Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
Meng Shi, Kelei Huang, Ruining He, Yinghua Jiang, Yun Zou, Jing Xu, Zhangfa Tong
Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology, School of Chemistry and Chemical Engineering, Guangxi University,Nanning 530004, China
Keywords: Esterification Isobutyl acetate Ionic liquid Kinetics
ABSTRACT As an important organic, isobutyl acetate (IbAc) has been widely used in industries because of its good biodegradability, low surface tension, and other properties. The industrial production of IbAc is usually catalyzed by sulfuric acid. However, the use of sulfuric acid has the drawbacks of causing considerable corrosion to equipment and being difficult to be separated. In this work, n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate ([HSO3-PMPY][CF3SO3]) Brönsted acidic ionic liquid(BAIL) was used as the catalyst and the catalytic activity, solubility, and corrosiveness were evaluated for the esterification of acetic acid with isobutanol.The reaction kinetics and chemical equilibrium were systemically studied.Compared to conventional acid catalysts,[HSO3-PMPY][CF3SO3] showed higher catalytic activity,more excellent reusability,more favorable phase separation,and non-corrosiveness.Three kinetic equations based on ideal homogeneous (IH), non-ideal homogeneous (NIH), and modified nonideal homogeneous (NIH-M) models were established and correlated with the experimental data to determine the parameters and errors. The NIH-M model exhibited the best agreement with the experimental data, owing to its prediction considering the non-ideality and the self-catalysis effect of acetic acid in this system.Besides,the error of NIH-M model fitting was mainly caused by the difference in solubility between [HSO3-PMPY][CF3SO3] with reactants and products in the reaction system.Furthermore,the applicability of the NIH-M model was investigated by simulating the esterification of acetic acid with three short-chain alcohols(ethanol,n-butanol,and isobutanol)catalyzed by BAILs.The NIH-M model displayed an acceptable simulation for this type of acetic acid esterification reaction catalyzed by BAILs at different ranges of the BAILs concentration and temperature. This study confirmed the industrial prospects of[HSO3-PMPY][CF3SO3] in isobutyl acetate production and the applicability of the NIH-M kinetic model in the esterification of acetic acid.
1. Introduction
As a green solvent,isobutyl acetate(IbAc)has been widely used in coating, food, pharmaceutical, cosmetics, and other industries,because of its good biodegradability, outstanding solvent activity,and low surface tension [1–4]. In industrial production, IbAc is commonly synthesized via the esterification of acetic acid (HAc)and isobutanol (IbOH) using sulfuric acid (H2SO4) as the catalyst[5,6]. Sulfuric acid is a low-cost catalyst with excellent catalytic activity. However, the practical operation still suffers from some drawbacks, including inevitable equipment corrosion, difficult product separation, and environmental pollution [7]. Therefore, it is necessary to develop novel catalysts with high catalytic activity to overcome the above issues in the synthesis of IbAc.
To conquer these problems that originated from the sulfuric acid catalyst, scholars have made effort to develop various catalysts, such as heteropoly acids, molecular sieves, ion-exchange resins, biological enzymes, and so on [8–11]. However, the practical applications of these catalysts are still limited due to their low conversion rate, poor stability, easy deactivation, and high mass transfer resistance [12,13]. Recently, ionic liquids (ILs), especially Brönsted acidic ionic liquids (BAILs), have exhibited promising potential in catalyzing the esterification or transesterification process to replace traditional mineral acids or ion-exchange resins,benefiting from their high catalytic activity, low corrosion, high thermal stability, favorable solvation, and good reusability [14–19]. The catalytic behaviors of BAILs for the esterification and transesterification reactions are closely related to the component of cations and anions [20–22]. Lunagariyaet al. [23] reported that the presence of sulfonic acid (—SO3H) functional group in the cation of BAILs could result in much higher activity in the esterification ofn-butanol with acetic acid than that of without —SO3H group. Ullahet al. [24] investigated the catalytic activity of 3-met hyl-1-(4-sulfo-butyl)-benzimidazolium ([BSMBIM]+) based BAILs with different anions (including [CH3SO3]-, [HSO4]-, [CF3CO2]-,and [CF3SO3]-) in the transesterification of vegetable oil with methanol. They found that [BSMBIM][CF3SO3] catalyst exhibited the highest activity of biodiesel production owing to its strong acidity and rapid proton ionization. Besides, Sunet al. [25] found that 1-butylsulfonic-3-methylimidazolium trifluoromethanesulfonate [BSMIM][CF3SO3] showed higher catalytic ability for the esterification reaction of ricinoleic acid with methanol than that ofp-toluene sulfonic acid(PTSA),H2SO4,and tosylate-based BAILs.Inspired by the above,the BAILs combining the—SO3H functionalized cation with[CF3SO3]-anion probably become an efficient catalyst to realize the esterification reactions.
For the esterification reactions, the study of reaction kinetics is important, which can provide the theoretical basis for the industrial application of catalysts [26]. There are several kinetic models widely used to describe esterification reactions behavior,including pseudo homogeneous (PH) model, ideal homogeneous (IH) model,nonideal homogeneous (NIH) model, Langmuir-Hinshelwood-Hou gen-Waston (LHHW) model, and Eley-Rideal (ER) model [27–30].However, due to the complexity of the esterification mechanism catalyzed by ionic liquids, there is still a gap between the prediction of the kinetic model and the reality [31–33]. Moreover, most researchs focused on using a specific kinetic model to describe an esterification reaction,while the applicability study of a kinetic model to a class of esterification reactions is still rare so far.
In this work, a novel ionic liquid catalyst,n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate ([HSO3-PMPY][CF3SO3]),was employed to catalyze the esterification of acetic acid and isobutanol to synthesize isobutyl acetate. The solubility of[HSO3-PMPY][CF3SO3] in the esterification system was studied using the σ-potential calculated based on the Conductor-like Screening Model for Realistic Solvents (COSMO-RS). Besides, the catalytic activity and corrosivity of [HSO3-PMPY][CF3SO3] were tested and compared with some common catalysts (H2SO4, PTSA,and Amberlyst 15). The chemical equilibrium and esterification reaction kinetic experiments catalyzed by [HSO3-PMPY][CF3SO3]were conducted. Based on the previous work of our group [34],the modified non-ideal homogeneous model (NIH-M) was employed to insight into the kinetic behavior, and compared with IH and NIH models. Furthermore, the applicability of the NIH-M model was explored by describing the kinetics of the esterification of acetic acid and three short-chain alcohols (ethanol,n-butanol,and isobutanol) catalyzed by BAILs.
2. Materials and Methods
2.1. Materials
Acetic acid (99.5%) and isobutanol (99.5%) were obtained from Sinopharm Chemical Reagent Co., Ltd. (China). Isobutyl acetate(99.0%), dimethyl sulfoxide (99.0%), sulphuric acid (98.0%), and Amberlyst 15 were purchased from Shanghai Macklin Biochemical Co.,Ltd.(China).P-toluene sulfonic acid(99.5%)was obtained from Damao Chemical Reagent Factory (China).N-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate (99.0%) was purchased from Shanghai Chengjie Chemical Co., Ltd. (China). All chemicals were of analytical grade and used without further purification.
2.2. Solubility analysis by COSMO-RS calculation
The σ-potential can be used to qualitatively analyze the solubility of [HSO3-PMPY][CF3SO3] with components in the esterification system of HAc and IbOH.The σ-potential of[HSO3-PMPY][CF3SO3],acetic acid,isobutanol,water,and isobutyl acetate were calculated by the COSMO-RS theory using the COSMOtherm 2021.The.cosmo files of IbAc, HAc, IbOH, and H2O were obtained directly from the database. Since [HSO3-PMPY][CF3SO3] was not involved in the database, its .cosmo file was calculated using the TURBOMOLE module with the def-TZVP basis set.
2.3. Esterification reaction
The esterification reaction was carried out in a 250 ml threenecked flask,as shown in Fig.S1 Supplementary Material The temperature and stirring speed of the mixture could be controlled by the oil bath with magnetic stirring, and samples were taken using syringes. In a typical run, HAc (1 mol) and catalyst (0.05 mol∙L-1)were taken in a three-necked flask, while IbOH (1 mol) was taken in another flask.After connecting the thermometer and condenser and sealing the flasks’ mouths, the two flasks were placed in the heat collecting constant temperature magnetic agitator to preheat.IbOH was quickly added to the three-necked flask when the temperature of the reactants was close to the reaction temperature(373.15 K). At this point, start stirring immediately (450 r∙min-1)and record the time. Samples (0.20 ml) were taken from the three-necked flask at regular intervals and cooled rapidly to 273.15 K to avoid any further reaction.The stirring rate,the molar ratio of reactants,catalyst concentration,and reaction temperature were adjusted for reaction kinetics experiments.
All samples were analyzed by a gas chromatograph(GC,Agilent 7820A) equipped with a thermal conductivity detector (TCD). The GC column was an HP-Innowax(30 m×320 μm×0.5 μm).Hydrogen was used as the carrier gas at a flow rate of 60 ml∙min-1. The temperatures of the injector and detector were set at 503.15 K.The amount of each injection was 0.2 μl. When the program started,the GC column box temperature was kept at 368.15 K for 4 min,then heated to 493.15 K at a rate of 55 K∙min-1.Finally,the GC column temperature was kept at 493.15 K for 2 min. The internal standard method was used to correct the experimental data with dimethyl sulfoxide as the internal standard substance.
The esterification reaction rate could be evaluated by the conversion rate of acetic acid (XHAc), using Eq. (1):
whereXHAcis the conversion of acetic acid;nHAc,0is the number of moles of acetic acid at the initial time,mol;nHAc,tis the number of moles of acetic acid at the testing time, mol.
The chemical equilibrium constant of the esterification reaction was determined experimentally in the range of 333.15–393.15 K with the initial molar ratio of HAc to IbOH of 1:1 and the [HSO3-PMPY][CF3SO3]concentration of 0.05 mol∙L-1.Samples were taken and analyzed at an interval of 1 h. The chemical equilibrium was reached when the content of the sample did not change.The molar fraction-based equilibrium constant (Ka) was calculated using Eq.(2).
where νiis the stoichiometric coefficient of componenti;aiis the activity of componenti;γiis the activity coefficient of componenti,which were calculated by the NRTL model;xiis the molar fraction of componenti.
The relationship between the natural logarithm values ofKa(lnKa) and the inverse absolute temperatures (1/T) could be obtained from the integral form of the van’t Hoff equation, as shown in Eq. (3):
where ΔrH0is the standard molar enthalpy of reaction, kJ∙mol-1;Ris the gas constant;T0andTare reaction temperatures, K.
2.4. Recycling test
The recyclability of[HSO3-PMPY][CF3SO3]used in the esterification reaction was investigated by recycling experiments which were conducted at 373.15 K with the initial molar ratio of HAc to IbOH of 1:1 and the catalyst concentration of 0.05 mol∙L-1. When the reaction finished, the mixture was cooled and kept still for a while.The heavy phase and ester phase were separated by a separator funnel. The heavy phase mainly includes the [HSO3-PMPY][CF3SO3],and the ester phase mainly contains the IbAc.Then,IbOH and deionized water were added separately to the heavy and ester phases. Most of the water and organic components in the heavy and ester phases were removed by a rotary evaporator at 373.15 K and a degree of vacuum of 0.085 MPa. Finally, the[HSO3-PMPY][CF3SO3] could be reused in the next cycle after removing the impurities residual in a vacuum drying oven at 393.15 K and a degree of vacuum of 0.085 MPa.The mass of recovered [HSO3-PMPY][CF3SO3] was recorded. To determine the structural changes between the fresh and recovered [HSO3-PMPY][CF3SO3] after the 5th run, the Fourier transform infrared spectroscopy (FT-IR) and nuclear magnetic resonance (NMR) analysis of fresh and recovered [HSO3-PMPY][CF3SO3] were performed.
2.5. Reaction kinetic modeling
The IH model can be expressed as Eq. (4). When the reaction system is non-ideal, the mole fraction of reactants and products can be replaced by the activity in the reaction kinetic equation.Therefore, the reaction kinetic equation based on the NIH model can be written as Eq. (5).
whereris the reaction rate,mol∙L-1∙min-1;ciis the concentration of componenti,mol∙L-1;nis the total moles of the reactants,mol;Vis the total volume of the reaction solutions, L;xiis the mole fraction of componenti;k+andk-are the forward and backward reaction rate constants, respectively, mol-1∙L∙min-1;aiis the activity of componenti.
When[HSO3-PMPY][CF3SO3]is used as the catalyst in the esterification reaction of HAc with IbOH, the catalytic activity of the esterification reaction comes from the hydrogen proton provided by the BAILs, but the catalysis of the BAILs and the self-catalysis effect of acetic acid cannot be ignored. Thus, our research group proposed the NIH-M model to describe the kinetics of the esterification reaction catalyzed by BAILs.The NIH-M model was modified by introducing the self-catalysis effect factor of acetic acid(d′)and catalyst concentration (ccat). As [HSO3-PMPY][CF3SO3] was a kind of Brönsted acid, the reaction rate should be linearly related tod′cHAc+ccat. AndcH+(Eq. (5)) could be replaced byd′cHAc+ccat.Thus, the reaction kinetic equation based on the NIH-M model can be expressed as Eq. (6):
wherek+andk-are unknown in the kinetic equations,which can be expressed by the Arrhenius equations in Eqs. (7) and (8),respectively.
whereandare the pre-exponential factors of forward and backward reaction, mol-1∙L∙min-1, respectively;Ea+andEa-are the activation energies of forward and backward reaction, J∙mol-1;Ris the gas constant;Tis reaction temperature, K.
The non-random two liquid(NRTL)model has been proven suitable for non-ideal correction of the aqueous system,so the activity coefficient (HAc, IbOH, IbAc, and H2O) can be calculated by the NRTL model equations. The binary interaction parameters and the non-randomness parameters were obtained from the Aspen Plus database.
To obtain the relationship betweenXHAcand time fitted by IH,NIH, and NIH-M models, the function between reaction rate andXHAcwas established, as shown in Eq. (9).
According to Eq. (9), the kinetic equations based on the IH model (Eq. (4)), NIH model (Eq. (5)), and NIH-M model (Eq. (6))were transformed into ordinary differential equations aboutXHAc,which were solved by fourth order to fifth order Runge-Kutta algorithm. Taking minimizing the mean absolute squared errors(MASE) between the experimental and calculated values of HAc conversion as the objective function. To obtain the unknown parameters of these kinetic equations for the esterification of HAc and IbOH catalyzed by [HSO3-PMPY][CF3SO3], the objective function was fitted by the nonlinear least-square method. The MASE is expressed as follows:
whereNis the number of data points;XHAc,cal,iis the calculated value of HAc conversion;XHAc,exp,iis the experimental value of HAc conversion.
3. Results and Discussion
3.1. [HSO3-PMPY][CF3SO3] as catalyst for esterification of HAc with IbOH
3.1.1. Catalytic activity
The basic properties of [HSO3-PMPY][CF3SO3] catalysts were listed in Table 1. [HSO3-PMPY][CF3SO3] is a solid at room temperature with a melting point of 346.65 K. The strength of Brönsted acids is usually measured by the Hammett acidity function (H0).TheH0value of[HSO3-PMPY][CF3SO3]is about 1.2 at 303.15 K(detail in Text S1,Supplementary Material).The decomposition of the[HSO3-PMPY][CF3SO3] sample starts at 553.15 K, indicating that the BAIL has good stability before 553.15 K (in Fig. S2).
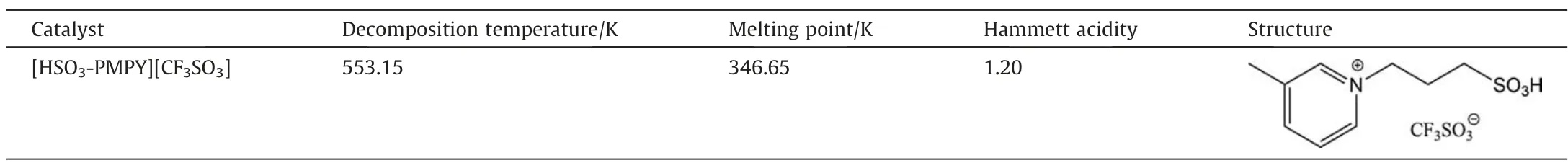
Table 1Basic properties of ionic liquid catalysts
To confirm the catalytic activity of[HSO3-PMPY][CF3SO3],esterification experiments were performed, as shown in Fig. 1(a). This esterification without catalyst still happened, while theXHAcwas low, only 25.6% after 225 min reaction. With [HSO3-PMPY][CF3SO3]as the catalyst,theXHAcreached 70.9%in 225 min,which was higher than that of conventional catalysts, such as H2SO4(68.2%), PTSA (68.5%), Amberlyst 15 (49.5%). The tiny difference in conversion rate of [HSO3-PMPY][CF3SO3] with H2SO4(or PTSA)was possibly assigned to the solubility of [HSO3-PMPY][CF3SO3]in the esterification system. Besides, the [HSO3-PMPY][CF3SO3]exhibited a rapid reaction chemical equilibrium like H2SO4and PTSA catalyst about 60 min, which was significantly faster than that of Amberlyst 15. Table S2 shows the results of the catalytic activity comparison on the [HSO3-PMPY][CF3SO3] with literature for the esterification reaction of acetic acid with isobutanol. Compared with the catalysts reported in the literature, the [HSO3-PMPY][CF3SO3]has a faster reaction rate within 1 h,a shorter time to reach the chemical equilibrium,and a higher conversion rate at equilibrium. These results revealed that [HSO3-PMPY][CF3SO3]ionic liquid containing —SO3H functionalized cation and[CF3SO3]-anion had excellent catalytic activity in the esterification of acetic acid with isobutanol.
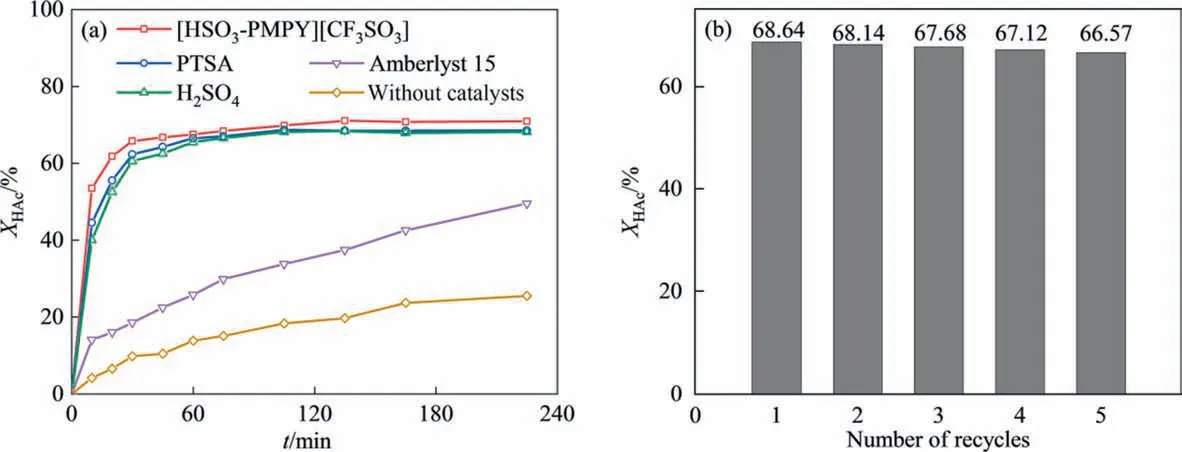
Fig. 1. (a) Catalytic activities of [HSO3-PMPY][CF3SO3], H2SO4, PTSA, and Amberlyst 15 under the same condition (hydrogen ion concentration: 0.033 mol∙L-1, stirring rate:800 r∙min-1, temperature: 363.15 K, and the initial molar ratio of HAc to IbOH: 1:1). (b) Recycling test of [HSO3-PMPY][CF3SO3] as the catalyst.
The catalyst recycling experimental result was shown in Fig. 1(b),which indicated that the decrease in theXHAcwas 2.07%when used the fifth time. The result showed that the catalyst activity of[HSO3-PMPY][CF3SO3] maintained almost its initial performance after five times cycles, which confirmed the excellent reusability of [HSO3-PMPY][CF3SO3]. [HSO3-PMPY][CF3SO3] has extremely low vapor pressure and will not be evaporated during the recovery process.The recovery rate of the[HSO3-PMPY][CF3SO3]was 98.93%after five times of continuous recycling (Table S3). The FT-IR and NMR results of fresh and recovered [HSO3-PMPY][CF3SO3] were shown in Fig. S3 and Fig. S4. These findings suggested that the[HSO3-PMPY][CF3SO3] has good stability in the esterification of acetic acid and isobutanol.
3.1.2. Solubility analysis based on COSMO-RS theory
The solubility between BAILs and the esterification reaction system would impact the interaction of molecules in the esterification system,thus possibly resulting in a change in the reaction pathway and catalytic ability[35].COSMO-RS has been proven as a valuable predictive method for the thermodynamic properties of liquid and liquid mixtures[36]. Extensive research efforts have reported that COSMO-RS can provide reasonable predictions of interaction energy and thermodynamic properties of complex mixtures involving ionic liquids [37]. Therefore, the solubilities of [HSO3-PMPY][CF3SO3] with reactants and products in the esterification of HAc with IbOH were known by σ-potential based on COSMORS theory.
As shown in Fig.2,there are three regions divided by the hydrogen bond threshold (σ = ±0.82 e∙nm-2), including the non-polar zone (-0.82 e∙nm-2<σ < 0.82 e∙nm-2), the hydrogen bond donor(HBD) zone (σ > 0.82 e∙nm-2), and the hydrogen bond acceptor(HBA)zone(σ<-0.82 e∙nm-2)[38].The smaller the radius of curvature of the σ-potential in the non-polar zone, the smaller the polarity of the substance. A higher negative value of σ-potential in the HBA zone or HBD zone indicates stronger interaction of components, while a higher positive value of σ-potential in HBD zone signifies an increase in repulsive behavior [39]. Therefore, [HSO3-PMPY][CF3SO3]and H2O showed similar polarity and strong hydrogen bond interaction with strong affinities,resulting in good intersolubility. Besides, a positive and increased valve of IbAc in the HBD range was observed (Fig. 2), which was different from that of [HSO3-PMPY][CF3SO3] and H2O. It could infer that IbAc possessed a repulsive interaction with H2O and [HSO3-PMPY][CF3SO3], the poor mutual solubility between IbAc and [HSO3-PMPY][CF3SO3]or H2O,which is beneficial to the phase separation of product. Otherwise, IbOH and HAc could be mutually soluble with other components (H2O, [HSO3-PMPY][CF3SO3], and IbAc) in the system to some extent. As a result, the excessive residue of IbOH or HAc might increase the mutual solubility of reactants with products after the esterification finished,which was not conducive to separation and purification.
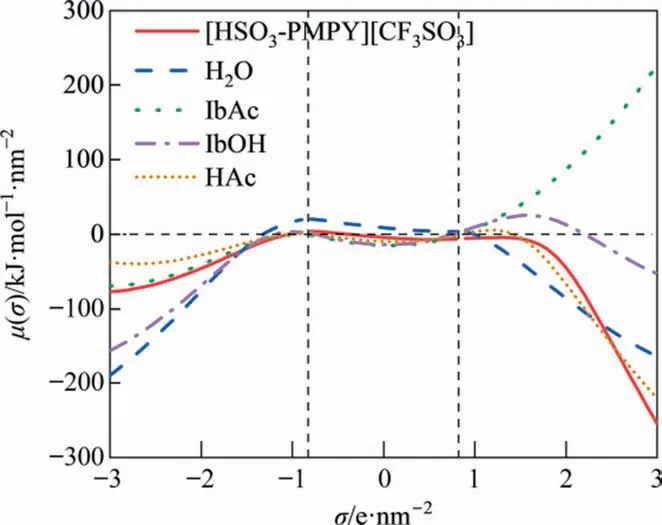
Fig. 2. σ-potential of [HSO3-PMPY][CF3SO3], H2O, IbAc, HAc, and IbOH. Vertical dashed lines represent the threshold value for the hydrogen-bond interaction(σHB = ±0.82 e∙nm-2).
According to the above analysis,the possible process of esterification of HAc and IbOH catalyzed by [HSO3-PMPY][CF3SO3] was deduced in Fig. 3. There were large volumes of IbOH and HAc in the early stage of this esterification reaction, which made the system to form a homogeneous phase. Then, [HSO3-PMPY][CF3SO3]made full use of its nature of anion and cation to catalyze the esterification reaction to produce isobutyl acetate and water [40,41].Due to their different solubility between [HSO3-PMPY][CF3SO3]and products,[HSO3-PMPY][CF3SO3]combined with water to form a heavy phase and promoted the separation of IbAc from the heavy phase to form an ester phase in the top part of the esterification system,which led to both phase equilibrium and chemical equilibrium constantly broken(Fig.S5).In this process,the IbAc was separated constantly into the ester phase and the reactants were consumed until reaching the reaction equilibrium,which probably was the reason for the higher conversion rate with [HSO3-PMPY][CF3SO3] as the catalyst than that of H2SO4and PTSA catalysts(Fig. 1(a)).
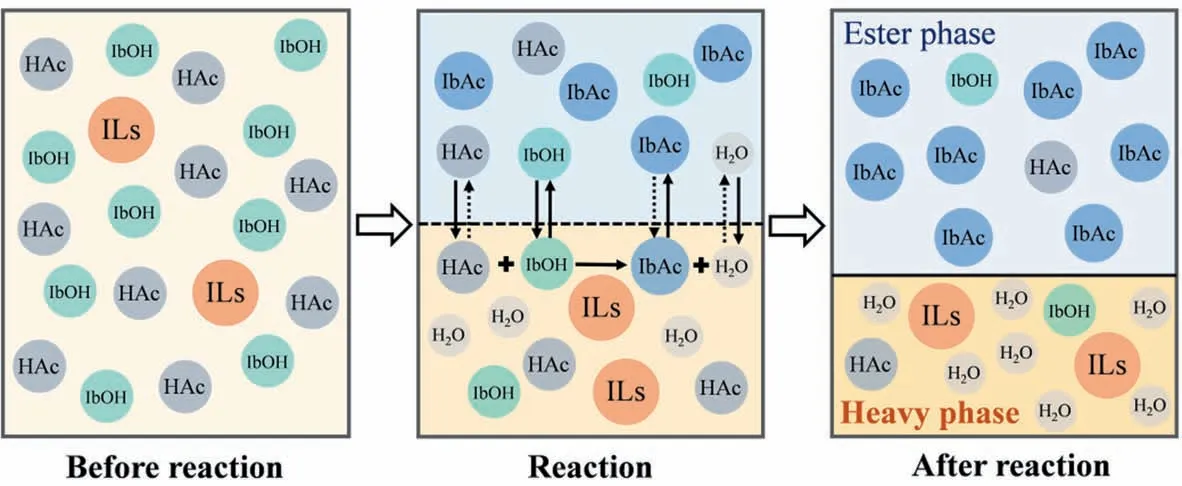
Fig. 3. Possible catalytic process of synthesizing IbAc by [HSO3-PMPY][CF3SO3].
3.1.3. Corrosion rates
The corrosion ability of H2SO4, PTSA, Amberlyst 15, and [HSO3-PMPY][CF3SO3] for S304 stainless steel was also tested, detailed in Text S2. As shown in Fig. 4, the S304 stainless steel blocks were severely corroded in H2SO4solution and PTSA solution, and the corrosion rates were 21.72 g∙m-2∙h-1and 23.64 g∙m-2∙h-1respectively. However, no corrosion behavior of the S304 stainless steel block was detected in the mixture of Amberlyst 15 and water. It was observed that the corrosion rate of S304 stainless steel in the [HSO3-PMPY][CF3SO3] solution had almost noncorrosive. This finding could be attributed to the fact that the cation of [HSO3-PMPY][CF3SO3]adsorbed on the surface of stainless steel via physical adsorption and chemisorption to reduce corrosion[42].On the anodic surface, due to electrostatic interactions and complexation of [HSO3-PMPY][CF3SO3] and Fe2+, [HSO3-PMPY]+adsorbed on the anode surface to form a dense protective layer, which effectively inhibited the anodic mild steel dissolution. While on the cathodic surface, the adsorption of [HSO3-PMPY]+showed a stronger preference over the competitive adsorption of H, which prevented cathodic hydrogen evolution reactions [43–45]. Therefore, using[HSO3-PMPY][CF3SO3]as the catalyst can solve the problem of corrosion of stainless steel equipment in isobutyl acetate production.
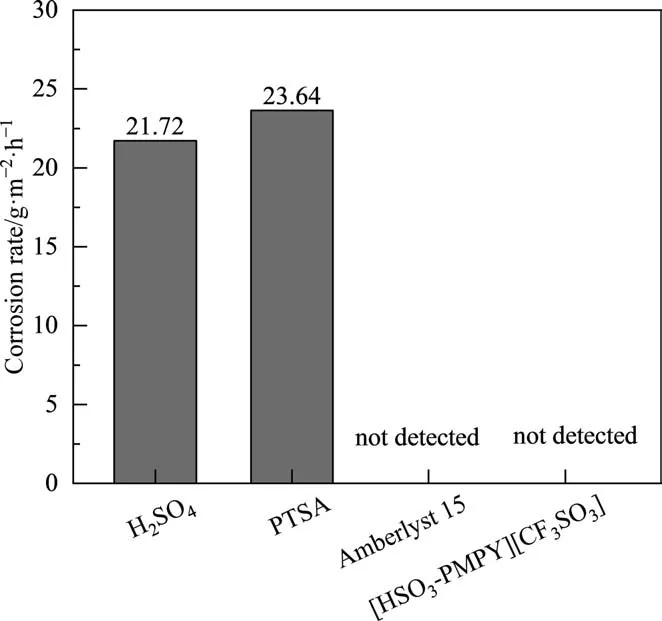
Fig. 4. Corrosion rates of different catalysts for S304 stainless steel blocks.
3.1.4. Chemical equilibrium
The relationship between the natural logarithm values ofKa(lnKa) and the inverse absolute temperatures (1/T) was shown in Fig. 5. The lnKawas linearly related to 1/T. The standard molar enthalpy of the reaction calculated was ΔrH0= -10.26 kJ∙mol-1.The ΔrH0also was calculated by the standard molar enthalpies of formation (from the NIST database), and the result was 9.53 kJ∙mol-1, which was similar to the standard molar enthalpy of reaction calculated in our experimental setup. These results showed that the chemical equilibrium experiment was reliable.Besides, due to the value of ΔrH0being slightly less than zero,the esterification reaction of acetic acid and isobutanol was slightly exothermic,and the small range of temperature change has only a slight effect on the equilibrium constant.
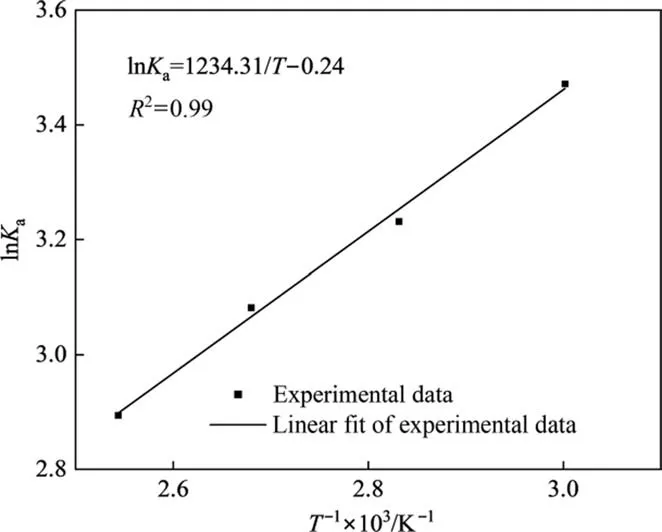
Fig. 5. Chemical equilibrium constants versus the reaction temperature (initial molar ratio of HAc to IbOH: 1:1 and [HSO3-PMPY][CF3SO3] concentration:0.05 mol∙L-1).
3.2. Kinetic experiments
3.2.1. Effect of stirring speed
Four different stirring speeds (150 r∙min-1, 300 r∙min-1, 450 r∙min-1,and 600 r∙min-1)were studied to find out the effect of stirring speed on the conversion of HAc.As shown in Fig. 6,when the stirring rate increased from 150 r∙min-1to 450 r∙min-1, the reaction rate increased significantly and the conversion of acetic acid increased from 46%to 51%in the first 10 min of reaction initiation,but the equilibrium conversion was same.When the stirring speed was higher than 450 r∙min-1,the increase in stirring speed had no significant effect on the reaction rate and the conversion of acetic acid, which could be attributed to the negligible external mass transfer resistance at this stirring speed.Consequently,the stirring speed of 450 r∙min-1was used for the subsequent reaction kinetics experiments to eliminate the influence of external mass transfer resistance.
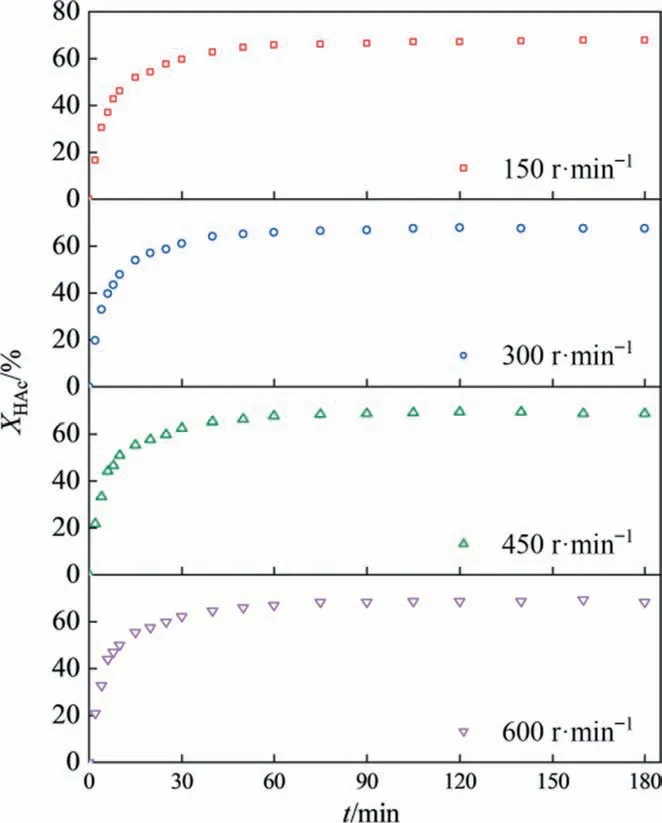
Fig. 6. Effect of stirring speed on acetic acid conversion (temperature: 373.15 K,[HSO3-PMPY][CF3SO3] concentration: 0.025 mol∙L-1, and initial molar ratio of HAc to IbOH: 1:1).
3.2.2. Effect of initial molar ratio of HAc to IbOH
Five different initial molar ratios of HAc to IbOH(2:1,1.5:1,1:1,1:1.5, and 1:2) were studied to find out the effect of initial molar ratio of HAc to IbOH on the conversion of HAc. As shown in Fig. 7, the conversion of HAc increased with increasing IbOH in the mixture. When the initial molar ratios of HAc to IbOH from 1:1 to 1:1.5 and 1:2,the increment of the conversion of HAc gradually decreased. The concentration of isobutyl acetate reached the highest when the initial molar ratio of HAc to IbOH was 1:1. The excessive reactants not only increased the cost of separation but also were not conducive to product separation and catalyst recycling. Therefore, the initial molar ratios of HAc to IbOH of 1:1 was chosen as the optimal reaction condition to obtain a high yield.
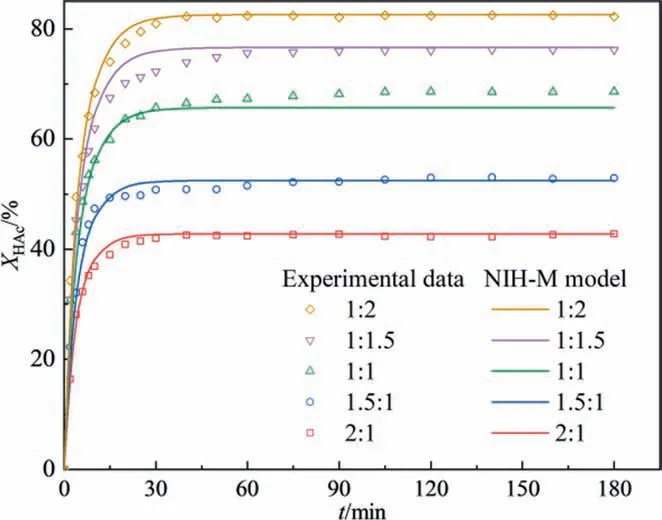
Fig. 7. Effect of initial molar ratio of HAc to IbOH on acetic acid conversion(temperature: 373.15 K, and [HSO3-PMPY][CF3SO3] concentration: 0.05 mol∙L-1).
3.2.3. Effect of catalyst concentration
Four different catalyst concentrations (0.0125 mol∙L-1,0.0250 mol∙L-1, 0.0500 mol∙L-1, and 0.0750 mol∙L-1) were studied to find out the effect of catalyst concentration on the conversion of HAc. As shown in Fig. 8(a), when the concentrations of [HSO3-PMPY][CF3SO3] increased from 0.0125 mol∙L-1to 0.0500 mol∙L-1,the reaction rate significantly increased and the reaction equilibrium time dramatically shortened. These were attributed to the presence of more catalytic active sites when the BAILs concentration increased, which speeded up the reaction rate. When the catalyst concentration was higher than 0.05 mol∙L-1, the increase in reaction rate was not obvious,which suggested the catalytic active sites tended to saturate. Therefore, the concentration of [HSO3-PMPY][CF3SO3]of 0.05 mol∙L-1was chosen as the optimal reaction condition. After 120 min of reaction, theXHAcwas found to be slowly enhanced, which might be owing to the [HSO3-PMPY][CF3SO3] promoting the phase separation of the esterification system and pushing forward movement of the chemical equilibrium.However,the amount of[HSO3-PMPY][CF3SO3]in the esterification system was small, so its effect on the chemical equilibrium was also little.
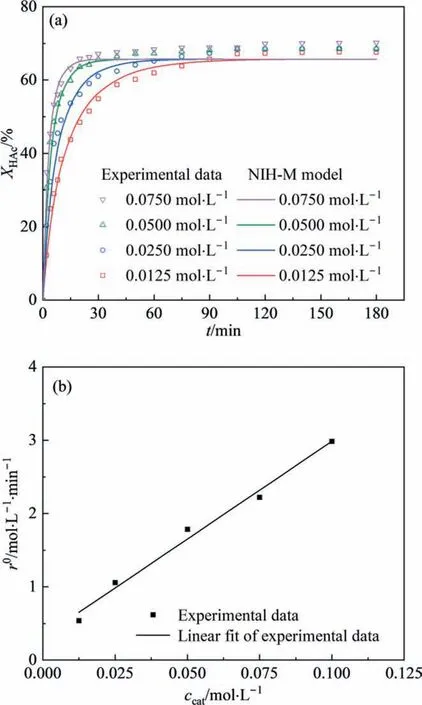
Fig. 8. (a) Effect of [HSO3-PMPY][CF3SO3] catalyst concentration on acetic acid conversion, (b) relation of initial reaction rate versus catalyst concentration,(temperature: 373.15 K, and the initial molar ratio of HAc to IbOH: 1:1).
In addition, the relationship between the initial reaction rate(r0) and the concentration of [HSO3-PMPY][CF3SO3] was also studied.The calculation method was the same as that used by Liaoet al. [46]. As shown in Fig. 8(b), ther0was linearly and positively correlated withccat(R2>0.98),and the function could be expressed as Eq. (11). In the reaction kinetic equation (Eq. (6)), the self-catalysis effect factor of acetic acid could be calculated asd′=a÷b÷cHAc,0=0.316÷26.7÷6.67=0.00177.
3.2.4. Effect of reaction temperature
Four different reaction temperatures (353.15 K, 363.15 K,373.15 K,and 383.15 K)were studied to find out the effect of reaction temperature on the conversion of HAc.As shown in Fig.9,the increasing reaction temperature from 353.15 K to 373.15 K was favorable to accelerate the esterification reaction, and the equilibrium time shortened from 60 min to 40 min. With the further increase of reaction temperature, the equilibrium time changed slightly. This is because the effective collisions of molecules were increased with increasing reaction temperature,which accelerated the reaction rate.And the standard molar enthalpy of the reaction calculated was ΔrH0= -10.26 kJ∙mol-1(Section 3.1.4),which suggested the small range of temperature change had only a slight effect on the chemical equilibrium. Therefore, according to the incremental change in reaction rate, the reaction temperature of 373.15 K was the optimal reaction condition.
3.3. Reaction kinetic model
The reaction kinetic parameters and errors of fitting experimental data of the esterification of acetic acid with isobutanol catalyzed by [HSO3-PMPY][CF3SO3] using IH, NIH, and NIH-M models were listed in Table 2. The MASE and the mean relative deviation (MRD) ofXHAc,cal,iandXHAc,exp,ifollowed an order of NIH-M model < NIH model < IH model. The NIH-M model had the highest fitting accuracy with MASE of 5.47 × 10-4and MRD of 3.48%,which indicated that the non-ideal correction of the reaction system by the NRTL model and the introduction of the selfcatalysis effect factor of acetic acid could improve the accuracy of the kinetic models. The obtained activation energy of the esterification reaction of HAc with IbOH catalyzed by [HSO3-PMPY][CF3SO3] (30.3 kJ∙mol-1) was lower than that catalyzed by Amberlyst 15(41.5 kJ∙mol-1)[6]and Amberlite IR-120(49 kJ∙mol-1)[47],suggesting a better catalytic activity of [HSO3-PMPY][CF3SO3] for the esterification reaction.
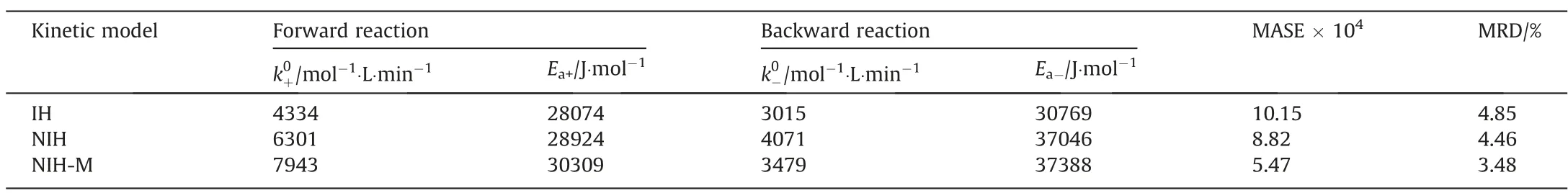
Table 2Parameters and errors of the kinetic models in the esterification of HAc and IbOH
The catalytic activity of[HSO3-PMPY][CF3SO3]depended on the structure of their cation and anion. Combined with the Hammett acidity, solubility, and reaction kinetic results, the reasons for the improvement in the catalytic activity of [HSO3-PMPY][CF3SO3]were analyzed from the following two aspects: (1) As a Brönsted strong acid, the [HSO3-PMPY][CF3SO3] could rapidly ionize a proton due to the negative induction effect of[CF3SO3]-and the—SO3-H functional group, which facilitated the combination of proton and carbonyl group of acetic acid to form a carbocation intermediate[19,48].Furthermore,the fluorine atom of[CF3SO3]-could generate a hydrogen bond with the hydroxyl group in the isobutanol molecule, which made nucleophilic substitution reaction between carbocation and isobutanol easier [40,49]. Therefore, the [HSO3-PMPY][CF3SO3] can improve the esterification reaction rate of acetic acid and isobutanol.(2)The difference in solubility between [HSO3-PMPY][CF3SO3] with reactants and products in the esterification reaction system pushed the movement of the chemical equilibrium to the product side and improved catalytic activity.
The reaction kinetic equation based on the NIH-M model could accurately describe the esterification of HAc and IbOH catalyzed by[HSO3-PMPY][CF3SO3], and was shown in Eq. (12):
The reason for the error in NIH-M model fitting could be analyzed by comparing the gap between NIH-M model fitting curves and experimental data. The NIH-M model fitting curves were drawn in the results of the kinetic experiments. In the first 30 min of reaction initiation, the NIH-M model fitting curves exhibited an excellent fit for the experimental data in changing reactant molar ratio (Fig. 7) and catalyst concentration (Fig. 8(a)),which could be ascribed to the NIH-M model considering the catalytic effect of functionalized groups (—SO3H and [CF3SO3]-) from[HSO3-PMPY][CF3SO3] and the self-catalysis effect factor of acetic acid. After 60 min of reaction, the NIH-M model fitting curves showed good agreement with experimental data with an initial molar ratio of reactants was not 1 (Fig. 7). Notably, the NIH-M model fitting curve with an equal molar of HAc and IbOH was significantly lower than the experimental data. This phenomenon could be explained by the phase separation and chemical equilibrium shift based on solubility analysis calculated by the COSMORS theory (Section 3.1.2). HAc and IbOH had certain mutual solubility with [HSO3-PMPY][CF3SO3], H2O, and IbAc. When the initial reactant molar of HAc and IbOH was unequal, excessive HAc or IbOH in the system certainly weakened the separation degree between the ester phase and the heavy phase, leading to a low equilibrium conversion rate. Besides, the simulated curves by the NIH-M model with different catalyst concentrations (Fig. 8(a))exhibited a lower fitting conversion than that of experimental data after 60 min of reaction.Meanwhile,with increasing[HSO3-PMPY][CF3SO3] catalyst concentration, the NIH-M model fitting error increased,which might be attributed to the enhanced phase separation degree. These results probably suggested the NIH-M model could not describe the positive promotion of equilibrium conversion caused by adding[HSO3-PMPY][CF3SO3]. In the kinetic experiment of studying temperature (Fig. 9), the NIH-M model fitting accuracy in the higher reaction temperature (373.15 K and 383.15 K) was better than that of lower temperature, which suggested that the[HSO3-PMPY][CF3SO3]catalyst had higher catalytic activity at high temperatures. The above results also confirmed that the speculation about the reaction process (Fig. 3) was reasonable.
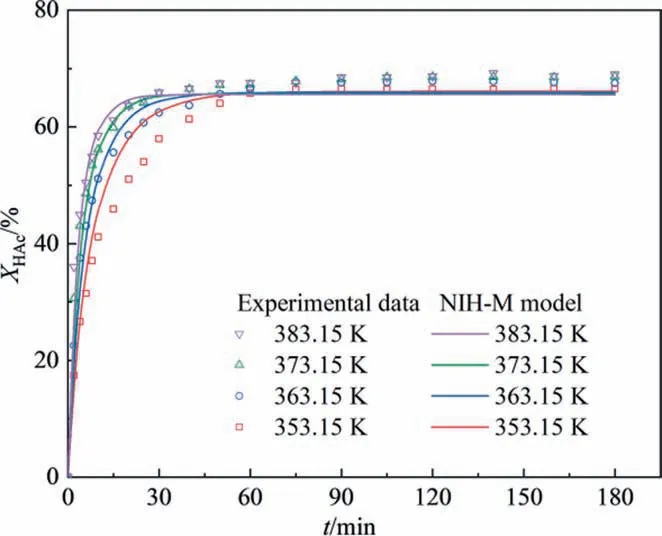
Fig. 9. Effect of reaction temperature on acetic acid conversion ([HSO3-PMPY][CF3SO3] concentration: 0.05 mol∙L-1, and the initial molar ratio of HAc to IbOH:1:1).
3.4. NIH-M kinetics model for acetic acid esterification reactions
Recently, BAILs used as catalysts have shown enormous potential in the reaction of acetic acid esterification [50,51]. It is necessary to develop a precise kinetics model to describe the catalytic reaction behavior for the subsequent industrialization application.Whether the NIH-M model can be applied to descript this type of acetic acid esterification reaction catalyzed by BAILs? Combined the previous works from our group [33,34], the applicability of the NIH-M model to describe the kinetics of esterification of acetic acid and three short-chain alcohols(ethanol,n-butanol,and isobutanol) catalyzed by BAILs were evaluated and analyzed. The reaction conditions of esterification of acetic acid with three shortchain alcohols (initial molar ratio of reactants of 1:1) catalyzed by BAILs were listed in Table 3. The MASE and MRD between the experimental data and the NIH-M model simulation data were calculated when the concentration of BAILs or temperature ofdifferent ranges were used as variables, further exploring the fitness degree of the NIH-M model under different reaction conditions.
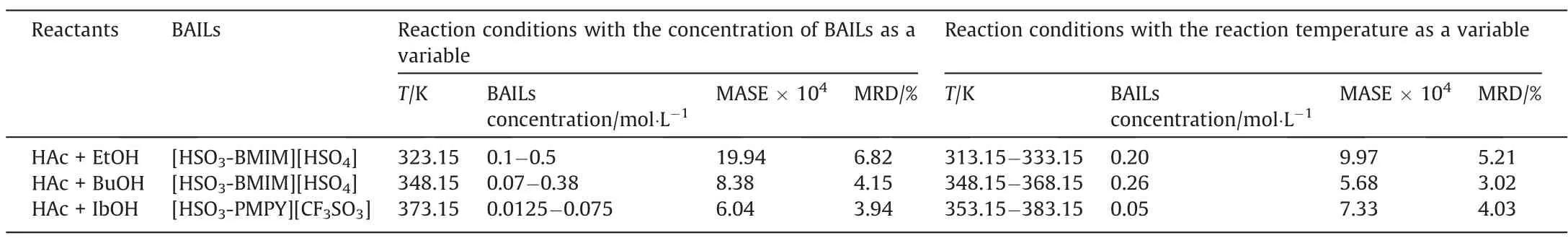
Table 3Reaction conditions of esterification of acetic acid with three short-chain alcohols, including ethanol (EtOH), n-butanol (BuOH), and isobutanol (IbOH)
The MASE and MRD decreased with the decrease of BAILs concentration when the concentration of BAILs was variable(Table 3).Such a result might be attributed to the fact that the positive promotion effect of BAILs on the chemical equilibrium was not considered in the NIH-M model. And the higher concentration of BAILs,the greater error.Besides,when the reaction temperature was variable, their MASE and MRD decreased obviously at the range of higher reaction temperature. These results showed that the NIHM model simulation results were in good agreement with the experimental data with the MASE of less than 2 × 10-3and the MRD of less than 7%, which were within the acceptable limit.Therefore, the NIH-M model can appropriately describe the acetic acid esterification kinetic behavior catalyzed by BAILs.
4. Conclusions
In this research,an efficient,non-corrosive,and reusable[HSO3-PMPY][CF3SO3]catalyst for the esterification of HAc and IbOH was presented.The solubility of[HSO3-PMPY][CF3SO3]in the esterification system was analyzed based on COSMO-RS theory,which confirmed that [HSO3-PMPY][CF3SO3] was easy to recycle and could promote the forward movement of chemical equilibrium. Compared with different catalysts (including H2SO4, PTSA, and Amberlyst 15), [HSO3-PMPY][CF3SO3] exhibited more excellent catalytic activity,more excellent reusability,and less corrosiveness.A rapid chemical equilibrium was reached with [HSO3-PMPY][CF3SO3],H2SO4, and PTSA, while a slightly higher equilibrium conversion over [HSO3-PMPY][CF3SO3] catalyst was observed because of the repulsion between [HSO3-PMPY][CF3SO3] and product IbAc.Besides, the kinetic experiments were carried out, meanwhile,the reaction kinetics equations based on IH,NIH,and NIH-M models were established to describe the esterification kinetics of HAc and IbOH.The results showed that the NIH-M model fitting curves were in good agreement with the experimental data with theMASEof 5.47×10-4and the MRD of 3.48%,owing to the modification of the non-ideality of the reaction system and self-catalysis effect of acetic acid.Moreover,when the concentration of BAILs or temperature of different ranges were used as variables,the NIH-M model was in good agreement with the experimental data for esterification of HAc and three short-chain alcohols with theMASEof less than 2×10-3and the MRD of less than 7%.This result further confirmed that the NIH-M model could appropriately describe the type of acetic acid esterification reaction kinetic behavior catalyzed by BAILs. Our study provided a new candidate to improve the industrial production of isobutyl acetate using ionic liquids catalysts and affords ideas for improving and applicating the kinetic models in the esterification reactions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22168004), the Natural Science Foundation of Guangxi Province (2017GXNSFDA198047), and the Dean Project of Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology (2019Z010, 2021Z013).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.022.
Nomenclature
aiactivity of componenti, mol∙L-1
ciconcentration of componenti, mol∙L-1
d′self-catalysis effect factor of acetic acid
Ea+,Ea–activation energies of forward and backward reaction,J∙mol-1
ΔrH0standard molar enthalpy of reaction, kJ∙mol-1
Kamolar fraction-based equilibrium constant
k+, k–the forward and backward reaction rate constants, mol-1-∙L∙min-1
,pre-exponential factors of forward and backward reaction,mol-1∙L∙min-1
MASE mean absolute squared errors
MRD mean relative deviation, %
Nnumber of data points
ntotal moles of the reactants, mol
nHAc,tnumber of moles of acetic acid at the testing time, mol
Rgas constant,R= 8.3145 J∙K-1∙mol-1
r0initial reaction rate, mol∙L-1∙min-1
rreaction rate, mol∙L-1∙min-1
Treaction temperatures, K
testerification reaction time, min
Vtotal volume of the reaction solutions, L
XHAcconversion of acetic acid, %
XHAc,cal,i,XHAc,exp,icalculated value and experimental value of HAc conversion, %
ximolar fraction of componenti
γiactivity coefficient of componenti
μ(σ) value of σ-potential displayed, kJ∙mol-1∙nm-1
σ shielding charge density, e∙nm-1
νistoichiometric coefficient of componenti
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
- Separation of lithium and nickel using ionic liquids and tributyl phosphate
