Separation of lithium and nickel using ionic liquids and tributyl phosphate
Kun Wang, Guoquan Zhang, Linye Li, Yuzhang Li, Xiangxin Liao, Pu Cheng, Mingzhi Luo
School of Chemical Engineering, Sichuan University, Chengdu 610065, China
Keywords: Ionic liquids Selective separation Solvent extraction Lithium
ABSTRACT With the vigorous development of the electronics industry, the consumption of lithium continues to increase, and more lithium needs to be mined to meet the development of the industry. The content of lithium in the solution is much higher than that of minerals, but the interference of impurity ions increases the difficulty of extracting lithium ions.Therefore,we prepared an imidazole-based ionic liquid(1-butyl-3-methylImidazolium bis(trifluoromethyl sulfonyl)imide) (IL) for efficient lithium extraction from aqueous solutions by solvent extraction.Using an extraction consisting of 10%IL,85%tributyl phosphate(TBP),and 5%dichloroethane and an organic to aqueous phase ratio(O/A)of 2/1,over 64.23%of Li were extracted, and the extraction rate after five-stage extraction could reach more than 96%. The addition of ammonium ions to the solution inhibited the extraction of Ni, and the separation coefficient between lithium and nickel approached infinity, showing a very perfect separation effect. Fouriertransform infrared spectroscopy and slope methods were used to analyze the changes that occurred during extraction,revealing possible extraction mechanisms. In addition,the LiCl solution generated during the preparation of ionic liquids was mixed with the stripping solution,and the battery-grade lithium carbonate was prepared by Na2CO3 precipitation, with a purity of 99.74%. This study provides an efficient and sustainable strategy for recovering lithium from the solution.
1. Introduction
As the lightest metal element,lithium has a wide range of applications in batteries, ceramic glass, adhesives, lubricants, metal alloys, aerospace, and other industrial fields, so it is regarded as a new type of strategic energy [1–3]. Lithium has become the key to develop industrial products, especially lithium-ion batteries used in electronic devices and electric vehicles, and the demand for lithium has increased significantly over the past few years[4]. From 2015 to 2024, global lithium-ion battery consumption for electric vehicles alone is expected to reach 221 billion USD[5–8]. Lithium in nature usually exists in the form of minerals and soluble salts. However, the content of lithium in the solution far exceeds that in minerals, and more than 85% of the lithium in the solution can be extracted and utilized [9]. Therefore, how to efficiently extract lithium from the solution was worth studying.
Lithium ions in solution can be recovered from several different processes, including precipitation, ion exchange, adsorption, and extraction [9,10]. Among these methods, solvent extraction was considered to be an effective technique for the separation of lithium from the solution [11]. One or more component dissolved in an aqueous phase is transferred to an organic phase through the contact between both phases. This method has the advantages of high selectivity, large production capacity, and ease of application for continuous operations.In industry,lithium was extracted from salt lakes using an organic phase composed of tributyl phosphate(TBP), FeCl3, and kerosene [12]. To prevent the hydrolysis of iron ions, the whole process can only be carried out under strong acid conditions, the equipment was severely corroded, and a large amount of acidic wastewater was produced. Therefore, it was urgent to develop new extractants or co-extractants for the extraction of lithium ions from solutions to improve reaction conditions.Ionic liquids (IL) are organic molten salts composed of ions with melting points close to or below room temperature, composed of asymmetric cations and anions [13,14]. They have the advantages of high selectivity,strong stability,and almost no volatility,and are almost-perfect organic solvents in solvent extraction, which has aroused the interest of many researchers[15–17].However,many factors need to be considered when selecting ionic liquids. Taking the widely used ionic liquid containingas an example, the HPF6that was formed during the stripping step can be lost to the aqueous phase due to the solubility of the acid HPF6in water.Ionic liquid 1-butyl-3-methylImidazoliume bis(trifluoromethyl sulfonyl)imide has high hydrophobicity and the loss of ionic liquid will decrease during the extraction and stripping process [18–21].
In recent years, researchers have carried out many studies on the extraction of lithium from lithium-containing solutions by taking advantage of the strong selectivity of ionic liquids.For example,Shiet al. [22] used 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) as a diluent and co-extractant to extract lithium from solutions with a very high ratio of lithium to magnesium. The extraction rate of lithium can reach more than 90%, the separation coefficient(βLi/Mg)can reach 125.However,the concentration of magnesium in the solution is much higher than that of lithium, and this system only reduces the ratio of magnesium to lithium, and subsequent steps are required to achieve complete separation. Because the cationic part of ionic liquid participated in the reaction, it caused the loss of ionic liquids, and the reuse of extractant was defective. To this end, Shiet al. [23] improved the functionalization of ionic liquids to avoid the loss of ionic liquids, but the separation effect of impurity ions was reduced. Masmoudiet al. [24] used HBTA to combine with 1-hexyl-3-methylimidazole bis(trifluoromethyl sulfonyl)imide to form an organic phase. The experimental results show that the selectivity of organic relative to lithium is higher than that of sodium, but the selectivity of calcium is poor. Yanget al. [25] used hydroxyl functionalized ionic liquid 1-hydroxyethyl-3-methylimidazolium bis(trifluoromethyl sulfonyl)imine([OHEMIM][NTf2])and neutral ligand trialkyl phosphine oxide (Cyanex923) as extractants. The single-stage extraction rate of lithium ions was more than 93%,and ion exchange was found to be dominant in the extraction process.Zhenget al.[26]prepared a carboxyl-functionalized ionic liquid (carboxymethyltrimethylammonium bis(trifluoromethyl)sulfonimide) to selective extract lithium from spent lithium-ion batteries. Under the optimal experimental conditions, the singlestage extraction rate was 63.2%,and the extraction rate of impurity ions also reached 5%.The above studies show that there have been some studies on the extraction of lithium by ionic liquids, but the composition and concentration of the target solution are different,which will have a great impact on the extraction process. At the same time,the extraction of organic relative to impurity ions needs to be suppressed,and it is necessary to further study the extraction of lithium by ionic liquids.
In this paper, imidazole-based ionic liquids were synthesized,mixed with tributyl phosphate and dichloroethane as organic phase, and the extraction of lithium ions from lithium and nickel acetate solutions was studied. The highlight of this experiment is that ammonium ion was introduced into the solution,which completely inhibited the extraction of organic relative nickel and achieved the perfect separation of lithium and nickel, while the extraction rate of lithium was kept at a high level. Gibb’s free energy, enthalpy change, and entropy change indicate that the extraction reactions were exothermic and spontaneous and reduced the disorder. In addition, the reusability of the organic phase was investigated. Finally, the lithium ion in the solution was precipitated by Na2CO3, and the purity of lithium carbonate reached 99.74%.This study provides an efficient method to recover lithium from lithium-containing solution.
2. Materials and Methods
2.1. Reagents
Nickel acetate(AR),lithium acetate dihydrate(AR),bis(trifluoro methane)sulfonimide lithium salt (99% purity), and 1-butyl-3-methylimidazolium chloride (97% purity) were purchased from Aladdin Industrial Co. (Shanghai, China). Ethyl acetate (AR), tributyl phosphate (99% purity), acetic acid (AR), sodium carbonate(99.8% purity), and dichloroethane (AR) were purchased from Chengdu Kelong Chemical Co., Ltd (China).All solutions of specific concentrations in the experiments were prepared or diluted with deionized water.
2.2. Preparation of ionic liquids
An aqueous solution containing 1-butyl-3-methylimidazolium chloride was added to a beaker; then bis(trifluoromethane)sulfoni mide lithium salt aqueous solution was slowly added with equal moles and the mixture was stirred at 25°C for 10 h.After the solution was left to stand for stratification, a separatory funnel was used to separate the two phases. The LiCl solution in the upper layer was collected for subsequent use, and the crude product of the ionic liquid was obtained in the lower layer. The ionic liquid was purified with ethyl acetate and washed with deionized water until no chloride ions remained in the deionized water (add nitric acid acidified AgNO3solution dropwise, no white precipitate was formed in deionized water).The ethyl acetate and water in the product were removed by distillation under reduced pressure and vacuum-dried at 75°C for 10 h to obtain a high-purity ionic liquid.The synthesized ionic liquids were characterized by infrared spectroscopy, high-resolution mass spectrometry, and hydrogen NMR spectroscopy. Fig. S1 (Supplementary Material) shows the highresolution mass spectrometry of the ionic liquid. Fig. S2 shows the Infrared spectra of ionic liquids and precursors.
2.3. Solvent extraction
Mix the solution and the organic phase in a certain proportion,stir in a constant temperature water bath and stabilize for some time, draw part of the aqueous phase with a syringe, and dilute it according to a certain multiple. Inductively coupled plasmaoptical emission spectrometer (ICP-OES) was used to measure the liquid components, and the content of each ion in the organic phase and the aqueous phase was calculated according to mass conservation.The extraction rate(E),distribution ratio(D),separation coefficient(β),and stripping rate(H)can be calculated according to Eqs. (1)–(4):
whereVoandVaqare the volumes of the organic and aqueous phases, respectively;CoandCaqrepresent the concentrations of the target metallic element in the organic and aqueous phases,respectively;DLiandDNirepresent the distribution ratios of the target element and impurity, respectively.
2.4. Analytical methods
The pH of the solution was measured using a P901 acidity meter(Shanghai Youke Instrument Co., Ltd., China). Fourier-transform infrared spectrometer (FT-IR, Nicolet iS10, Thermo-Fisher) was used to detect the shifts in the absorption peaks. The concentrations of the metallic elements were determined by ICP-OES (ICAP 7400, Thermo Scientific, USA). The particle morphology, and crystalline structure were characterizedviascanning electron microscopy (SEM, Hitachi SU3500, Japan), X-ray diffractometer(XRD6100, Shimadzu, Japan).
3. Results and Discussion
3.1. Exploration of extraction factors
Deionized water was used to prepare an acetate solution with a Li+concentration of 0.35 g∙L–1and a Ni2+concentration of 0.15 g∙L–1as the extraction water phase.This is based on the concentration of lithium and nickel in the leaching solution of spent lithium-ion batteries. In the previous work, we separated aluminum, cobalt and manganese from the leaching solution [27].
3.1.1.Effect of the concentration of ammonium acetate(CH3COONH4)
Separation of metals with close ionic radii is extremely challenging (e.g., Li, Mg from salt lake brines), and although ionic liquids excel in this regard, they also extract impurity ions. The ionic radius of lithium is 0.076 nm, and that of nickel is 0.069 nm,which are very close.To reduce the impurity ions entering the organic phase, ammonium ions (ionic radius: 0.143 nm)were introduced in this experiment to inhibit the extraction of Ni from the organic phase. The organic phase was composed of 10%ionic liquid, 5% dichloroethane, and 85% TBP. The organic phase was mixed with the water phase and reacted for 15 min at 25 °C,pH=5.40,and organic to aqueous phase ratio(O/A)was 2.Table 1 shows the effects of different concentrations of CH3COONH4in solution on the extraction rate(E),distribution ratio(D),and separation coefficient (β) of lithium and nickel. When the CH3COONH4concentration was 0,the extraction rate of lithium reaches 78.93%,but the extraction rate of nickel also reaches 29.11%. The separation coefficient between lithium and nickel was only 9.12, and the separation effect was extremely poor. However, with the increase in the concentration of CH3COONH4, the extraction rate of lithium and nickel decreased. When the concentration of CH3-COONH4reaches 0.12 mol∙L–1, the extraction rate of lithium was 64.23%, while the extraction rate of nickel decreases to 0, and the separation coefficient approaches infinity, indicating that lithium and nickel have been completely separated. After adding 0.12 mol∙L–1CH3COONH4, the extraction rate of lithium was reduced by 14.7%, while the extraction rate of nickel was reduced by 29.11%, which was nearly twice that of lithium. Therefore,0.12 mol∙L–1CH3COONH4was added to the solution in the subsequent experiment.
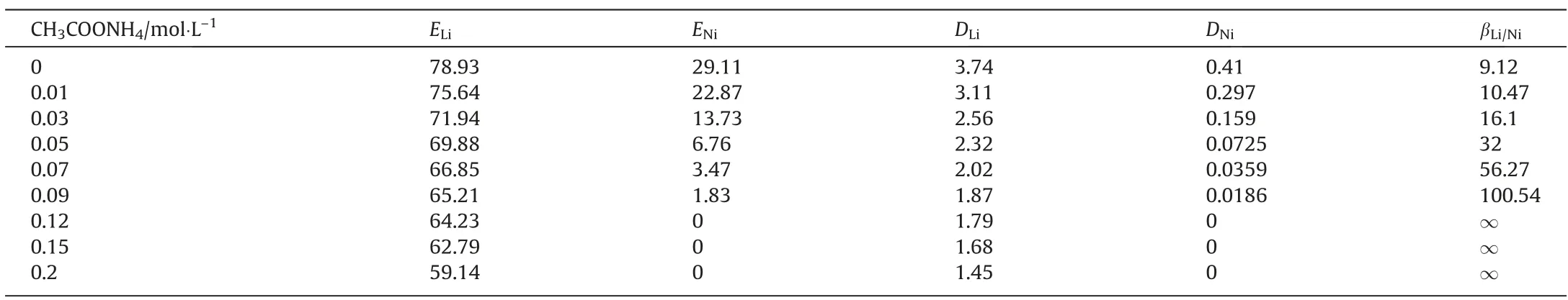
Table 1Effects of different concentrations of CH3COONH4 on the extraction of lithium and nickel
3.1.2. Effect of the volume ratio of ionic liquid
To determine the effect of lithium extraction from the solution of ionic liquids, organic phases consisting of different concentrations of ionic liquids(0%–25%)were prepared for the experiments.During the whole process, the TBP concentration was kept constant, and dichloroethane was added to keep the total volume of the organic phase constant. As shown in Fig. 1, when no ionic liquid was added to the organic phase, the extraction rate of organic relative lithium was 0,indicating that there was no extraction effect at this time,which was consistent with the experimental results of [26]. When the ionic liquid concentration in the organic phase was increased to 10%, the extraction rate was directly increased from 0% to 64.23%, and the distribution ratio(DLi)was 1.79.This is because the presence of ionic liquid enhances the coordination of lithium ions with the extractant. However,when the ionic liquid concentration was increased to 25%, the extraction efficiency decreased rather than increased. This may be due to the increase of ionic liquid content in the organic phase,which leads to an increase in the viscosity of the organic phase,which affects the diffusion of lithium ions in the organic phase[28]. Although the extraction rate reached the maximum(65.01%) at 15% ionic liquid concentration, which was the result of adding 5% ionic liquid, the extraction rate was only increased by 0.78%. Therefore, choosing an ionic liquid concentration of 10% for experiments can effectively selectively extract lithium from the solution.
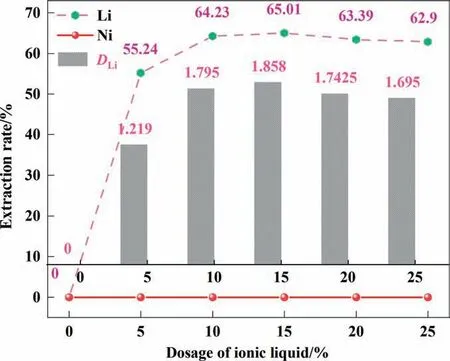
Fig. 1. Effect of the volume ratio of ionic liquid.
3.1.3. Effect of the organic to aqueous phase ratio and pH
In the extraction system with organic phase 10% IL + 85%TBP + 5% dichloroethane, the extraction experiment was carried out under the conditions of O/A = 2/1, time 15 min and temperature 25°C.The pH value of the experimental solution was adjusted by acetic acid and ammonia,and the effect of pH value of aqueous phase on the extraction rate of lithium was investigated.As can be seen from Table 2, the extraction efficiency of organic relative lithium ions remains almost unchanged at different pH values,fluctuating between 63%and 65%.This shows that H+will not compete with lithium ions during the extraction process, and the extraction efficiency of organic matter relative to Ni2+is still 0,and the separation factor between lithium and nickel tends to infinity. If the pH value of the aqueous phase is higher (>7), Ni2+may be precipitated. In order to prevent the influence of pH on Ni2+, the pH of the solution was adjusted to 5.40.
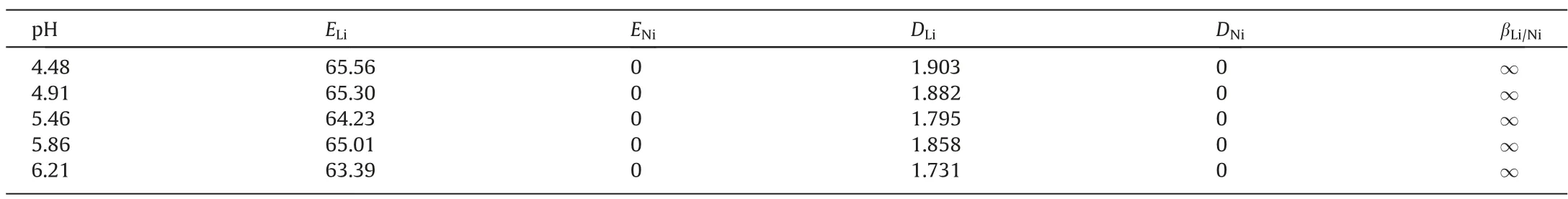
Table 2Effects of pH on the extraction of lithium and nickel
It can be seen in Fig. 2 that with the increase of O/A ratio, the extraction rate and distribution ratio of lithium gradually increase.When O/A was 1/2, the extraction rate of lithium is only 42.11%,and the distribution ratio (DLi) was 0.727; although the extraction rate of nickel was 0, the extraction rate of lithium is at a lower level. When the O/A was increased to 2, the extraction rate of lithium increased from 42.11%to 64.23%,and the distribution ratio was 1.798,which achieved an ideal effect.This is because the number of ionic liquids and TBP in the organic phase increases,the contact area between the organic phase and the aqueous phase increases, and the probability of solvent molecules colliding with lithium ions is also significantly increased.
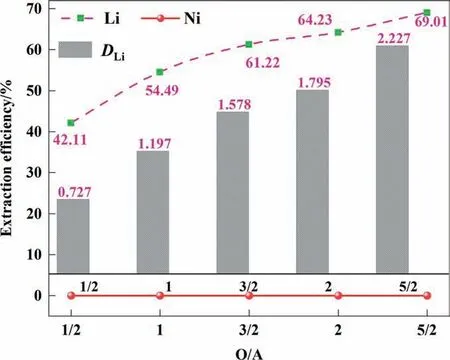
Fig. 2. Effect of the O/A ratio.
3.1.4. Effects of the temperature and time
Fig. 3(a) shows the trend of the extraction rate with temperature. Increasing the reaction temperature will aggravate the collision between metal ions and solvent molecules, which seems to improve the extraction efficiency. However, the experimental results are contrary to the previous conjecture, and the extraction rate is greatly reduced.This may be because the organic phase contains dichloroethane,which can change the viscosity and density of the organic phase and make the phase separation easier; the increase in temperature makes the dichloroethane volatilize,increasing the viscosity of the organic phase, which was not conducive to extraction [29]. Therefore, 25 °C was chosen as the optimal extraction temperature.
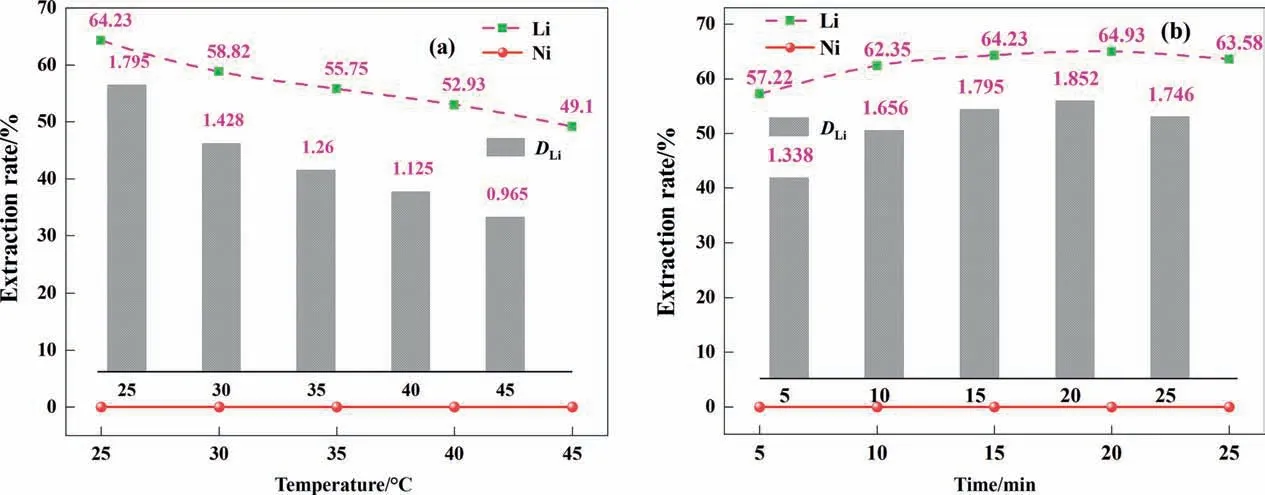
Fig. 3. Effects of different factors on the extraction efficiency of Li, Ni: (a) temperature; (b) time.
3.1.5. Theoretical series of extraction
Under the optimal experimental conditions, the extraction isotherm experiment was performed by changing the O/A ratio, and the O/A ratio varied between 1/2 and 7/1. As shown in Fig. 4, the McCabe-Thiele diagram for lithium extraction was determined.The theoretical number of countercurrent extraction stages was determined to be 5, and the extraction rate of lithium reached 96% after five-stage countercurrent extraction.
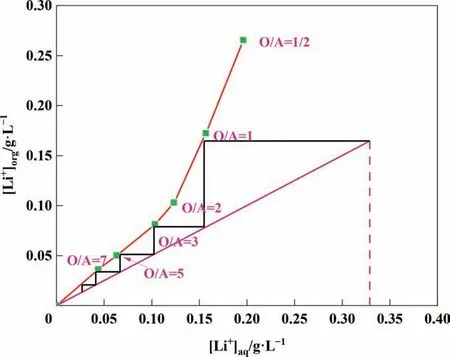
Fig. 4. Extraction isothermal curve at 25 °C.
3.2. Stripping and reuse of extractant.
During the preparation of ionic liquids, the resulting upper aqueous phase was a pure solution of LiCl. Considering that other impurities cannot be introduced when the back-extraction liquid and the water phase are mixed, HCl was selected as the backextraction agent. The effects of HCl concentration and O/A ratio on stripping rate were studied.From Fig. 5(a),it can be found that when O/A=1,the stripping rate increases with the increase of HCl concentration,reaching 95.29%at 1.2 mol∙L–1.However,if the concentration of hydrochloric acid was too high,the equipment will be corroded(in industry,TBP,FeCl3, and kerosene are used as extractants to extract lithium[12].During stripping,the concentration of hydrochloric acid was 3–6 mol∙L–1, and the service life of the equipment was greatly reduced).From Fig.5(a),(b),it can be seen that the stripping rate can reach 88.2%when the HCl concentration was 0.4 mol∙L–1, and the stripping rate was 94.81% when the O/A ratio was 1/2. Therefore,0.4 mol∙L–1HCl was selected as the stripping agent.
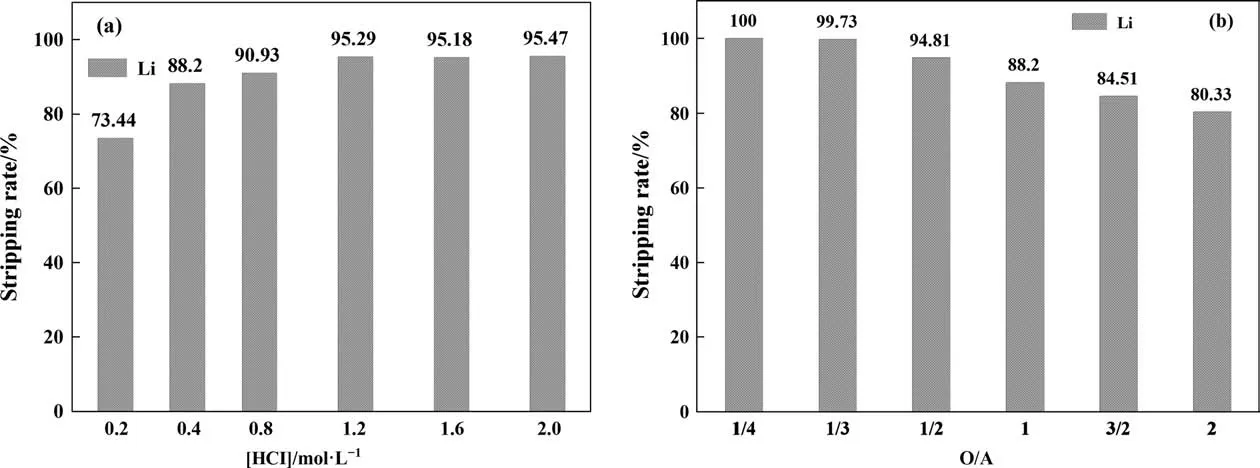
Fig. 5. (a) O/A = 1, the concentration of HCl was changed; (b) [HCl] = 0.4 mol∙L–1, the O/A ratio was changed.
For all[Tf2N]–based IL,Mazanet al.[30]found that the amount of the IL anions in the aqueous phase increases with increasing the acid concentration. This indicates that during the stripping step,the anions of the ionic liquid will enter the aqueous phase. After mixing the raffinate and stripping solution,the anions and cations recombine in the aqueous phase to form new ionic liquid molecules. In this way, it is possible to recover the IL molecules lost to the aqueous phases.
3.3. Extraction mechanisms
3.3.1. Infrared spectroscopy analysis
Infrared spectroscopy can provide information about the functional groups of substances. The infrared spectra of the organic phase before and after extraction were measured and compared.Fig. 6(a) shows the infrared spectrum of the fresh organic phase.3475 cm-1is the characteristic peak of O–H,because the ionic liquid has strong water absorption and can quickly absorb water in the air. 3153 cm-1and 3107 cm-1are the characteristic peaks of stretching vibration of C–H on the imidazole ring, 2962 cm-1and 2876 cm-1can be attributed to the saturated C–H stretching vibration. Near 1574 cm-1and 1466 cm-1are imidazole ring skeleton vibrations; 1139 cm-1is the characteristic peak of C—F on the anion in ionic liquid. 1271 cm-1is the characteristic peak of P=O, and 1028 cm-1can be attributed to the vibration of P—O—C [31].
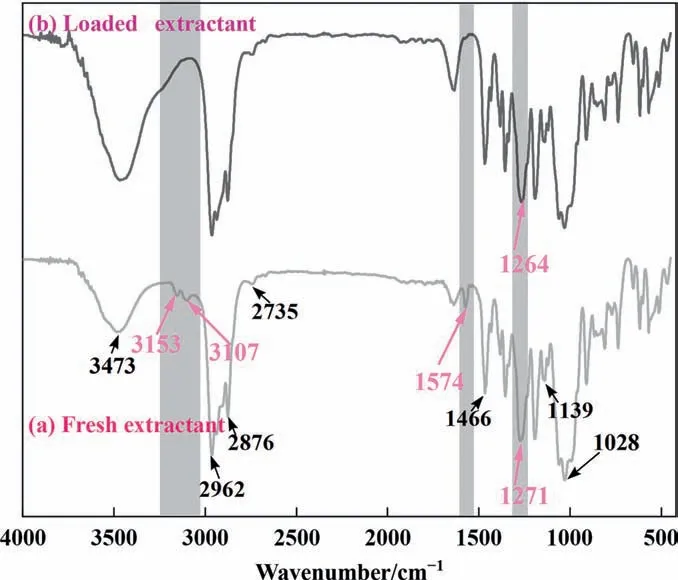
Fig. 6. Infrared spectra of (a) fresh extractant, and (b) loaded extractant.
Fig.6(b)was the infrared peak of the organic phase after extraction. The position of P=O is shifted to a lower wavenumber by 7 cm-1, which indicates that the O atoms on P=O may interact with Li+in the water phase,which reduces the vibration frequency of P=O. In addition, the characteristic peaks located on the imidazole ring disappeared (3153 cm-1, 3107 cm-1, and 1574 cm-1),which indicated the concentration of the cationic part of the ionic liquid in the organic phase decreased, and cation exchange may have occurred during the extraction process [32]. The position of C–F did not change, indicating that Li+did not interact with the anion.
3.3.2. Slope method analysis
When the concentration of Li was much lower than the concentration of TBP,the reaction equilibrium constant was constant at a certain temperature, and the concentration of TBP in the system was close to the initial value after the extraction was completed[26]. Therefore, a 0.03 mol∙L–1lithium ion solution was used as the aqueous phase for slope analysis. The concentration of TBP was varied from 40.0% to 80.0% at an O/A ratio of 2:1, the concentration of ionic liquid was 10%, and the extraction period was15 min at 25 °C using dichloroethane as the diluent to keep the total volume constant, and the corresponding distribution ratio was calculated according to the experimental results. Take lgDLias the ordinate and lg[TBP] as the abscissa to fit the curve, as shown in Table 3.
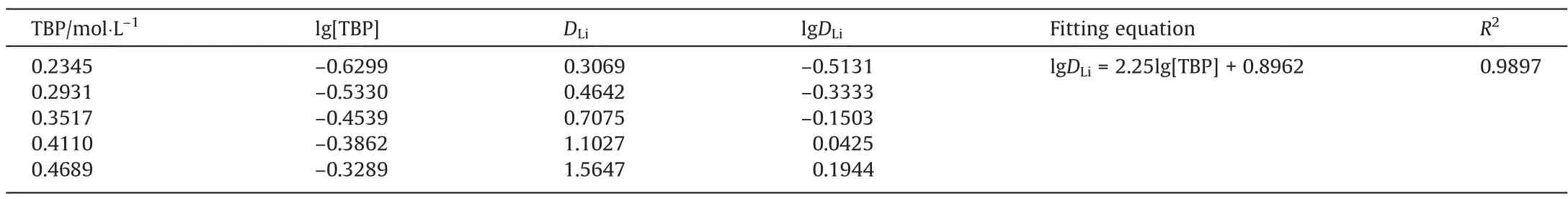
Table 3Distribution ratios and fitting equation for different TBP concentrations
After the linear fitting of lgDLi–lg[TBP], the slope is 2.25, which is close to the integer 2, indicating that 2 TBP molecules are required to participate in the reaction to extract one Li+. Referring to the reported studies, the extraction mechanism of imidazole ionic liquids is generally cation exchange, the cations of ionic liquids enter the aqueous phase as the exchange part, and lithium ions enter the organic phase after combining with tributyl phosphate[20].Based on the research of Masmoudiet al.[33],the slope method and the results obtained by infrared spectroscopy, the extraction equation was finally determined as shown in Eq. (5).
3.3.3. Calculation of thermodynamic parameters
According to the change of extraction rate at different temperatures,the corresponding distribution ratio was calculated,and the curve was fitted with lgDLias the ordinate and 1000/Tas the abscissa, as shown in Fig. 7.
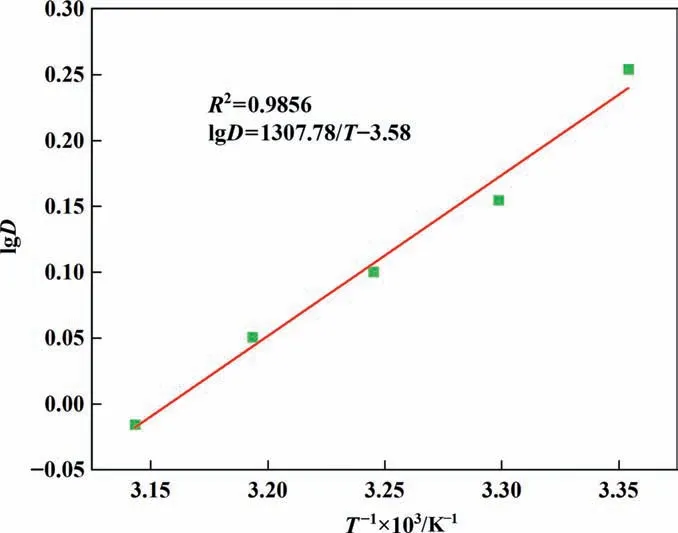
Fig. 7. The plot of lgD vs. 1000/T. Temperature range 298.15–318.15 K, organic to aqueous phase ratio (O/A) 2:1.
The change in enthalpy (ΔH) during extraction can be calculated using the van’t Hoff equation:
whereRis the universal gas constant, andCis the integration constant.
The slope of the fitted curve is –ΔH/2.303R, from which the value of ΔHcan be calculated to be–25.04 kJ∙mol-1.ΔHis less than 0,indicating that this is an exothermic reaction,and low temperature is beneficial to the reaction, which can also be proved from Fig. 3(a). According to Eqs. (8) and (9), the values of ΔGand ΔSare –5.12 kJ∙mol-1and 66.83 J∙mol-1∙K-1, respectively, indicating that the extraction reactions were spontaneous and the degree of disorder was weakened.
3.4. Preparation of battery-grade lithium carbonate
The stripping solution was mixed with the LiCl solution produced in the process of preparing ionic liquid, and the concentration of LiCl solution was concentrated to about 1.5 mol∙L–1by evaporating solvent. The solution was heated to 90 °C, and an equimolar Na2CO3solution was slowly added dropwise and stirred for 1 h.Filter while hot and wash with boiling water to completely remove Na ions attached to Li2CO3.
Fig.8 (a)shows the white powder of Li2CO3.The phase composition of the product was analyzed by X-ray diffractometer, and only Li2CO3. Phase existed without other impurities, as shown in Fig. 8 (b). Fig. 8 (c) and (d) show that Li2CO3was formed by the accumulation of sheetlike substances with a smooth surface, with some defects on the surface.This may be caused by the collision of substances during stirring. After testing, the purity of Li2CO3was 99.74%.
4. Conclusions
The innovation of this experiment is the introduction of ammonium ions into the solution, which completely inhibits the extraction of Ni. The separation coefficient between lithium and nickel approached infinity,showing a very perfect separation effect.Ionic liquids were prepared by ion exchange, and the organic phase composed of IL, TBP, and dichloroethane was studied to separate lithium ions from Li and Ni solutions. Under optimal conditions(10% IL, 85% TBP, 5% dichloroethane, O/A = 2, 25 °C), the singlestage extraction rate reaches 64.23%,and more than 96%of lithium ions can be extracted after 5-stage extraction. The extraction mechanism was investigated by slope method, and FTIR spectroscopy. The cationic part of the ionic liquid is exchanged with lithium ions, and 2 molecules of TBP are required to extract one lithium ion. 0.4 mol∙L–1HCl solution can strip 94.81% of Li in the organic phase, which greatly reduces the concentration of stripping solution, increases the service life of the equipment. The back-extracted liquid was mixed with the LiCl solution produced during the preparation of ionic liquid,and the Li2CO3with a purity of 99.74%was prepared by precipitation with an equimolar Na2CO3solution.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22008161), Sichuan Science and Technology Program (2022YFQ0037).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.023.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
