feed and filtrate was significantly different, as presented in Fig. 10(a), in which the filtered liquid was clarified and transparent, suggesting the excellent removal efficiency. Furthermore, the cycled filtration experiment was conducted using a chemical cleaning procedure. As shown in Fig. S4 and Table S2, the oil droplets on the membrane surface were efficiently eliminated after cleaning and the porous surface could be clearly observed.In addition,the content of C element on the SiC membrane surface significantly decreased after cleaning. Moreover, the flux recovery ratio of S-NaA, S-SDBS, and S-WG in the 3rd cycle were 81%, 85%, and 82% (Fig. S5), respectively, suggesting good regeneration capabilities of these reaction bonded membranes.
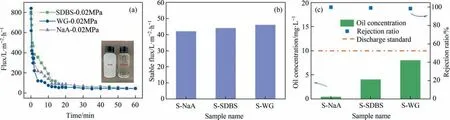
Fig.10. O/W emulsion separation performance of reaction bonded SiC membranes:(a)time-dependent permeate flux of various SiC membranes using O/W emulsion with a concentration of 500 mg∙L–1 at 0.02 MPa,where the insets show the optical photos of feed and permeate after S-NaA membrane treatment at 0.02 MPa,(b)stable flux,and(c)rejection rate and oil concentration in the permeate, where the dash line refers to the discharge standard (10 mg∙L–1).
As displayed in Table 2, results of ceramic membranes used in oil-in-water separation are summarized in terms of mean pore size, operating conditions and separation performance, and these ceramic membranes exhibit excellent oil interception ratio of over 90%.Among them,SiC membranes exhibit exceptional hydrophilicity and superoleophobicity,allowing for efficient separation of oil–water emulsions with high oil concentration [58] and viscosity,even under low transmembrane pressure. The SiC membrane prepared in this study has been found to have superior oil rejection ratios compared to other SiC membranes and alumina membranes,specifically under ultra-low transmembrane pressure (0.02 MPa).Therefore, the reaction bonded SiC membranes can be applied in low-pressure filtration process for the high-efficient separation of oil-in-water emulsion.
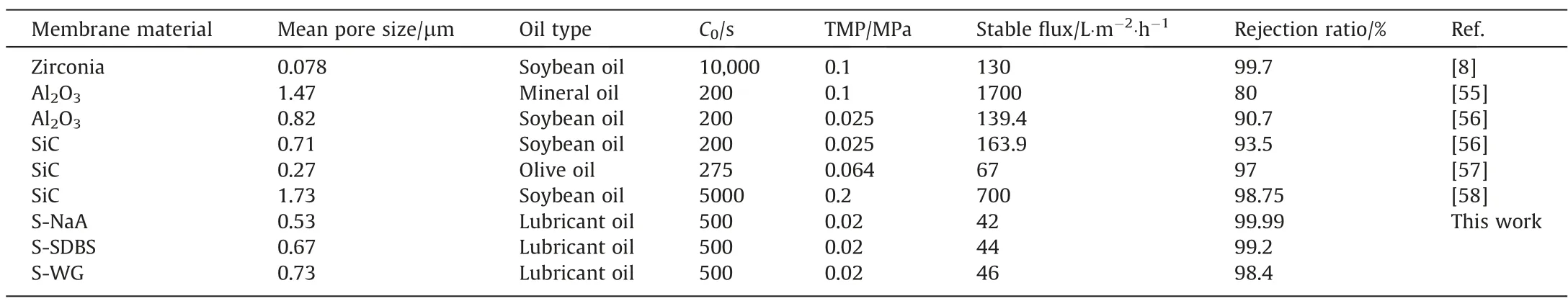
Table 2Comparison of oil–water separation performance of ceramic membrane
4. Conclusions
A comparative study on three types of reaction-bonded SiC membranes was conducted by choosing sodium-containing compounds of different original states as sintering additives,i.e.,solid-state NaA residue, and liquid-state WG and SDBS. It was found that the original state of the sintering additives significantly influenced the microstructure of prepared SiC membranes.Among them,the liquid-state additives(i.e.,WG and SDBS)had better dispersion and fluidity, which can better promote the surface oxidation of SiC particles, resulting in the lower open porosity, large pore size and higher bending strength of SiC membranes. On the contrary, SiC membranes prepared with the solid-state additives(NaA residue) showed higher open porosity and smaller average pore size.When applied in O/W emulsion separation at low transmembrane pressure (0.02 MPa), S-NaA membrane delivered a higher rejection ratio (99.99%) compared with other two types of SiC membranes. Importantly, the three types of SiC membranes can effectively separate the oil–water emulsion with the oil concentration in all filtrates satisfying the discharge standards(<10 mg∙L–1).This work disclosed the comprehensive effects of initial state of sintering additives on SiC membranes, providing valuable guidelines for on-demand fabrication of porous ceramic membranes.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2022YFB3805002),the National Natural Science Foundation of China (21838005),the Innovative Research Groups of the National Natural Science Foundation of China (21921006), the Natural Science Foundation of Jiangsu Province(BK20220345)and Youth Science and Technology Talents Lifting Project of Jiangsu Association of Science and Technology (105019ZS_007).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.05.010.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
- Separation of lithium and nickel using ionic liquids and tributyl phosphate
