A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
Guoliang Zeng, Danni Ye, Xingfang Zhang, Fengfeng Gao,, Xiaogang Hao,, Jun Li, Zhong Liu
1 College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, China
2 Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China
Keywords: Electrochemically switched ion exchange Potential-responsive ion-pump system NiHCF film electrode Cesium ions
ABSTRACT A nickel hexacyanoferrate(NiHCF)film electrode was prepared with NiHCF,conductive carbon black,and polyvinylidene difluoride, which was coated on graphite plate substrate for selective extraction of Cs+ions by using electrochemically switched ion exchange (ESIX) technology. A potential-responsive ionpump system for efficient extraction of Cs+ ions was designed, and the effect of wet film thicknesses,charging modes, flow rates, and chamber widths on Cs+ ions extraction performance was investigated.In the system, the adsorption capacity and removal percentage of Cs+ ions on the NiHCF film electrode reached as high as 147.69 mg∙g-1 and 92.47%, respectively. Furthermore, the NiHCF film electrode showed high selectivity for Cs+ions and stability.After seven cycles of adsorption/desorption,the desorption percentage could reach about 100%.The excellent Cs+extraction performance should be attributed to the strong driving force produced by the potential-responsive ion-pumping effect in the ESIX process,as well as the low ion transfer resistance of the film electrode which is caused by the special crystal structure of NiHCF.In addition,the NiHCF film electrode was implemented to work together with the bismuth oxybromide (BiOBr) film electrode to accomplish the simultaneous extraction of Cs+ and Br–. And the adsorption capacity and removal percentage of Br–ions on the BiOBr film electrode reached 69.53 mg∙g-1 and 77.32%, correspondingly. It is expected that such a potential-responsive ion-pump system based on NiHCF and BiOBr film electrodes could be used for the selective extraction and concentration of Cs+ and Br– ions from salt lake brine..
1. Introduction
Cesium (Cs) is an important and expensive alkali metal, which is widely used in national defense,pharmaceuticals,and fiber optic communication systems. Therefore, the extraction of Cs is an important topic that has attracted the special attention of numerous researchers.Currently,the source of Cs depends mainly on the exploitation of ores.However,it is increasingly difficult to produce Cs from ores to fulfill the market demand, resulting in the high price of Cs [1]. Furthermore, the methods to extract Cs from ores such as high-temperature calcination and strong acid and alkali leaching cause serious environmental pollution. Fortunately, the reserves of Cs in geothermal water, oil field brine, and salt lake brine are extremely high, a large number of natural salt lakes contain abundant Cs resources in China, and the reserves are among the highest in the world, but have not been fully utilized[2].Compared to the complex ore roasting and leaching processes,Cs in salt lakes exists in the ionic state and can be directly separated and concentrated. Hence, it is urgent and necessary to use the Cs resources in the salt lake brine to alleviate the demand for Cs resources. However, in the salt lake brine, Cs+usually coexists with other alkali metal ions such as Rb+,K+, Na+,and Li+[3].These coexisting ions and Cs+are quite similar in their physical and chemical properties,especially the hydration ion radii are in order of Cs+(0.325 nm), Rb+(0.329 nm), K+(0.330 nm), Na+(0.360 nm),Ca2+(0.410 nm) and Mg2+(0.425 nm), increasing the difficulty of separating Cs+from salt lake brine [4]. So far, several methods for Cs+extraction have been reported, including chemical precipitation, adsorption, ion exchange, solvent extraction,etc.[5–9]. All these methods usually suffer from the disadvantages, such as secondary pollution, high cost, low efficiency, and so on [10].
Currently,electrochemically switched ion exchange(ESIX),as a novel, environment-friendly, and highly efficient ion separation technology, has attracted increasing attention from researchers due to its advantages of high selectivity, low concentration extraction, and controllable rate [11,12]. In the ESIX process, the adsorption and desorption of ions can be achieved by adjusting the redox state of the ESIX film, which is simple and convenient,as well as effective in preventing secondary contamination [13–17]. Up to now, we have researched and developed ESIX films for the separation of many different target ions such as Li+, Cs+, Pb2+,Ni2+, F–, Cl–, Br–, I–and PO43–[18–31]. However, as of present, ESIX technology is still in the laboratory stage, and most researchers focus on the development of ESIX film materials and the research of related mechanisms,while little work has been done on the fabrication of large-area film electrodes and industrial applications.
To realize the effective scale-up of ESIX film electrodes, some electroactive ion exchange materials with simple preparation,low cost, and excellent performance are urgently needed. Nickel hexacyanoferrate (NiHCF), as a special face-centered cubic structure, has exchangeable K+, whose cell parameters match the size of the hydrated alkali metal cations, and the affinity order is Cs+> Rb+> K+> Na+> Li+[32–34]. Moreover, with the action of the applied potential, the potential-responsive ion-pumping effect of NiHCF could be exacted. When an oxidation potential is imposed on the NiHCF,Fe2+in the NiHCF is oxidized to Fe3+,thus the cations in the lattice are released to maintain electric neutrality.Inversely,when a reduction potential is applied to the NiHCF,Fe3+is reduced to Fe2+,thus the cations in the solution are absorbed.What’s more,NiHCF has a low-cost and relatively simple preparation process,which is promising for the industrialization of Cs+separation.However, it is difficult to coat NiHCF to form a film because of its weak adhesion. Polyvinylidene difluoride (PVDF), as a binder, has excellent properties such as thermal stability,chemical resistance,and high mechanical strength [35,36]. Thus, PVDF is used to enhance the firmness of the NiHCF film electrodes. In addition,conductive carbon black (CB) is also added to enhance the electrical conductivity during the preparation of the NiHCF film electrode. CB is the most popular conductive additive due to its low cost, high electrical conductivity, and chemical stability [37].
In addition, a plate-and-frame type film module consisting of a pressing plate, inlet (outlet) plate, spacer, and film electrode is designed, as shown in Fig. 1 [38]. Among them, the pressing plate is used to fasten each part and prevent the liquid from leaking.The inlet plate and outlet plate are used for introducing the solution into and out of the device.The spacer is designed to separate other components and homogenize the solution. As well, the film electrode is energized and selectively extracts the target ions from the solution under the effect of an electric field. Typically, a platinum electrode is often used as a counter electrode to the film electrode, which not only wastes electricity but also tends to produce side reactions.Therefore,a bismuth oxybromide(BiOBr)film electrode was used as the counter electrode of the NiHCF film electrode to achieve simultaneous extraction of Cs+and Br–.In this way,most side reactions could be avoided and more products obtained without electricity wastage.
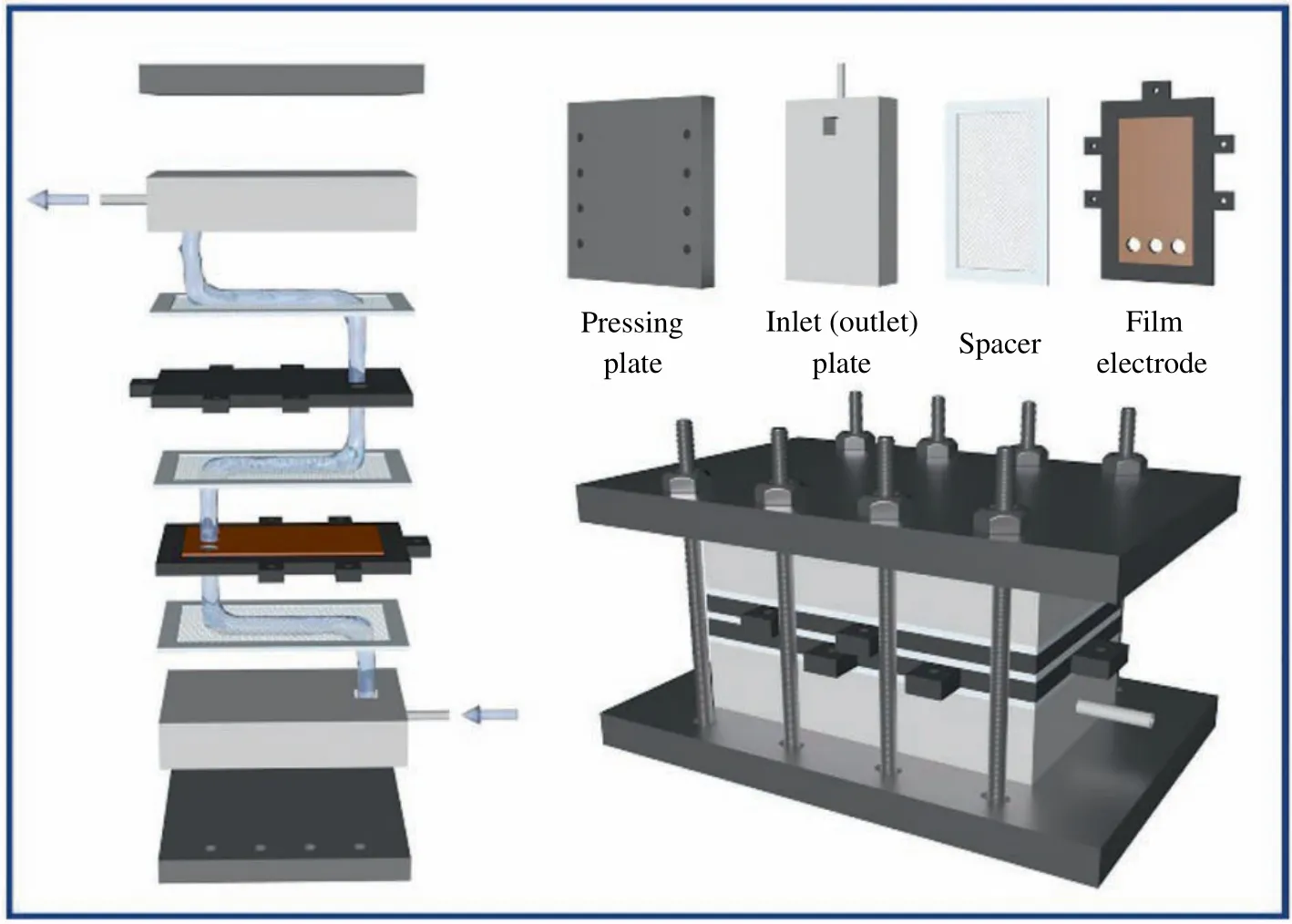
Fig. 1. Schematic diagram of the film device.
In this study,NiHCF was synthesized for the preparation of ESIX film electrodes by the co-precipitation method, and produced as a slurry with PVDF and CB.The slurry was coated on a graphite plate to produce a NiHCF film electrode with an effective film area of 4 cm×8 cm.A film module applicable to ESIX technology was also designed, in which a NiHCF film electrode and a BiOBr film electrode were used for the simultaneous extraction of Cs+and Br–ions. Herein, the effect of wet film thicknesses, charging modes,flow rates and chamber widths on Cs+ions extraction performance were compared.In addition,the desorption of the NiHCF film electrode for Cs+in different desorption solutions and the selectivity of the film electrode for Cs+in mixed solution was also investigated.Finally, the long-term working stability of the film electrode was investigated to ensure its industrial application.
2. Experimental
2.1. Reagents and materials
Nickel sulfate (NiSO4∙6H2O, 98.5%) was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Potassium ferricyanide (K3Fe(CN)6, 99.5%) was procured from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Absolute ethanol(C2H6O,99.7%)and concentrated sulfuric acid(H2SO4, 98.0%)were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai,China).N-Methylpyrrolidone (C5H9NO, 99.0%) was bought from Tianjin Beichen District Fangzheng Reagent Factory (Tianjin,China). Graphite plate (6.0 cm × 10.0 cm × 0.5 cm) was acquired from Beijing Jinglong Special Carbon Technology Co., Ltd. (Beijing,China). Other chemicals were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). All reagents were analytical grade and used without further purification.
2.2. Synthesis and pretreatment of materials
A co-precipitation method was employed to synthesize nickel hexacyanoferrate (NiHCF), which is an analog of Prussian blue[20,39]. As-prepared 0.1 mol∙L–1K3Fe(CN)6solution was added dropwise to 0.1 mol∙L–1NiSO4solution for co-precipitation. And all of the above solutions contained 20% ethanol. The solution was kept under stirring by a magnetic stirrer during the coprecipitation for 12 h. Finally, the precipitate was centrifuged,washed with deionized water and absolute ethanol respectively,and then dried for 48 h in an oven at 50°C.After ball milling,NiHCF powder was obtained.
After the concentrated sulfuric acid was diluted to 0.1 mol∙L–1,the graphite plate was soaked in the dilute sulfuric acid solution for 2 h, and then soaked in absolute ethanol for 1 h after being washed with deionized water. After rinsing with deionized water and drying for 24 h in an oven at 50 °C, the dry graphite plate was prepared for use.
2.3. Preparation of NiHCF film electrode
The film electrodes were made of active material NiHCF,CB,and PVDF with a mass ratio of 8:1:1. First, 0.375 g PVDF was mixed with 12.5 gN-methyl-2-pyrrolidinone (NMP) solution, and heated in a water bath at 40 °C with magnetic stirring until PVDF was completely dissolved in NMP. The PVDF/NMP solution was added to the NiHCF/CB mixture and ball milled at a frequency of 40 Hz for 6 h. Subsequently, the prepared slurry was coated on the pretreated graphite plate by a film coater, and the NiHCF film electrode was obtained after drying. Here, the effective film area was 4 cm × 8 cm. The schematic diagram of the preparation of the NiHCF film electrode is shown in Fig. 2.
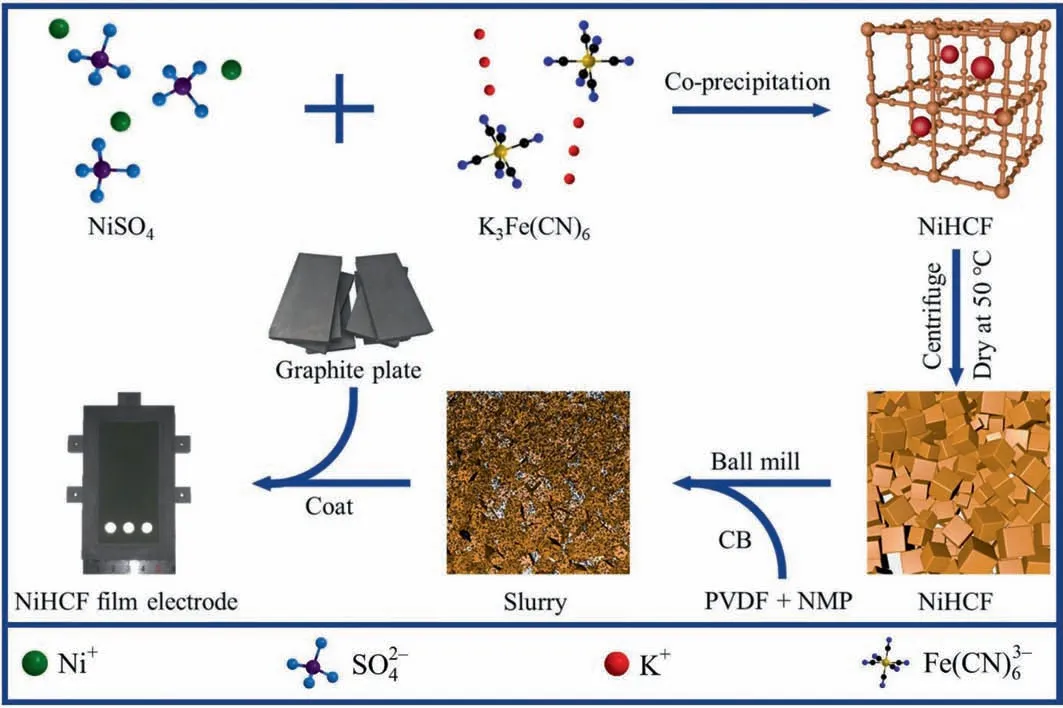
Fig. 2. Schematic diagram of the preparation of NiHCF film electrode.
In addition, BiOBr powder was prepared by room temperature hydrolysis method using Bi(NO3)3and KBr as raw materials, and the BiOBr film electrode was prepared by a similar method as that of the NiHCF film electrode [40,41].
2.4. Design of film module
The film module consisted of pressing plates, inlet (outlet)plate, spacer, and film electrode, as shown in Fig. 1. NiHCF film electrodes and BiOBr film electrodes were paired and assembled in the film module, with chambers separated by spacers between each part, and an oxidation/reduction potential was applied to the film electrodes. As shown in Fig. 3, the original liquid entered from the storage tank through the peristaltic pump to the inlet plate, then flowed through the chambers in turn, and finally returned to the storage tank through the outlet plate.
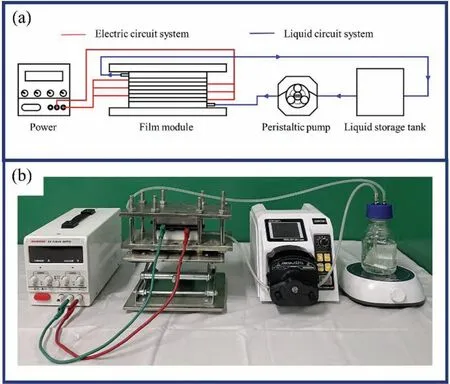
Fig. 3. (a) Schematic of ESIX system and (b) picture of the self-designed device for NiHCF and BiOBr film electrodes.
2.5. Electrochemical and adsorption/desorption performance test
The electrochemical performance of the NiHCF film electrode was tested in a three-electrodes system with the prepared NiHCF film electrode, graphite plate, and Ag/AgCl electrode as the working, counter, and reference electrodes, respectively. Herein, the electrochemical performance was tested and analyzed by cyclic voltammetry (CV) and electrochemical impedance spectroscopy(EIS).
The adsorption/desorption performance of the NiHCF film electrode was tested in a two-electrodes system in the designed and constructed film module. In a solution containing 1.5 mmol∙L–1CsBr,the adsorption of Cs+on NiHCF film electrodes with different wet film thicknesses(100,200,300,400 μm)was investigated.The adsorption of Cs+on NiHCF film electrode was investigated at 400 μm wet film thickness under different charging modes (static adsorption,pulse potential,constant potential),different flow rates(5, 10, 20, 40 ml∙min-1) and different chamber widths (2, 4, 6,8 mm). In addition, the desorption efficiency of NiHCF film electrodes in different desorption solutions was investigated. The adsorption quantityQA(mg∙g-1) of the NiHCF film electrode was calculated by the following Eq. (1):
wherec0(mg∙L–1)andce(mg∙L–1)are the initial and final concentrations of Cs+,respectively;V(L)is the volume of the solution,andm(g) is the mass of the electroactive materials on the film electrode.
When a positive potential was applied to the working electrode,the electrochemical desorption of Cs+was carried out to achieve the regeneration of the film electrode. The desorption percentageRD(%) was calculated by using Eq. (2):
whereQDandQA(mg∙g-1) are the amounts of Cs+desorption and adsorption by film electrode, respectively.
To explore the adsorption selectivity of Cs+with the existence of other cations, competitive adsorption experiments were carried out.The distribution coefficientKD(ml∙g-1)of the NiHCF film electrode for each cation was calculated by using Eq. (3):
whereC0(mg∙L–1) andCe(mg∙L–1) are the initial and final concentrations of cations,respectively;V(L)is the volume of the solution,andm(g) is the mass of the electroactive materials on the film electrode.
Therefore, the separation factor α1,2of Cs+(i1) to other cations(i2) was calculated by Eq. (4):
2.6. Characterizations
The electrochemical performance of the NiHCF film electrode was tested with a multi-potentiostat (VMP3, Princeton, USA) controlled by EC-Lab software. The microstructures of materials and NiHCF film electrodes were characterized by a scanning electron microscope (SEM, JSM-6510LV, JEOL, Japan). The crystalline structure of NiHCF was detected by X-ray diffraction (XRD, SmartLab,Rigaku, Japan). Fourier transform infrared (FT-IR) spectrum was recorded by FTIR-8400 spectrometer (Shimadzu, Japan) during the range of 400–4000 cm-1.The cation concentration in the solution was analyzed by an atomic absorption spectrometer (AAS,TAS-990, Beijing Purkinje General Instrument Co., Ltd., China).
3. Results and Discussion
3.1. ESIX mechanism of the NiHCF film electrode
Fig. 4 illustrates the structure of the NiHCF film electrode and the adsorption/desorption mechanism of Cs+during the ESIX process. The extraction of Cs+from the solution by NiHCF film electrode is mainly based on the special potential-responsive ionpumping effect of NiHCF in the ESIX process [42,43]. Driven by the potential on the film electrode,the valence changes of iron ions in the NiHCF structure cause the intercalation and deintercalation of ions in the lattice, which enables the transfer of Cs+against the concentration gradient. When a reduction potential is applied to the film electrode, Fe3+is reduced to Fe2+, and Cs+in solution is selectively inserted into the lattice of NiHCF to maintain the electric neutrality of the film electrode. On the contrary, when an oxidation potential is applied to the film electrode,Fe2+is oxidized to Fe3+,thus causing the Cs+in the lattice to be released into the solution to maintain the electric neutrality of the film electrode.Therefore, the adsorption/desorption of Cs+by the NiHCF film electrode is achieved by adjusting the redox potential on the film electrode for the purpose of extraction of Cs+[39,44,45]. In addition, NiHCF extracts Cs+from the solution and also suffers from cation selfexchange behavior. The K+existing in the crystal framework of NiHCF occurs in self-exchange behavior with the Cs+in solution due to the effect of concentration difference and affinity order,hence the Cs+in solution is adsorbed into the NiHCF[46].The reaction equations of cation self-exchange behavior are as follows:
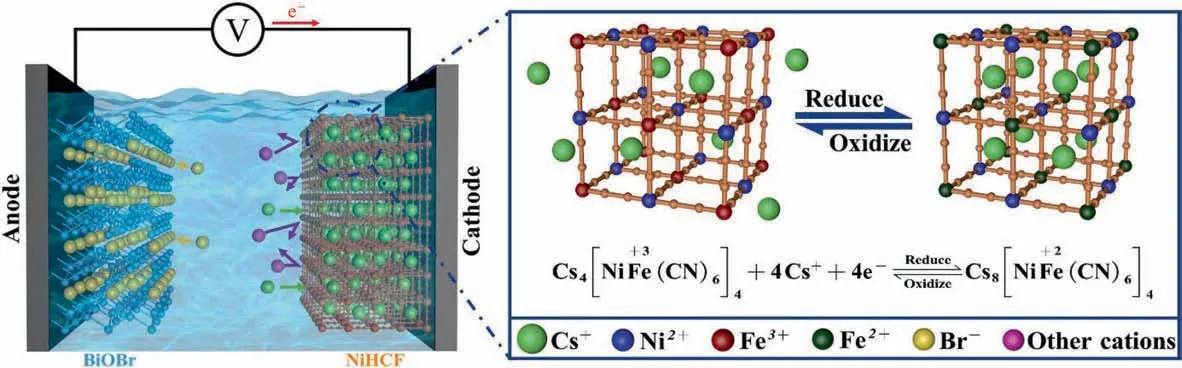
Fig. 4. The ESIX mechanism of NiHCF for the extraction of Cs+.
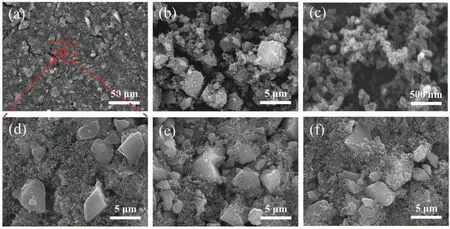
Fig.5. SEM images of(a,d)NiHCF film electrode,(b)NiHCF powder,(c)CB powder,(e)NiHCF film electrode after adsorption of Cs+,(f)NiHCF film electrode after desorption of Cs+.
3.2. Morphology and structure of NiHCF film electrode
3.2.1. SEM analysis
The microstructures of NiHCF powder, CB powder, and NiHCF film electrode were characterized by SEM, and the results are shown in Fig. 5. It can be seen that the NiHCF powder exhibited a typical crystalline particle structure (Fig. 5(b)), and the CB powder exhibited a fine spherical structure (Fig. 5(c)). Fig. 5(a) and (d)show the SEM images of NiHCF film electrodes at different magnifications, and one can see the materials were evenly mixed and bonded together. It was significant that there were numerous cracks on the surface of the film, which facilitated the entry of the solution into the film electrode and the contact with the electroactive material. Fig. 5(e) displays the image of the NiHCF film electrode after the adsorption of Cs+, and Fig. 5(f) shows the SEM image of the NiHCF film electrode after the desorption of Cs+. It can be seen that the micro-morphology of the electrode was almost identical to that before adsorption, indicating that the adsorption/desorption could not destroy its crystalline structure.It is proved that the NiHCF film electrode has great stability. In addition, the SEM image of the BiOBr film electrode was shown in Fig. S1 (in Supplementary Material).
3.2.2. XRD and FT-IR analysis
The crystalline structure of NiHCF powder was characterized by XRD analysis. As shown in Fig. 6(a), the XRD pattern of NiHCF matched well with the standard pattern (PDF# 086-0501).Well-resolved diffraction peaks of NiHCF and its analogs were presented at 2θ of 14.99°,17.35°,24.63°,35.03°,39.37°,43.24°,50.42°,53.73°, 56.85°, 65.72° and 68.56°. These diffraction peaks could be indexed for (1 1 1), (2 0 0), (2 2 0), (4 0 0), (4 2 0), (4 2 2), (4 4 0),(6 0 0),(6 2 0),(6 4 0),and(6 4 2)crystal planes of NiHCF,respectively. It confirms the successful preparation of NiHCF, and the sharp and strong diffraction peaks indicate a well crystalline structure [47–49].
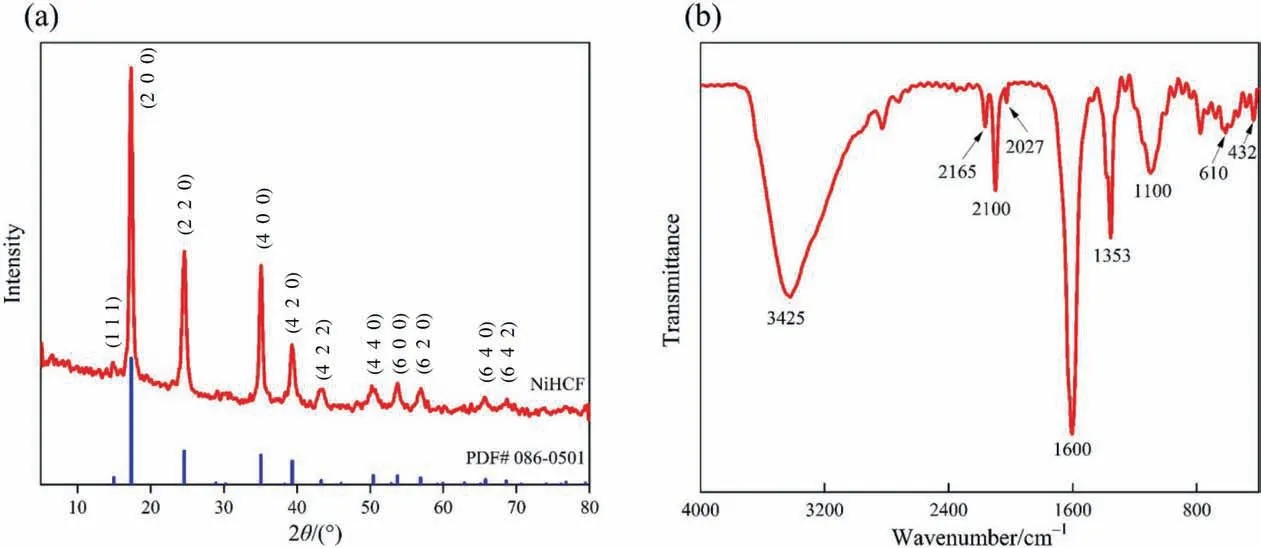
Fig. 6. (a) X-ray diffraction pattern and (b) Fourier transform infrared spectrum of NiHCF.
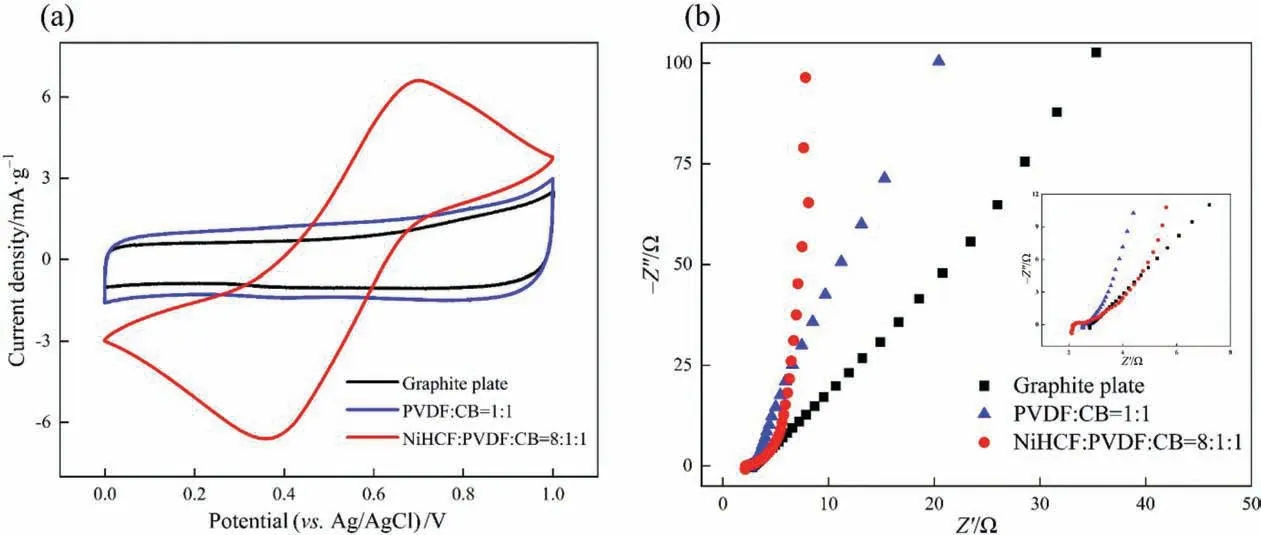
Fig.7. (a)CV curves of graphite plate,PVDF/CB film electrode,and NiHCF film electrode and(b)electrochemical impedance spectra of graphite plate,PVDF/CB film electrode,and NiHCF film electrode in the 1.0 mol∙L–1 KNO3 solution.
In addition, further analysis of the synthesized NiHCF powder was performed by FT-IR spectrometer, as shown in Fig. 6(b). In the spectrum of NiHCF, the absorption peaks located at 3425 and 1600 cm-1corresponded to the stretching vibration and bending vibration of O—H can be observed. Whereas the absorption peaks at 2165 and 2100 cm-1are attributed to C≡N groups stretching.The free C≡N group usually exhibits a stretching vibration absorption peak at 2027 cm-1.When the CN group forms a complex with the transition metals,the complex depends on the oxidation state,electronegativity, and coordination number of the bonded metal.Therefore, the absorption peaks at 2165 and 2100 cm-1might be related to Fe(II)—C≡N—Ni(II) and Fe(III)—C≡N—Ni(II) [48]. It is proved that the presence of Fe(II)/Fe(III) species in the framework of NiHCF and this further confirms the successful synthesis of NiHCF. The absorption peak at 1353 cm-1is attributed to C—N stretching vibrations, and the peak at 1100 cm-1might belong to the stretching vibrations of the SO42–remaining during the preparation process [50]. The absorption peaks at 610 and 432 cm-1are attributed to Fe—CN bending vibration and Fe—CN stretching vibration, respectively [47]. In addition, the XRD pattern of BiOBr was shown in Fig. S2.
3.3. Electrochemical performance of NiHCF film electrode
Cyclic voltammetry (CV) was performed in 1.0 mol∙L–1KNO3solution at a scan rate of 20 mV∙min-1. As shown in Fig. 7(a), one can see that the CV curve (black) corresponding to the graphite plate and the CV curve (blue) of the PVDF/CB film electrode presented a typical electrical double layer effect, and there was no redox peak.It demonstrated the graphite plate,PVDF and CB were stable without redox reaction.In addition,the electroactive area of the PVDF/CB film electrode was larger than that of the graphite plate,indicating that the addition of CB improved the capacitance.Whereas,the CV curve(red)of the NiHCF film electrode had a pair of highly symmetrical redox peaks based on the redox of Fe2+/Fe3+in the electrolyte solution, which showed excellent reversibility and a large capacitance.
Fig. 7(b) shows the electrochemical impedance spectroscopy(EIS)of the graphite plate,PVDF/CB film electrode,and NiHCF film electrode.Herein,the semicircular diameter in the high-frequency region represented the charge transfer resistance associated with the Faraday reaction at the interface between the film electrode and the electrolyte. The straight line in the low-frequency region was caused by the Warburg impedance of the ions on the electrode, and the slope of the straight line corresponded to the diffusion resistance of the ions in the electrolyte as they diffuse to the surface of the electrodes.
As shown in Fig. 7(b), the semicircles of the graphite plate,PVDF/CB film electrode, and NiHCF film electrode were small or almost absent, which is because the charge transfer resistance was greatly reduced and the conductivity was enhanced by using the graphite plate as the substrate. In addition, in the lowfrequency region, it could be seen that the slope of the three electrodes was great than one, and the slope of the NiHCF film electrode was approximately parallel to the imaginary axis, which proves that the diffusion resistance of the NiHCF film electrode was small. It is beneficial for the ion adsorption of NiHCF film electrodes.
3.4. Process parameters
3.4.1. Effect of wet film thickness on adsorption performance
The adsorption performance of Cs+on NiHCF film electrodes with different wet film thicknesses was tested in a solution containing 1.5 mmol∙L–1CsBr.As shown in Fig.8,the adsorption quantities of these NiHCF film electrodes reached equilibrium after about 4 h, and the adsorption quantities of different film thicknesses vary little. As can be seen from Fig. 8(a), the film electrode with a wet film thickness of 300 μm performed best and the adsorption quantity was 149.96 mg∙g-1. The film electrode with a wet film thickness of 200 μm performed worst but the adsorption quantity was still 137.86 mg∙g-1. According to the analysis, the reason may be that the diffusion resistance of the NiHCF film electrode is quite small. Therefore, the change of diffusion resistance caused by increasing the film thickness in a certain range is quite weak.
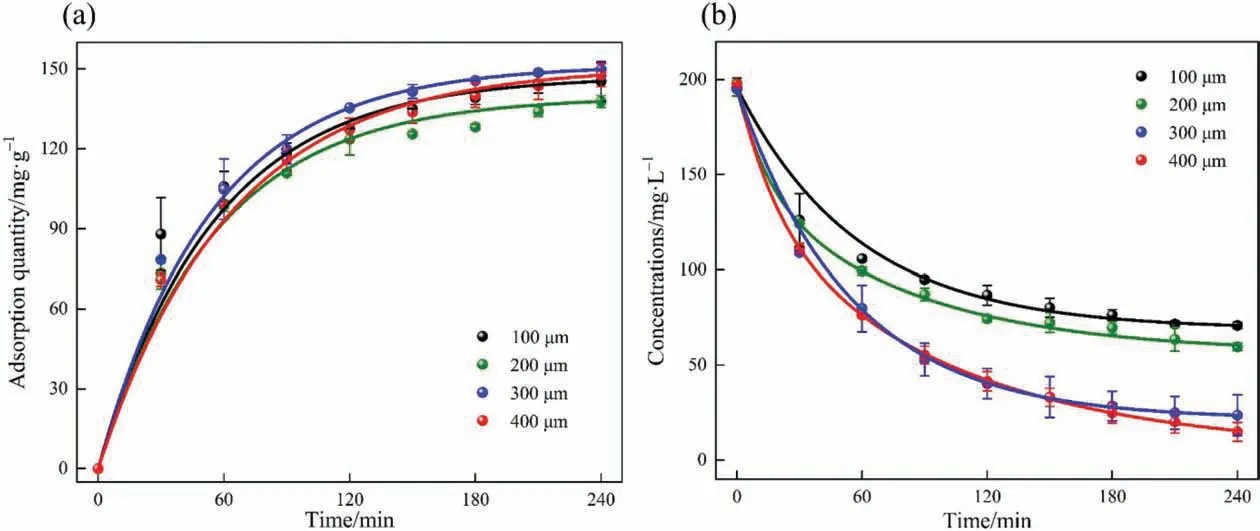
Fig. 8. The curves of (a) adsorption quantity of NiHCF film electrode and (b) Cs+ concentration in the solution.
However, from the Cs+concentration change curve (see Fig. 8(b)),the Cs+concentration decreased from 198.06 to 14.91 mg∙L–1,and the adsorption percentage could reach 92.47%with a wet film thickness of 400 μm. Whereas, the adsorption percentage was 87.93% for the electrode with 300 μm wet film thickness. It is due to the fact that increasing film thickness represents the increase in the active material,which can provide more active sites for Cs+ions. Therefore,for industrial applications, it may be better to choose a film electrode with a thicker wet film thickness that could adsorb more Cs+in the same time frame,which is beneficial to improve economic efficiency.
3.4.2. Effect of charging mode on adsorption performance
The effects of different charging modes on the adsorption of Cs+on the wet film electrode with a thickness of 400 μm were investigated. As shown in Fig. 9(a), the adsorption effects of applying 1.0 V constant potential, 1.0/0 V pulse potential (switching time was 30/2 s),and static adsorption on the film electrode were compared.The adsorption effect of applying constant potential was the best and is much better than that of static adsorption without applying potential.It is proved that the adsorption of ions in solution can be improved significantly by applying a certain potential on the film electrode.It is because the potential applied on the film electrode can activate the potential-responsive ion-pumping effect of NiHCF, which induces the adsorption of Cs+from the solution into the film electrode. And when the pulse potential was applied,the charging time was less than the constant potential condition in the same working time. Moreover, during the power-off period,some of the Cs+which was adsorbed on the film may return to the solution under the effect of the concentration difference. It results in the adsorption quantity of pulse potential being less than that of the constant potential condition.In addition,it can be seen that NiHCF still had a certain amount of Cs+adsorption under static adsorption conditions, which is due to the cation self-exchange behavior of NiHCF, the K+in the crystal structure of NiHCF occurs self-exchange behavior with the Cs+in solution since the effect of concentration difference and affinity order.
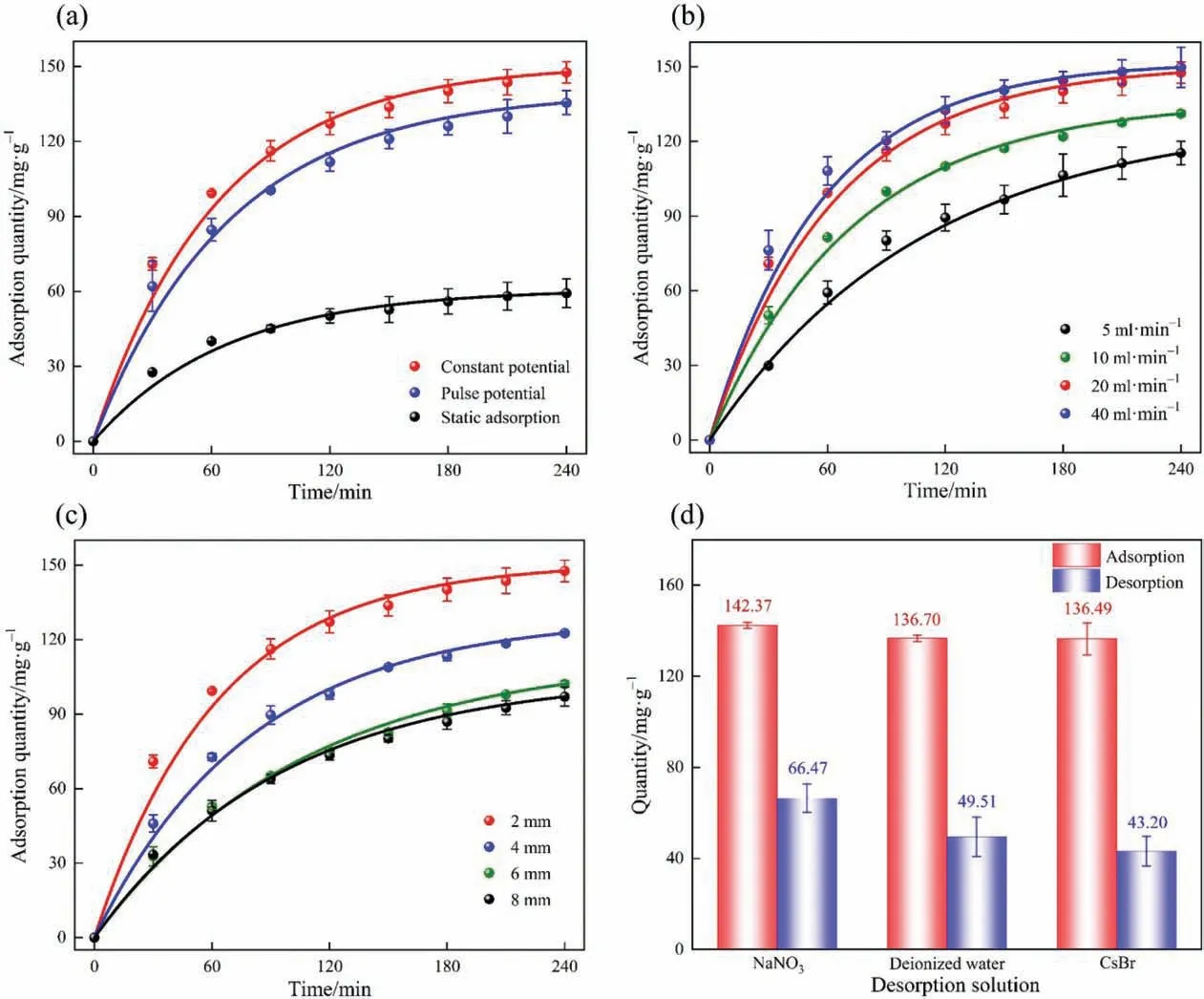
Fig. 9. The adsorption quantities of Cs+ at (a) different charging modes, (b) different flow rates, and (c) different chamber widths on the NiHCF film electrode. (d) The desorption effect of the NiHCF film electrode in different desorption solution.
3.4.3. Effect of flow rate on adsorption performance
As shown in Fig. 9(b),it can be observed that the change of the flow rate in the range of 5–20 ml∙min-1was obvious to the adsorption of ions. However, when the flow rate doubled from 20 to 40 ml∙min-1, the adsorption quantity hardly changed. When the flow rate is lower, the slow advection cannot keep synchronized with the relatively rapid ions diffusion, which leads to a low adsorption quantity [51]. And with the increase of the flow rate,the advection and the diffusion gradually kept synchronized,which matches the renewal rate of ions with the adsorption rate and improves the adsorption efficiency.However,there was no significant difference in the adsorption quantities when the flow rate was 20 ml∙min-1or 40 ml∙min-1, in terms of economic efficiency,the flow rate of 20 ml∙min-1is a more suitable choice for the system.
3.4.4. Effect of chamber width on adsorption performance
The influence of different chamber widths on the adsorption performance of Cs+was investigated. It can be seen from Fig. 9(c)that the adsorption quantity of the NiHCF film electrode on Cs+decreases with the increase in the chamber width. It may be due to the fact that as the chamber width increases,the slow diffusion cannot keep pace with the relatively quick advection,and the ions in the solution leave the chamber with advection before they are adsorbed, thus the adsorption efficiency is reduced significantly.In addition, when the chamber width is too small, the cathode and anode film electrodes may touch each other easily and result in a short circuit. Therefore, 2 mm is the most suitable chamber width for this system.
At the optimal conditions,the adsorption capacity of Cs+on the NiHCF film electrode could be as high as 147.69 mg∙g-1, and the removal percentage reached 92.47%. Furthermore, the adsorption capacity and removal percentage of Br–on the BiOBr film electrode could reach 69.53 mg∙g-1and 77.32%, respectively (Fig. S3).
3.4.5. Effect of desorption solution on desorption performance
The Cs+desorption of NiHCF film electrodes in different desorption solutions was investigated.Three different desorption solution were selected: 10.0 mmol∙L–1NaNO3solution, deionized water,and 1.5 mmol∙L–1CsBr solution. As shown in Fig. 9(d), the desorption efficiency in three different desorption solution was 46.69%,36.22%, and 31.65%, respectively. The excellent desorption efficiency in NaNO3solution is to be expected due to the higher conductivity caused by the higher electrolyte content of NaNO3solution. In deionized water, the desorption was poor in the early stage,but with the increasing number of ions in water,the conductivity increased and the desorption effect improved. However,there was a certain amount of Cs+in 1.5 mmol∙L–1CsBr solution,which would inhibit the desorption of Cs+from the film electrode,so the desorption effect was slightly lower than that of the former two.Therefore,NaNO3solution was chosen as the desorption solution in this study.
3.5. Selectivity and stability of NiHCF film electrode
For eliminating the interference of PVDF and CB on Cs+adsorption, blank experiments were performed. Here, Cs+adsorption experiments were performed in the device using PVDF/CB film electrodes.As can be seen in Fig.10(a),the PVDF/CB film electrode is unable to adsorb Cs+both with and without applying an electrical potential on the PVDF/CB film electrode.
To investigate the selectivity of NiHCF film electrodes for Cs+, a batch of selective adsorption experiments was carried out in a mixed solution containing the same concentration of Cs+, Rb+, K+,Na+, Li+, Mg2+, Ca2+, Sr2+, and Y3+. Fig. 10(b) shows the distribution coefficients of different cations, which are completely consistent with the affinity order of NiHCF for alkali metal cations.The distribution coefficient of NiHCF film electrode for Rb+is similar to that of Cs+due to the fact that the physical properties,chemical properties, and hydrated ion radii of Rb+and Cs+are extremely similar,making it difficult to achieve separation. The separation factors for these ions are shown in Table 1. In practical application, two high value-added ions, Cs+and Rb+, can be chosen to be extracted from the solution simultaneously.

Table 1Distribution coefficients and separation factors of the NiHCF film electrode for Cs+ vs. other competitive cations
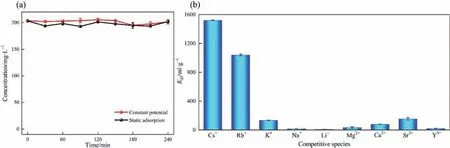
Fig. 10. (a) Cs+ concentration variation curves of blank experiments by PVDF/CB film electrodes. (b) Selectivity of NiHCF film electrode.
To evaluate the stability of the NiHCF film electrode, the longterm electrochemical stability test was performed by circulating 1200 cycles of cyclic voltammetry in 1.0 mol∙L–1KNO3solution at a scan rate of 50 mV∙s-1,and the repeated adsorption/desorption cycles were tested in 200 ml 1.5 mmol∙L–1CsBr solution and 10.0 mmol∙L–1NaNO3solution respectively for seven cycles. As shown in Fig. 11(a), even after 1200 cycles of cyclic voltammetry,the charging capacity of the NiHCF film electrode remained more than 80%. This shows that the NiHCF film electrode has excellent electrochemical stability. As shown in Fig. 11(b), the adsorption quantity retained at 94.83%of its initial value even after seven successive cycles. Furthermore, it is worth noting that the desorption quantity gradually increased as the number of cycles increased.This is due to the NiHCF on the film electrode being activated gradually as the number of cycles increased.As shown in Fig.12,there are eight lattices in each cell of the prepared NiHCF,among which four lattices are embedded with K+ions. During the adsorptionprocess, each NiHCF cell absorbs four Cs+ions into the empty lattices and exchanges the existing four K+ions with the Cs+ions in solution, and the exchanged four Cs+ions are fixed in the lattices without being released. When the K+ions in the lattices are replaced by Cs+ions, the NiHCF is transformed into ‘‘activated NiHCF”. In the first adsorption process, some of the Cs+ions in the solution are embedded in the empty NiHCF lattices, while others are exchanged with the K+ions in the lattices. And during the desorption process, the Cs+ions exchanged with K+are unable to desorb, thus the desorption percentage is only 50%. In the subsequent adsorption processes, the unactivated NiHCF is activated successively. Some of the Cs+ions in the solution are adsorbed by the previously activated NiHCF, while others are adsorbed by the unactivated NiHCF. In this case, the desorption percentage increases with the number of activated NiHCF,since the desorption percentage of the activated NiHCF is about 100%. After all the NiHCF on the film electrode is activated, all K+ions in the lattices are replaced by Cs+ions, and thereafter, all Cs+ions adsorbed by NiHCF can be desorbed, hence the desorption percentage reaches as high as 100%. It can be proved that NiHCF film electrode has excellent stability and regeneration performance.
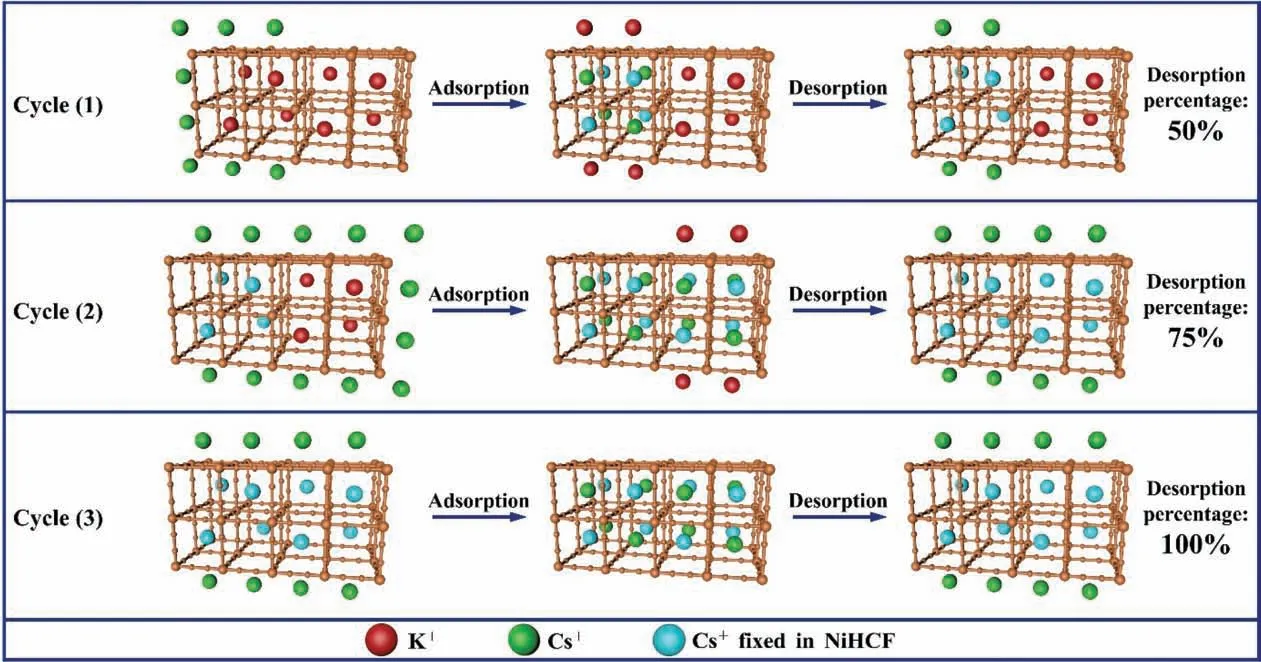
Fig. 12. Schematic illustration of the adsorption and desorption of Cs+ ions on the NiHCF film electrode during the adsorption/desorption cycles.
3.6. The comparison of adsorption performance for Cs+ with different materials
Table 2 compares the adsorption performance for Cs+in this work and other literature, and it can be seen that this work has excellent adsorption capacity. However,in addition to the adsorption capacity, other properties such as adsorption time, treatment volume,and initial concentration must also be considered.In comparison, this work has a larger adsorption capacity and a shorter adsorption time at a larger treatment volume and a lower initial concentration.
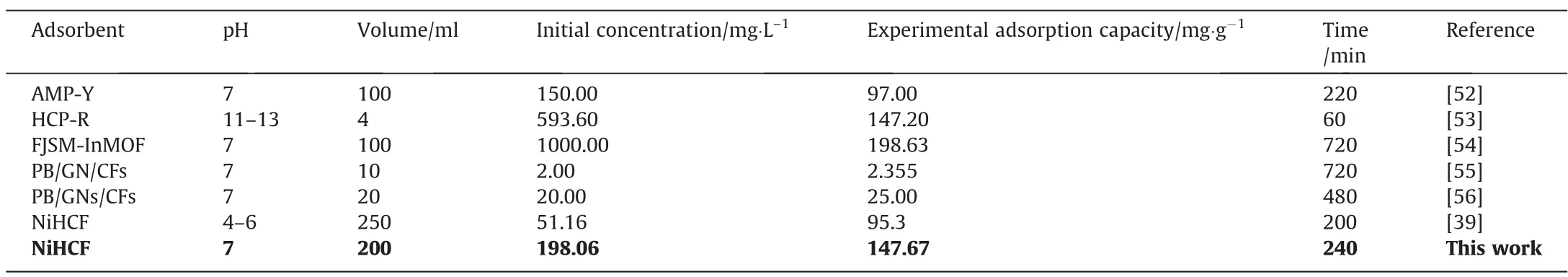
Table 2Performance comparison of adsorption for Cs+ in this work and references
4. Conclusions
A NiHCF film electrode with a potential-responsive ionpumping effect was prepared with NiHCF, CB, and PVDF, and the selective extraction of Cs+was successfully achieved by using ESIX technology.In addition,a film module for the potential-responsive ion-pump system was designed and assembled.In the system,the adsorption capacity and the adsorption percentage of Cs+ions on the obtained film electrode reached as high as 147.69 mg∙g-1and 92.47%,respectively.And the adsorption capacity and removal percentage of Br–ions on the BiOBr film electrode reached 69.53 mg∙g-1and 77.32%, correspondingly. The optimum wet film thickness,charging mode, flow rate, and chamber thickness of the NiHCF film electrode in the system was found to be 400 μm, constant potential, 20 ml∙min-1and 2 mm, respectively. The NiHCF film electrodes remained selectivity for Cs+and Rb+in complex solution systems and exhibit excellent stability. After seven cycles of adsorption/desorption, the desorption percentage of the NiHCF film electrode could reach about 100%, which demonstrated an outstanding regeneration performance. Thus, the potentialresponsive ion-pump system based on NiHCF and BiOBr film electrodes can be used for the extraction and concentration of Cs+and Br–ions from salt lake brine in a practical process.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (22108188, U21A20303, U20A20141), and CAS Project for Young Scientists in Basic Research (YSBR-039).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.007.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- Separation of lithium and nickel using ionic liquids and tributyl phosphate
