COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
Jie Wei, Chengcai Zhao, Ming Chen, Fengying Liu, Limin Zhao, Hui Zhao,, Zhong-Yong Yuan
1 School of Materials Science and Engineering, Liaocheng University, Liaocheng 252000, China
2 College of Chemistry and Chemical Engineering, Xinyang Normal University, Xinyang 464000, China
3 Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), School of Materials Science and Engineering, Nankai University, Tianjin 300350, China
Keywords: COF Co@NC Composites Electrochemistry Catalysis Zn-air battery
ABSTRACT Precise modulation of the structure and composition of electrocatalysts is critical for promoting the kinetically sluggish process of oxygen reduction reaction (ORR) and oxygen evolution reaction (OER).Covalent organic frameworks (COF) offer a novel way to create highly efficient electrocatalysts due to their tunable composition, structure and surface area. Herein, we report a high-efficiency bifunctional electrocatalyst comprising Co nanoparticles embedded within N-doped carbons(Co@NCs)for Zn-air batteries(ZABs).The Co@NC is yielded via the coordination of a triazine COF with Co-containing precursors and subsequent calcination under inert atmosphere. The as-prepared Co@NC exhibits remarkable ORR/OER performance and great potential in rechargeable ZABs.The liquid ZAB constructed with Co@NC provides both high specific capacity and power density.Remarkably,the ZAB exhibits a voltage gap of 0.8 V during discharge and charge cycles and high stability for 220 h compared to the Pt/C-assembled battery.This strategy for regulating electrocatalytic activities of COF-derived carbon materials could be expanded for creating various carbon catalysts.
1. Introduction
With the increasing energy needs and damaging environment,the rechargeable Zn-air battery featuring environmental friendliness,low cost and high energy density has aroused much attention as a promising energy device. It is acknowledged that the energy conversion performance of Zn-air batteries (ZABs) is greatly affected by the oxygen evolution reaction(OER)and oxygen reduction reaction (ORR) happened at the cathode during the charging and discharging processes [1–3]. However, both the OER and ORR possess sluggish kinetics, which need to be further improved by using electrocatalysts. The currently available electrocatalysts are noble metals such as IrO2/RuO2for OER and Pt/Pd for ORR [4–6].Although the noble metal catalysts exhibit remarkable electrocatalytic activity, their high cost, inferior bifunctional catalytic property and poor stability seriously hinder the extensively commercial applications. Herein, there is a pressing need to explore low-cost and sustainable electrocatalysts with high performance for both OER and ORR.
Carbonaceous materials, especially heteroatom-doped carbon materials, have received much attention owing to their great potential in various energy storage and conversion processes.One prominent illustration is nitrogen-doped carbons,which have indicated widespread application in the field of electrocatalysis[7].However, their electrochemical properties still need to be further improved compared to noble-metal catalysts. Embedding transition metal nanoparticles into nitrogen-doped carbon materials(TM@NC)has been considered as a promising strategy due to their highly synergistic effect between TM-N-C moieties and controllable N-doped framework. For instance, the triphenylimidazolecontaining polymer-derived FeNi, CoNi or FeCo nanoparticlesencapsulated N-doped carbon electrocatalysts have shown promising bifunctional ORR/OER properties [8–10]. The incorporation of TM ions gave rise to the formation of TM nanoparticles and TMNxactive sites which can improve the electrocatalytic process.Moreover, it features low cost and superior stability compared with noble metal catalysts. The carbons can protect it from corrosion in the electrolyte. However, it is difficult to control the structural uniformity of TM/N-C. Besides, exploring a novel type of carbon-based TM/N catalyst with good conductivity and porosity and investigating its structure-performance relationship is still a challenge.
As is known,both the ORR and OER reactions occur at the threephase interface of gas, liquid and solid. Thus, increasing the number of active sites and improving their utilization efficiency is imperative for boosting the activities of electrocatalysts, which can be achieved by the structural and morphological engineering[11–13]. The morphological and structural engineering can give the catalyst an ideal structure with accessible active sites and good mass transportation, thus yielding the promising catalytic performance. Covalent organic framework (COF) is an ideal platform for dedicated structural and morphological engineering owing to its structure diversity, large specific surface area, high porosity and flexible functionality,which allows them to be applied in various fields such as photocatalysis,electrocatalysis, gas storage and fluorescence detection [14–16]. The controllable structure can be obtained by choosing different building blocks and linkage motifs,allowing the facile engineering of electrocatalyst. Besides, COFs with N-containing ligand can be turned into N-doped carbon materials by calcination under inert atmosphere [17–19]. Transition metal ions can be easily coordinated with the N-containing ligands to obtain TM/N-C materials,showing superb electrocatalytic activity [20–22]. As such, using COFs as the platform is accessible to investigate the structure-performance relationship. However, the bifunctionality towards both ORR and OER is relatively scarce and the catalytic performance needs to be further improved.Moreover,the effect of TM content on catalytic performance should also be explored.
Therefore, we report a COF-derived Co nanoparticles@N-doped carbon (Co@NC) electrocatalyst through an impregnation method and a subsequent annealing process. As revealed, the structure and morphology of the catalyst is markedly modulated by varying the adding amount of Co precursor.Thanks to the unique morphology and the synergistic effect among the catalytical active sites,the resultant catalyst with an optimal Co content exhibits superior activity towards the ORR in 0.1 mol∙L–1KOH electrolyte, which even surpassed that of the commercial Pt/C catalyst. The high OER activity can also be achieved. When the catalyst was used in a home-made ZAB, a large peak power density and energy density and superb long-term durability were obtained, indicating its promising potential in the industrial applications.
2. Experimental
2.1. Material synthesis
0.1 mmol(21 mg)of 1,3,5-triformylphloroglucinol(Tp)was dissolved in the mixture of 1,4-dioxane(2 ml)and mesitylene(1 ml).0.15 mmol(27.9 mg)of 5,5′-diamino-2,2′-bipyridine(Bpy)was dissolved in the mixture of mesitylene (1 ml), 36% (mass) of acetic acid (300 μl) and 1,4-dioxane (2 ml), which was introduced into the former Tp solution. The solution mixture was then kept inside an autoclave under a condition at 120 °C for 72 h and further cooled to room temperature. A dark red product was obtained using filtration and washed thoroughly with tetrahydrofuran and extracted by Soxhlet extractor. Then the collected solid was subjected to drying at 80 °C in a vacuum to remove the solvent and signified as COF.
50 mg of the COF was dispersed in a solution of methanol(10 ml) and a certain amount of cobalt acetate tetrahydrate(Co(OAc)2∙4H2O) and vigorous stirred for 4 h. Subsequently, the Co2+-absorbed COF was collected by centrifugation and dried at 60 °C for 12 h. Finally, the powder was pyrolyzed at 800 °C for 2 h under N2atmosphere.After cooling down to room temperature,the obtained product was denoted as Co@NC.In addition,the products with various Co contents were synthesized under the identical conditions besides Co2+concentrations (0.17 mmol∙L–1,0.34 mmol∙L–1and 0.51 mmol∙L–1), signified as Co@NC-x(x= 1, 2,3). For the purpose of comparison, the N-doped carbons (denoted as NC) was also prepared using the same synthesis procedure in the absence of Co2+.
2.2. Characterization
X-ray diffraction (XRD) patterns were obtained on a Bruker D8 Focus diffractometer using Cu-Kα radiation. The microstructures and morphologies of samples were conducted with transmission Electron Microscope (TEM, Jeol JEM-3200FS) and field emission scanning electron microscope (FESEM, Jeol JSF-7500L). X-ray photoelectron spectra(XPS)data were obtained on a Kratos Axis Ultra DLD spectrometer using a Al X-ray source (1486.6 eV). Specific surface area and pore size distribution were obtained by N2adsorption–desorption analysis on a BELSORP-Max Microtrac BEL analyzer. Raman spectra were achieved on a Thermo-Fisher Scientific DXR spectrometer (532 nm excitation laser).
2.3. Electrochemical measurements
The electrochemical measurements were conducted on the electrochemical workstations (Gamry Reference 3000 and Reference 600+) using a three-electrode system. The catalyst powder(5 mg) was ultrasonically dispersed into a mix solution of 1 ml Milli-Q water/isopropanol (1/4, volume ratio) containing 20 μl of 0.5% (mass) Nafion solution. After ultrasonication, the catalyst ink (10 μl) was taken out and dropped onto a rotating disk electrode(RDE)with the catalyst loading of 0.255 mg∙cm-2.A graphitic rod as the counter electrode, the as-obtained RDE acted as the working electrode and an Ag/AgCl electrode filled with saturated aqueous KCl solution as the reference electrode in the presence of 0.1 mol∙L–1KOH aqueous solution (pH = 13) as electrolyte. The reversible hydrogen electrode (RHE) was used to normalize all the collected potentials (ERHE=EAg/AgCl+ 0.059pH + 0.196).
Cycle voltammetry (CV) and linear sweep voltammetry (LSV)plots for the ORR were obtained in O2-saturated 0.1 mol∙L–1KOH electrolyte with the scan rate of 20 mV∙s-1and 5 mV∙s-1, respectively.The LSV plots for the OER were measured in 0.1 M KOH electrolyte with the scan rate of 5 mV∙s-1. The electrochemical impedance spectroscopy (EIS) measurements were performed from 0.01 to 105Hz. The transferred electron number (n) and percentage of peroxide (cHO2–, %) were obtained by rotating ring-disk electrode(RRDE)measurements.The value ofcHO2–andnwas determined based on the following equations:
whereNis the current collection coefficient of Pt ring(0.37),idis the disk current, andiris the ring current.
In a clear, strong voice, Sonali sang to her fellow passengers. She then walked up and down the aisle with one of the crewmembers, receiving the smiles, thanks and love of all the United passengers. At the end of the flight, who stood on top of a box at the door with the flight attendant, thanking everyone and saying good-bye? Our Sonali!
For the Zn-air battery test, a Zn-air battery was constructed by using the catalyst-loaded carbon paper with a loading of 1 mg∙cm-2as an cathode, the Zn plate as the anode and a aqueous solution containing 6 mol∙L–1KOH+0.02 mol∙L–1Zn(CH3COO)2as the electrolyte. The polarization curve tests were conducted by using LSV at a scan rate of 5 mV∙s-1and potential range of 0.5–2.5 V.
3. Results and Discussion
3.1. Preparation and structural characteristics
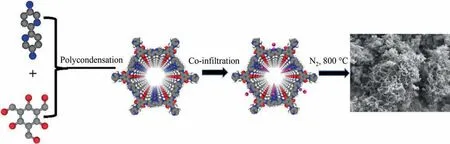
Fig. 1. Schematic representation of Co@NC fabrication.
As schematically indicated in Fig.1,the nitrogen-rich COF material (TpBpy) was first prepared by reacting the organic linkers Tp and BpyviaSchiff base reaction.As shown in Fig.S1 in Supplementary Material, the small-angle X-ray diffraction (XRD) of TpBpy displayed an intense peak at 3.6°, belonging to the (1 0 0) plane.The diffraction peak at 26° was ascribed to the (0 0 1) plane that relates to the π–π stacking between COF layers[23].Subsequently,Co2+-absorbed TpBpy(Co-TpBpy)were obtained by introducing the TpBpy powder into cobalt acetate solution at room temperature.For comparison, the TpBpy without cobalt ions and Co-TpBpy(Co-TpBpy-1, Co-TpBpy-2 and Co-TpBpy-3) with different cobalt content were prepared in the same way. Finally, NC and Co@NC with different Co contents (Co@NC-1, Co@NC-2 and Co@NC-3)were obtained by calcining the TpBpy and Co-TpBpy at 800 °C under N2atmosphere, respectively. At the calcination step, the COFs were transformed to N-doped porous carbons, and the adsorbed Co2+was reduced into metallic Co.The formed Co atoms diffused through the carbons and agglomerated into Co nanoparticles, yielding the Co@NC materials.
Field emission scanning electron microscopy (FESEM) was applied to analyze the morphological features of the assynthesized materials. Fig. 2(a) shows the NC possesses a shortrod morphology. The length of the short rod is about several tens of nanometers. After Co-infiltration, the resultant Co@NC-1 presents a coexistence of granular and rod morphology (Fig. S2(b)).The length of the rod increases with the increase of cobalt content.As illustrated in Fig. 2(b), Co@NC-2 presents a rod morphology with many Co nanoparticles on it. The length of the rod is about several hundred nanometers. The study of the elemental distribution of the Co@NC-2 using EDS elemental mapping images manifests the existence of C, N, Co and O elements (Fig. S3). Moreover,all the elements are homogenously scattered on the sample surface.As shown in Fig.2(c)and(d),the average size of Co nanoparticles is ~25 nm. The Co@NC-3 shows a sheet-like morphology with further increase of Co content (Fig. S2(d)). X-ray diffraction(XRD)further identified the phase components of samples.As plotted in Fig.2(f),the diffractions of NC at 24.4°and 44°are indexed as(1 1 0) and (0 0 2) of graphite (JCPDS no. 75-1621), indicating the successful conversion of TpBpy to carbon materials [17]. For the Co@NC materials, the diffraction peaks at 44.5° and 51.5° and 76°were ascribed to (1 1 1), (1 0 0) and (1 1 0) planes of metallic Co(JCPDS no. 15-0806), respectively [20]. The broad diffraction peak at ~24° can be ascribed to (0 0 2) plane of graphite carbon, originating from the calcination of COFs. These results suggest the Co species are chemically coordinated to the N-doped carbons, most probably in the form of Co—C and/or Co—N bonds.
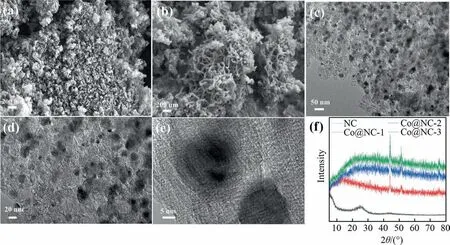
Fig. 2. FESEM images of NC (a) and Co@NC-2 (b); TEM images of Co@NC-2 (c, d); HRTEM image of Co@NC-2 (e); XRD patterns of the as-prepared samples (f).
Raman spectra (Fig. 3(a)) presented that the D band at~1341 cm-1and G band at 1579 cm-1, indicating the presence of both graphitic carbon and extrinsic defects [24]. The intensity ratio between D and G bands(ID/IG)of Co@NC-1(1.01)was slightly increased compared to that of NC(1.0),which is related with more defect structures in the carbon framework. TheID/IGvalue for Co@NC-2 and Co@NC-3 were determined to be 1.03 and 1.11,which are much higher than that of Co@NC-1, indicating a large quantity of Co atoms might be doped in the carbon framework.N2sorption isotherms were collected to investigate the porous structure and specific surface area of the as-synthesized samples.As depicted in Fig. 3(b), all the isotherms exhibit a typical type II isotherm, indicating the existence of micropores, mesopores and macropores. It is clear that applying COF as the precursor can not only promote the uniform dispersion of metal nanoparticles but also render the samples a good porosity. The Co@NC-1 possesses an inferior pore structure compared to NC sample. In the case of Co@NC-2 and Co@NC-3 materials, the isotherms are similar with that of Co@NC-1 but with an additional evident increase in N2adsorption capacity at low relative pressure, demonstrating that an additional microporosity was introduced by the increasing content of Co. The N2uptake at high relative pressure also sharply increases due to the stack of nanorods, manifesting the presence of a large amount of macropores. The detailed structural properties, as determined from the hysteresis loops for the samples, are illustrated in Table S1.The pore volume and calculated surface area of Co@NC-1 (0.22 cm3∙g-1, 41.6 m2∙g-1) are lower than that of NC (0.77 cm3∙g-1, 330.8 m2∙g-1). However, the pore volume and surface area were increased with the increase of Co content(1.03 cm3∙g-1, 250 m2∙g-1for Co@NC-2 and 1.08 cm3∙g-1,271.4 m2∙g-1for Co@NC-3), signifying the formation of cobalt nanoparticles can effectively enhance the accessibility of pores.As depicted in Fig.S4 and Table S1,Co@NC-2 and Co@NC-3 possess hierarchical porous structures with micropores, mesopores and macropores, which can facilitate the mass transportation and the enhancement of electrocatalytic activity.
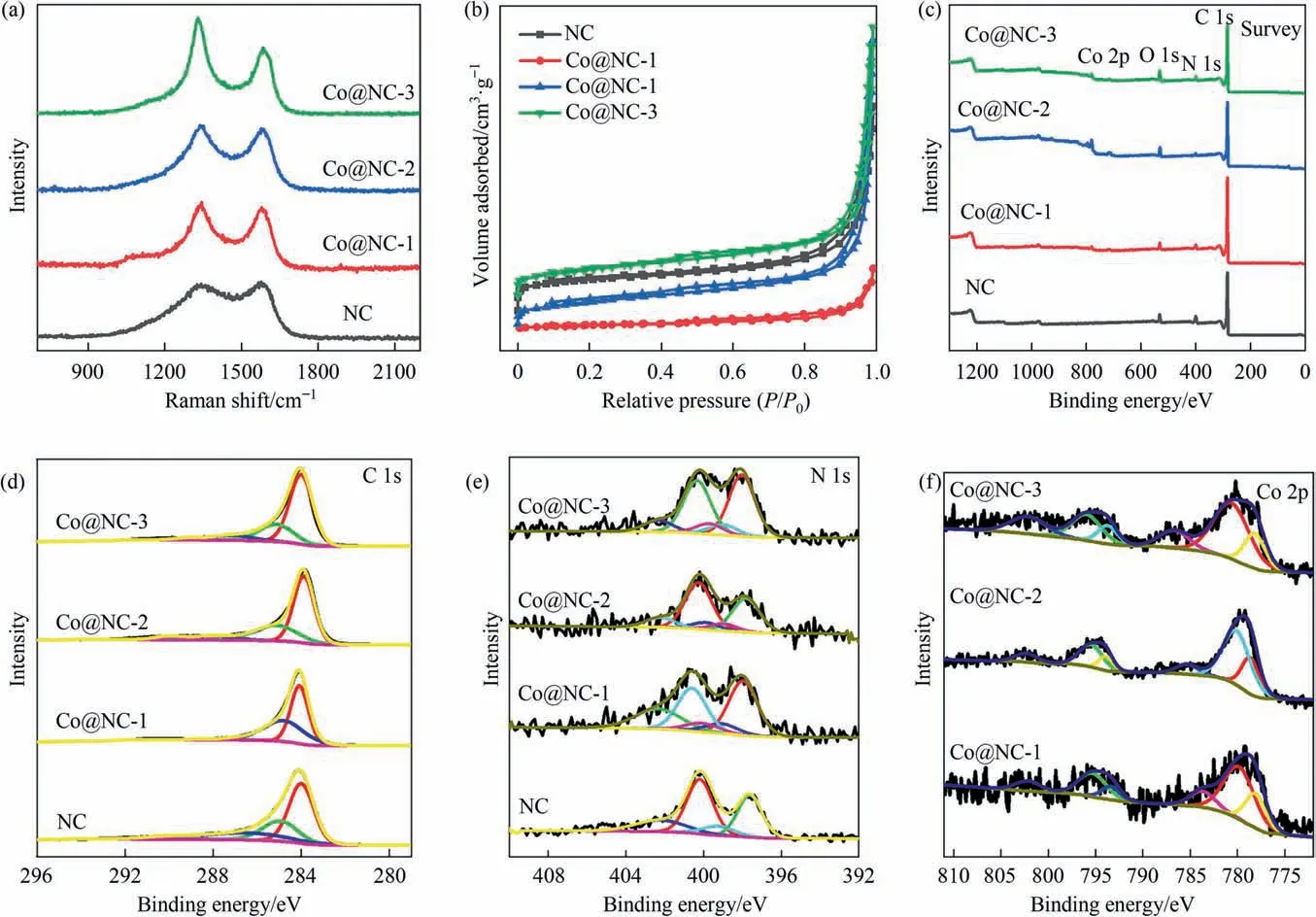
Fig. 3. Raman spectra (a), N2 sorption isotherm curves (b) and XPS survey spectra (c) of samples. High-resolution XPS C 1s (d), N 1s (e) and Co 2p (f) spectra of samples.
X-ray photoelectron spectroscopy (XPS) analyses were performed to explore the chemical constitution of the as-synthesized samples. NC exhibits C, N and O elements while all the Co@NC samples exhibit Co, C, N and O elements, proving the presence of Co species in Co@NC samples (Fig. 3(c)). The element contents of samples are presented in Table S2. Notably, the Co@NC-2 possessed the N content of 5.93% (atom), which is the highest among all the samples. It indicates that the suitable amount of Co is crucial to ensure the high-content N-doping in the Co@NC sample.High-resolution XPS spectra were taken to clarify the chemical states at the surfaces of the materials. The highresolution XPS spectra of C1s can be deconvoluted into three peaks(Fig. 3(d)). The peaks at 288.1 eV, 285.6 eV and 284.6 eV were ascribed to the O—C—O, C—N and C=C,respectively. Fig.3(e) indicates high-resolution XPS spectra of N 1s. The peaks at 402.3 eV,400.3 eV, 399.8 eV, 399.0 eV and 398.0 eV were assigned to pyridinic-N-oxide species,graphitic N,Co—Nx,pyrrolic N and pyridinic N, respectively [25,26]. The pyridinic N species can not only facilitate the ORR process by a 4e--like pathway over a wide pH range but also promote the catalytic performance for the alkaline OER [27]. For both ORR and OER, Co—Nxsite was also considered to be effective active site. Moreover, the Co atom in Co—Nxbond should be bound with pyridinic N because Co bound to pyrrolic N appears at a binding energy of ~398.5 eV[28–30].The graphitic N can improve the electron transfer process during the ORR and OER[31]. As observed in Table S3, the graphitic N (37.74% (atom)) and Co—Nx(10.9% (atom)) contents of Co@NC-2 are higher than those of other samples, which are expected to contribute significantly to the enhancement of electrocatalytic activity. The Co 2p spectra(Fig. 3(f)) was divided into six peaks of Co—N (795.4 eV), Co—Co(793.8 eV, 778.7 eV), Co—O (780.3 eV) and two satellite peak(802.3 eV,785.3 eV)[32–34].The fitted peaks of Co 2p further vindicated the presence of metallic Co, which was in agreement with the result of XRD. The results demonstrated Co was successfully incorporated into the carbon support.
3.2. Electrocatalytic characteristics
To explore the catalytic activity of the as-synthesized samples,electrochemical tests in 0.1 mol∙L–1KOH solution were carried out.The cyclic voltammetry(CV)results manifest that the cathodic peak for ORR located at 0.77 V for the NC material(Fig.4(a)).After introducing Co (Co@NC-1), this cathodic peak appears at 0.82 V,which has a 50 mV positive shift in comparison with that of the NC sample. This cathodic peak for ORR is the same with that of Pt/C catalyst. The cathodic peak current obtained for the Co@NC-1 is 1.56 mA∙cm-2that is larger than that for the Pt/C(0.87 mA∙cm-2)and NC (0.82 mA∙cm-2). The results indicate that the Co incorporated electrocatalyst has an impressive ORR activity in alkaline electrolyte. The cathodic peak potential of the Co@NC-2 (0.84 V)is more positive than other catalysts, signifying the Co@NC-2 might exhibit a superior ORR activity. The ORR activities of different catalysts were further evaluated by linear sweep voltammetry(LSV) measurement with the rotating rate of 1600 r∙min-1in KOH solution.
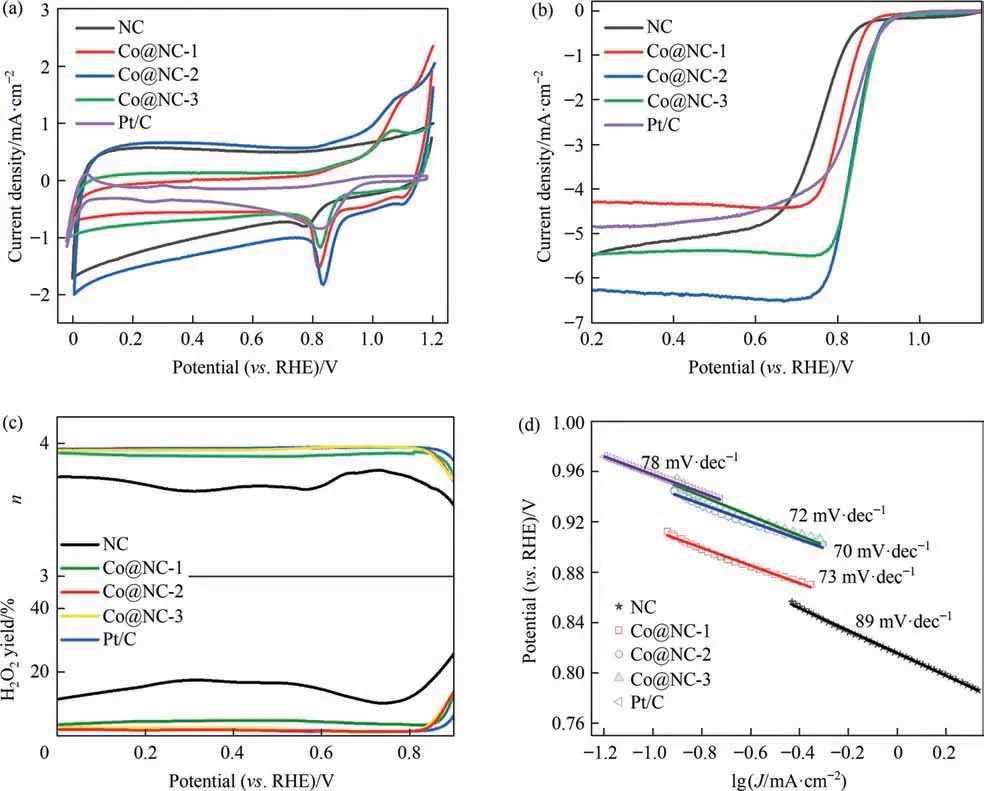
Fig.4. Electrocatalytic ORR performance of the as-prepared samples.(a)CV curves of the as-prepared samples and Pt/C for ORR in 0.1 mol∙L–1 KOH solution at 20 mV∙s-1 scan rate.(b)LSV curves of the samples and Pt/C for ORR in 0.1 mol∙L–1 KOH solution at an RDE rotation rate of 1600 r∙min-1 and a scan rate of 10 mV∙s-1.(c)The yield of H2O2 and transferred electron number (n) of the as-prepared samples as well as Pt/C for ORR in 0.1 mol∙L–1 KOH solution. (d) Tafel plots of the Pt/C and samples for ORR.
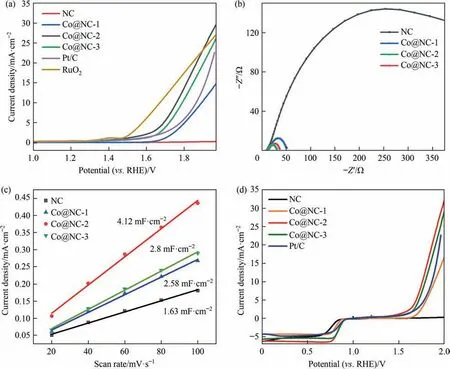
Fig.5. OER electrocatalytic performance of the as-synthesized samples.(a)LSV polarization plots of the as-prepared catalysts,Pt/C and RuO2 in 0.1 mol∙L–1 KOH with a scan rate of 5 mV∙s-1.(b)EIS Nyquist plots for the as-prepared catalysts.(c)Capacitive current densities measured at 1.07 V vs.various scan rates of the catalysts.(d)LSV plots of Pt/C and the as-prepared catalysts measured in the potential range of both OER and ORR.
The OER activity of the catalysts in 0.1 mol∙L–1KOH was evaluated. As presented in Fig. 5(a), Co@NC-1 exhibits a remarkably higher OER activity than that of NC catalyst with an overpotential of 660 mV to offer 10 mA∙cm-2, manifesting the important role of Co in the OER activity.Co@NC-2 needs an overpotential of 520 mV to achieve 10 mA∙cm-2, which is inferior to that of Co@NC-1,Co@NC-3 and Pt/C. The result demonstrates the Co content is important to the enhancement of OER activity.The EIS Nyquist plot of the catalysts is also presented in Fig. 5(b).The smaller polarization resistance of the Co@NC-2 indicates the faster electron transfer process and higher charge transfer kinetics that supports the OER performance.The electrochemical surface area(ECSA)of catalysts was also calculated by estimating the electrical double layer capacitance (Cdl) (Fig. 5(c)). TheCdlwas evaluated by measuring the CV in the non-faradic region at a scan rate of 20–100 mV∙s-1(Fig. S6). The calculatedCdlof Co@NC-2 is 4.12 mF∙cm-2, which is larger than that of the other catalysts.Thus, the Co@NC-2 exhibits a high electrochemical catalytic surface area. The bifunctionality activity was elucidated by calculating the differences(ΔE=Ej=10–Ehalf-wave) between ORR half-wave potentials for ORR and OER potentials at 10 mA∙cm-2. As depicted in Fig. 5(d), the Co@NC-2 presents the smallest ΔEvalue of 89 mV, indicating the superior bifunctional activity towards OER and ORR in alkaline electrolyte.
Next, Co@NC-2 was used as the air cathode in liquid ZABs. The Co@NC-2-assembled ZAB has the open-circuit voltage of 1.45 V(vs.Zn) (Fig. 6(a)). Fig. 6(b) compares the charge and discharge polarization curves of Pt/C and Co@NC-2 assembled ZABs. Co@NC-2 has the smallest voltage gap and highest power density.The maximal power density of 100 mW∙cm-2outperforms 40 mW∙cm-2of Pt/C + Pt/C (Fig. 6(c)). The performance of Co@NC-2-based ZAB is superior to many other reported ZABs (Table S5). The energy density of 780 mA∙h∙g-1outperforms 748 mA∙h∙g-1of Pt/C + Pt/C(Fig. 6(d)). At a current density of 10 mA∙cm-2, the cyclability in ZABs was measured using a galvanostatic discharge–charge method.As depicted in Fig.6(e),ZABs with Co@NC-2 have an initial potential of 1.16 V for discharge and 1.96 V for charge,respectively.Besides, the voltage gap increases by 0.05 V after 220 h of operation, indicating its robust corrosion resistance. The voltage gap of Pt/C + Pt/C reference expanded rapidly from 0.94 V to 1.53 V after 165 h. Furthermore, it was found that a commercial LED lamp brand can be lit by two Co@NC-2-assembled ZABs connected in series, indicating the feasibility of Co@NC-2 in practical application.
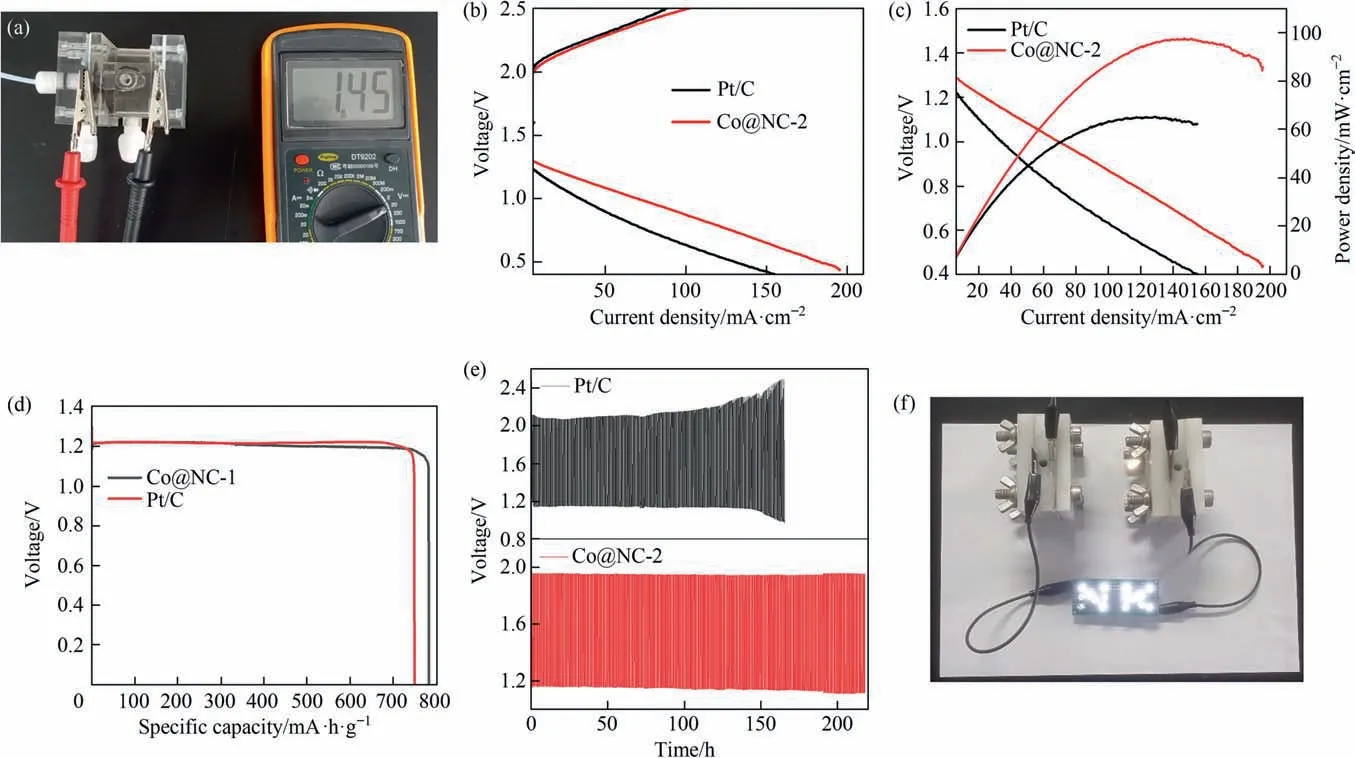
Fig. 6. Application of Co@NC-2 in liquid ZABs. (a) Open circuit voltage. (b) Galvanodynamic charge/discharge polarization curves of the ZABs with the Pt/C and Co@NC-2 cathodes.(c)The discharge polarization curves and the power densities of the ZABs with the Co@NC-2 and Pt/C cathodes.(d)Specific capacity of the Pt/C and Co@NC-2-based ZABs. (e) Discharge–charge cycling curves of the Co@NC-2-assembled ZAB. (f) Digital image of a LED lamp brand lit by two Co@NC-2-assembled ZABs connected in series.
At stated above,the physicochemical and electrochemical properties of Co@NC materials have been characterized and analyzed.The Co@NC-2 exhibits superb bifunctionality towards both ORR and OER and can be applied as the cathode material in ZABs with high application prospects. The performance can be attributable to some essential aspects.Firstly,the hierarchical porous structure brought by the COF-derived strategy greatly alleviates the electrolyte transportation issues.The combination of micropore,mesopore and macropore is favorable for the increase of active sites and acceleration of mass transfer process. Notably, controlled ligand environment and heteroatom-doping can be realized through this strategy, thus can tune the adsorption/desorption barriers of oxygen intermediates to improve the catalytic performance.Secondly,N-doping often plays an important role in regulating the d band center of Co metal sites. The energy barrier of the ratedetermining step of both OER and ORR can be reduced by Ndoping [35]. Thirdly, Co2+can coordinate with the organic ligands in COF,which gives rise to the uniform distribution of Co nanoparticles and novel Co—N coordination matrix.The introduction of Co can increase the defect degree of the materials. In addition, the suitable amount of small Co nanoparticles offers sufficient reaction sites for fast electrochemical reaction. The adsorption of O2and oxygenated intermediates can be enhanced on Co—Nxsites, which significantly improves the electrocatalytic performance. With the variation in Co content, the N-doping content also changes.Co@NC-2 possesses the optimal N-doping content,which serves as active sites to promote the catalytic process.Thus,the superb electrocatalytic performance is attributable to the synergistic effect between the Co—Nxactive sites and N-doped hierarchically porous structure.
4. Conclusions
A hybrid electrocatalyst with conformal Co nanoparticles incorporated hierarchical porous carbons has been developed through a COF-derived strategy,which provided remarkable bifunctional catalytic performances towards both OER and ORR in alkaline solution. The hierarchical porous structure offers a fast electron and ion diffusion pathway,while the suitable amount of Co nanoparticles and the optimal N-doping content facilitate sufficient reaction sites for fast electrocatalytic reactions.As a result,the as-prepared catalyst (Co@NC-2) presents superb performances towards both ORR and OER. Moreover, a liquid ZAB using Co@NC-2 as the cathode was constructed, which offered an open circuit potential of 1.45 V, a peak power density of 100 mW∙cm-2, an energy density of 780 mA∙h∙g-1and good cycling stability, outperforming the Pt/C-assembled ZAB. The synthesis strategy to derive hybrid carbonbased materials paves a promising way for the sustainable and green energy storage devices.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2019PB013) and the Training Program of Innovation and Entrepreneurship for Undergraduates of Liaocheng University (CXCY2022277).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.015.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
- Separation of lithium and nickel using ionic liquids and tributyl phosphate
