Facile synthesis of efficient pentaethylenehexamine-phosphotungsticacid heterogeneous catalysts for oxidative desulfurization
Chongfu Wu, Changsheng Chen, Zhaoyang Qi, Jie Chen, Qinglian Wang, Changshen Ye, Ting Qiu
College of Chemical Engineering, Fuzhou University, Fuzhou 350108, China
Qingyuan Innovation Laboratory, Quanzhou 362801, China
Engineering Research Center of Reactive Distillation, Fujian Province Higher Education Institutes, Fuzhou 350108, China
Keywords: Oxidation Catalyst Desulfurization Fuel Phosphotungstic acid Amino
ABSTRACT The ultra-deep desulfurization of oil needs to be solved urgently due to various problems,including environmental pollution and environmental protection requirements. Oxidative desulfurization (ODS) was considered to be the most promising technology. The facile synthesis of highly efficient and stable HPW-based heterogeneous catalysts for oxidative desulfurization is still a challenging task.In this paper,pentamethylene hexamine(PEHA)and phosphotungstic acid(HPW)were combined by a simple one-step method to prepare a heterogeneous catalyst of PEHA-HPW for the production of ultra-deep desulfurization fuel oil. The composite material exhibited excellent catalytic activity and high recyclability, which could reach a 100% dibenzothiophene (DBT) removal rate in 30 min and be recycled at least 5 times.Experiments and DFT simulations were used to better examine the ODS mechanism of PEHA-HPW. It was proved that the rich amino groups on the surface of PEHA-HPW play a crucial role. This work provides a simple and feasible way for the manufacture of efficient HPW-based catalysts.
1. Introduction
Sulfur-containing compounds widely found in fuel oil would produce sulfur oxide (SOx) after combustion, which is harmful to human beings and the environment [1,2]. Consequently, the removal of sulfides from fuel oil is of great importance in protecting the environment[3].Several techniques,such as hydrodesulfurization (HDS), adsorption desulfurization (ADS), and oxidative desulfurization (ODS), have been applied to remove sulfurcontaining compounds from fuel oil [1,4]. At present, the conventional HDS process is a well-established method at the industrial level, which is widely used and is effective to remove aliphatic and acyclic sulfur-containing organic compounds to a concentration of 500 mg∙kg-1[1,5].However,the HDS process requires harsh reaction conditions (high temperature and pressure) and high operational costs,and it is inefficient in achieving a desulfurization efficiency down to 10 mg∙kg-1, which is a new level of the sulfur content in fuel required by many regulations [6–9]. Amongst all the aforementioned alternative desulfurization methods, the ODS process has attracted extensive attention as a promising technique due to its mild reaction conditions(low temperature and pressure)and higher selectivity in eliminating aromatic sulfur compounds,compared to the HDS process [9,10]. Specifically, the ODS is a two-step process that includes an oxidation procedure and a liquid extraction process with aid of a polar solvent as the extractant.The oxidant used in the first step would donate one or two oxygen atoms, thus contributing to the formation of a corresponding sulfoxide or sulfone,respectively,which could be therefore effectively extracted and removed by the polar solvent (shown in Fig. S1,Supplementary Material ) [11].
Polyoxometalates (POMs), unique types of a nanoscale metaloxo cluster, have been employed as excellent catalysts in numerous oxidative reactions due to their redox properties[6].However,POMs are usually soluble in many polar solvents, causing difficulties in recovery, separation, and recycling of the catalysts, which affects their applications in systems that require environmentally friendly, efficient transformations and sustainable development[12,13].
A simple and effective strategy to design POM-based heterogeneous catalysts is to form insoluble ionic materials by combining appropriate inorganic or organic cations. For example, the complexation of POMs with large cations such as Cs+[14], Cu+[15],Ag+[16], and Zn2+[11] could produce insoluble POMs solids for acid-catalyzed or base-catalyzed organic reactions in polar organic solvents.Compared with inorganic cations,organic compounds act as cations that have unique advantages in the modification of POMs.Organic amines were often employed to modify heteropolyacids for preparing POMs-based catalysts with tunable surface area and redox properties.
Geet al.[17]prepared quinine modified phosphovanadomolybdate Q-V2for the H2O2mediated hydroxylation of benzene to phenol, affording a yield of 23.6%. Longet al.[18] utilized 4,4-bipyridine (bipy) to modify Kegging structured molybdovanadophosphoric acid (PMoV1), producing an organic–inorganic hybrid heterogeneous catalyst bipy-PMoV1and presenting an excellent catalytic performance for direct hydroxylation of benzene to phenol by O2with a phenol yield of 7.8%. Zhaoet al.[19]reported an acid-base bifunctional heteropoly acid nanocatalyst by the self-assembly of lysine and phosphotungstic acid, which has presented high activity for the simultaneous transesterification and esterification due to having the bifunctional acid and base together.
In addition,it has also been found that the introduction of—NH2groups capable of attracting thiophene sulfides into the catalyst is beneficial for desulfurization.Zhanget al.[20]introduce the amino groups into UiO-66(Zr) to help improve the adsorption performance of thiophene benzothiophene, due to the hydrogen bond interaction between amino groups and sulfides. Chenet al.[21]prepared the amine-modified SiO2hybrid aerogel desulfurization adsorbents with varied Si/N molar ratios (SiO2-NH2-n). SiO2-NH2-nexhibited a significantly higher adsorption capacity for thiophenes than SiO2aerogel because of the hydrogen bonding between thiophenes and amino groups.
In general, it is a feasible and effective solution to use organic amine to modify phosphotungstic acid (HPW) to construct the heterogeneous catalyst while attracting thiophene sulfides to improve the oxidative desulfurization activity of the catalyst. In this paper, pentaethylenehexamine (PEHA) as an organic amine substance rich in amino groups was chosen to modify phosphotungstic acid. The catalyst was systematically characterized and its catalytic performance was assessed in the oxidant desulfurization of model oil containing dibenzothiophene(DBT)as the typical refractory sulfur compound. PEHA-HPW can achieve complete desulfurization within 30 min under mild conditions.Both experiments and DFT calculations confirmed that the adsorptioncatalytic synergy between PEHA and HPW improved the ODS activity, and the amino group introduced by PEHA could help attract DBT and H2O2.
2. Experimental
2.1. Materials
Pentaethylenehexamine (PEHA), phosphotungstic acid(H3PW12O40), ethanol, acetonitrile (MeCN),n-octane (C8H18),dibenzothiophene (DBT), were purchased from Shanghai Aladdin Industrial Corp (China) and used without further purification.
2.2. Syntheses of PEHA-HPW
PEHA-HPW is synthesized by a simple method of stirring at room temperature. The synthetic route is described as follows:firstly, 1 mmol PEHA was dissolved in 5 g absolute ethanol and 0.1 mmol HPW was added into 5 g absolute ethanol, respectively.Afterward,both above solutions were mixed with vigorous stirring.The final product was separated by filtration and washed with ethanol to remove residual reactants. Then the products were obtained after drying at 70 °C for 12 h and it was simply denoted as PEHA-HPW.
2.3. Catalytic tests
The 1000 mg∙kg-1sulfur-containing model oil was prepared by dissolving a certain amount of DBT inn-octane. The oxidative desulfurization process consists of two main stages:the first stage is extraction, where acetonitrile, model oil, and catalyst are added to the bottle together and stirred for 20 min to achieve equilibrium in the extraction.The second stage is the oxidative reaction,which is initiated by adding H2O2and analyzed at a specific time after the reaction.The amount of acetonitrile and model oil is added according to a certain mass ratio, the amount of catalyst is a certain percentage of the mass of the model oil,and the O/S ratio is the ratio of H2O2mole to DBT mole in the model oil.A representative ODS procedure is as follows:briefly,PEHA-HPW(0.02 g),acetonitrile(2 g),and model oil(2 g)were placed in a 10 ml glass bottle.The mixture was vigorously stirred at the preset temperature for 20 min then aqueous H2O2(63 μl) was added into the bottle. The agitation was stopped at a certain time, and the mixture was cooled until the liquid–liquid phase splitting. After that, the sample was taken to be analyzed by gas chromatography on a SHIMADZU with an FID detector. For the recycling experiments, the PEHA-HPW catalyst was simply centrifuged and then directly subjected to the next oxidative desulfurization run.
Removal rate (η) is defined based on the number of Scompounds removed from model oil, which can be obtained according to:
in whichC0andCtrepresent the initial and final S-compound concentrations inn-octane.
3. Results and Discussion
3.1. Structures and characterization
Facile synthesized PEHA-HPW composite was characterized by FT-IR (Fig. 1(a)). The broad band at 3423 cm-1was assigned tostretching vibration [22]. The band at 1455 cm-1and the small band at 1009 cm-1corresponded to asymmetric bending of C—H and bending vibration of the C—N [23], respectively. And the N—H vibration peaks were shown at 750 cm-1and 1607 cm-1[24].All of these characteristic peaks indicated the existence of the PEHA group.It can be seen from Fig.S2 that the bands related to P—Oa, M—Ob—M, and M—Oc—M for PEHA-HPW were at 1078 cm-1, 884 cm-1, 807 cm-1, respectively [25], which were consistent with that for HPW. However, the band for M—Odhad an obvious red shift from 982 cm-1to 940 cm-1, confirming that the interaction between amine groups and HPW would weaken the bond energy of M—Od, which was beneficial to improving oxidative activity [26].
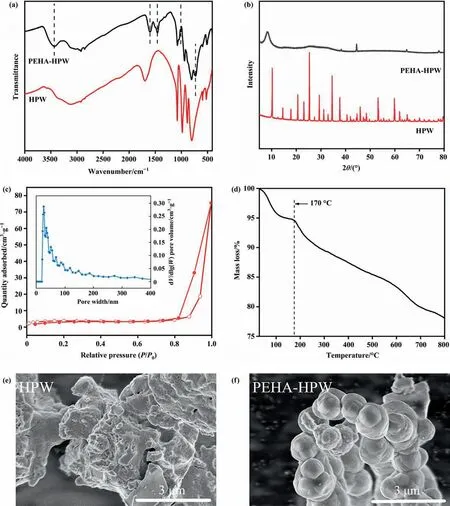
Fig. 1. (a) FTIR spectra of HPW and PEHA-HPW, (b) XRD patterns of HPW and PEHA-HPW. (c) Nitrogen adsorption–desorption isotherms of PEHA-HPW, (d) TGA curves of PEHA-HPW, and SEM image of the samples: (e) HPW, (f) PEHA-HPW.
The powder XRD patterns of HPW and PEHA-HPW have also undergone great changes (Fig. 1(b)). Compared with a set of well-resolved sharp diffraction peaks featuring the secondary structure of the P—W—O crystal constructed by Keggin anions of HPW, all these peaks disappeared on the XRD pattern of PEHAHPW, and a new broad Bragg reflection emerged at the low angle,indicating that PEHA-HPW has a semi-amorphous spherical-like nanoparticle structure, which involves the self-assembly of amine and HPW anions through the intermolecular interaction between PEHA and HPW. Similar characteristic absorption peaks of FT-IR spectra and diffraction peaks of XRD could be found in the composites formed by other polyamine-modified HPW (Fig. S3, Fig. S4).
The specific Brunauer-Emmett-Teller (BET) surface areas and porous structures of PEHA-HPW are characterized by nitrogen sorption experiments. As shown in Fig. 1(c), the nitrogen sorption isotherm of PEHA-HPW was type IV with a clear H1-type hysteresis loop in the relative pressureP/P0ranging from 0.8 to 1.0, indicating the formation of typical mesostructured. The pore size distribution curve presented a wide pore size distribution with the most probable pore sizes centered at 17.2 nm.The results indicated that PEHA-HPW had a high BET surface area of 105.7 m2∙g-1,a total pore volume of 0.57 cm3∙g-1,and an average pore diameter of 21.6 nm, which was greatly improved compared to the original HPW with a BET surface area of less than 10 m2∙g-1[27,28]. In short, the mesoporous catalyst with a large BET surface area was obtained through the combination of PEHA and HPW.The thermal stability can be confirmed by TGA(Fig.1(d))that the slight weight loss was caused by the evaporation of the adsorption water when the temperature is lower than 100 °C. The temperature of starting thermal degradation is about 170 °C, which was satisfactory for practical application.
As depicted in Fig. 1(e) and (f), the SEM images of HPW and PEHA-HPW revealed a radically distinct morphology.HPW showed an irregular crystal state, while PEHA-HPW showed an analogous spherical appearance. The primary particles of PEHA-HPW were about 1 μm in diameter,which may be a reflection of the interconnected PEHA-HPW secondary structure.
XPS was used to characterize the elemental composition and interactions between components of PEHA-HPW (Fig. S5), and peaks of C, O, N, W, and P could be observed in the full spectrum,which could prove the existence of several elements.It can be seen that the characteristic peaks of W4f in the material are located at 34.2 eV, 36.4 eV and 40.0 eV, which are the characteristic peaks of W4f, corresponding to the +6 valence of W [29]. The binding energies of 529.7 eV and 531.2 eV in the O1s spectrum belong to the W—O—W and W—O—P bonds, respectively [30]. It is evident that two resolved peaks of the N 1s signal appear at 398.5 and 400.6 eV. The peak at 398.5 eV is likely from alkylamines, while another peak at 400.6 eV can be attributed to the protonation of amine groups in PEHA, providing evidence for electrostatic or hydrogen bonding interactions between PW and PEHA [31].
3.2. Oxidative desulfurization performances of PEHA-HPW
Furtherly, the catalytic performance of facile-synthesized PEHA-HPW catalyst was discussed in the oxidative desulfurization process. The effect of reaction time on oxidative desulfurization was illustrated in Fig. 2(a). Before the oxidation reaction started,the desulfurization rate reached 54% after extraction for 20 min.The total desulfurization rate increases with the increase in reaction time and complete desulfurization were achieved within 30 min after the addition of H2O2.As far as we know,it was a satisfactory result in the ODS process. Moreover, the components in the MeCN phase and oil phase were analyzed by GC–MS (Fig. S6,Fig. S7) and it was found that only the response of DBTO2in the MeCN phase was detected after 30 min of reaction.It showed that PEHA-HPW could completely convert all DBT into DBTO2, and DBTO2can also be extracted thoroughly by MeCN.
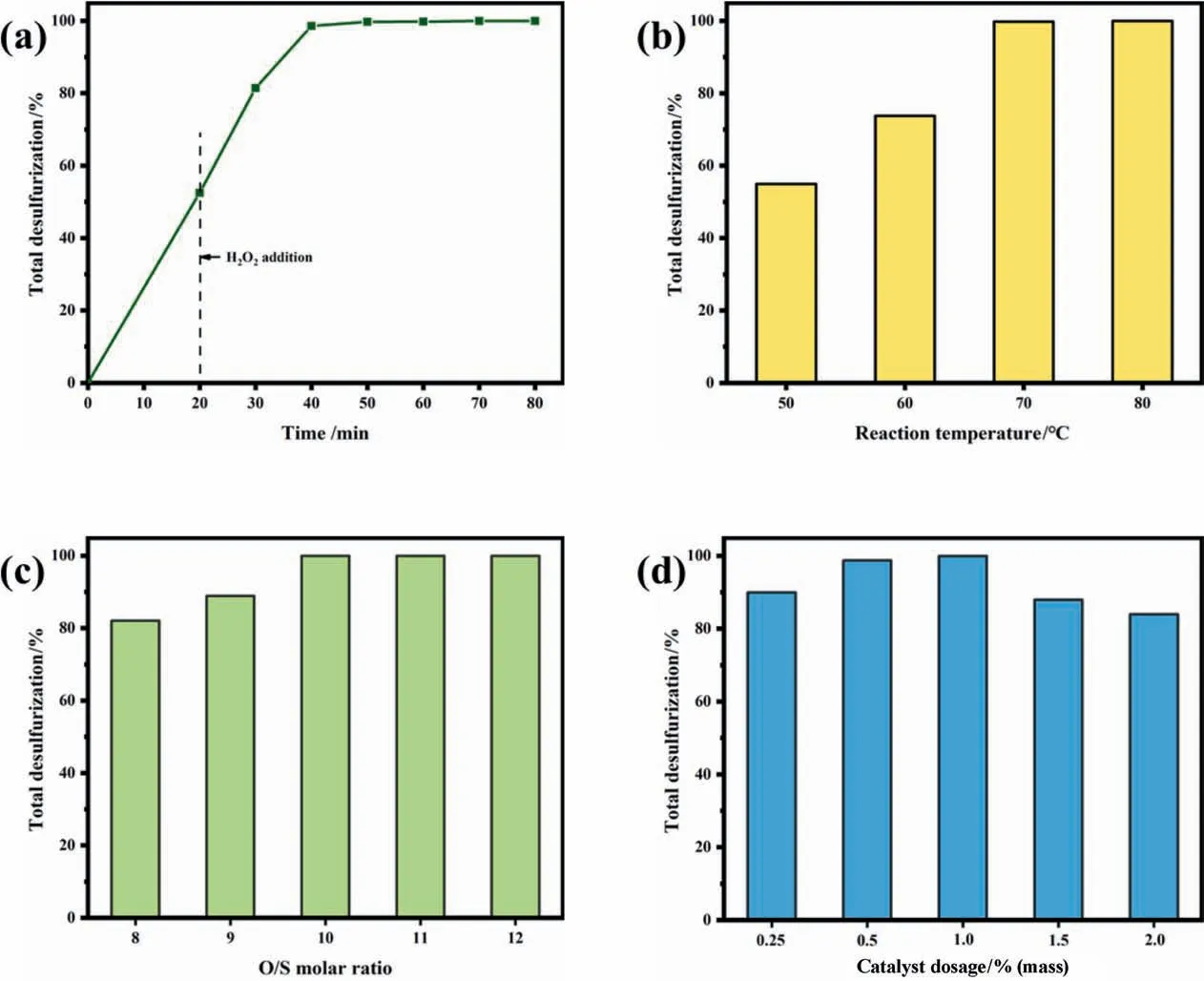
Fig. 2. The influences of process parameters on oxidative desulfurization: (a) reaction time, (b) reaction temperature, (c) O/S molar ratio, and (d) catalyst dosage.
The reaction temperature was conducted at a temperature ranging from 50 °C to 80 °C (Fig. 2(b)). The catalyst could also maintain a good nature at this temperature according to the previous TG analysis. As the temperature increases, the desulfurization rate would also increase. It was obvious that the removal rate could approach 100% at 70 °C within 30 min, which may be selected as the optimal reaction temperature in this ODS system.
The influence of catalyst dosage on the ODS was depicted in Fig. 2(c). the DBT conversion rapidly rose with an increasing amount of catalyst and reached 100%conversion when the catalyst dosage was 1.0% (mass). But an excessive amount of catalyst was harmful to the reaction rate because it might be that more catalysts would promote self-decomposition of H2O2, generating oxygen and water, which was not only uninvolved in the progress of the oxidation reaction but also caused waste of the catalysts [32].For this reason, it can be seen that the amount of H2O2was also a key factor in the ODS process.It is desirable to apply as few oxidants as possible in the ODS process to attain complete desulfurization and achieve a high economic value. But H2O2has nonproductive decomposition in the ODS process,it was necessary to afford a high O/S molar ratio[33,34].Fig.2(d)showed the effect of the O/S molar ratio. With the increase of the O/S molar ratio from 8 to 12, the DBT conversion correspondingly increased from 65% to 100%. When the O/S molar ratio exceeded 10, complete removal of DBT could be achieved within 30 min. Therefore, by optimizing the ODS process, the appropriate ODS conditions are 70 °C reaction temperature, 1.0% (mass) catalyst dosage, and a 10:1 O/S molar ratio. Under these conditions, desulfurization can be completed within 30 min.
In addition, the amount of MeCN and the stirring rate in the reaction were also investigated (Fig. S8, Fig. S9), respectively.Reducing the amount of MeCN is helpful for the subsequent treatment of ODS,but prolonging the time to complete desulfurization.In order to obtain better results,the experiment was carried out at 1:1(model oil mass/MeCN mass).The stirring rate affects the mass transfer and reaction rate, but the effect is not obvious when the stirring rate is above 500 r∙min-1, and the experiments were chosen to be performed at 500 r∙min-1.
Kinetic experiments of the catalytic ODS were performed at 50,60,65,and 70°C(Fig.S7).In the presence of excess H2O2,a plot of ln(C0/Ct)versusreaction time displayed a linear relationship which confirms that the oxidation reaction of DBT follows pseudo-firstorder reaction kinetics. The apparent activation energy obtained for the desulfurization reaction is 109.5 kJ∙mol-1. It can be seen from Fig. S8 that no significant decrease in catalytic activity was observed and the sulfur removal rate could attain 96.5% after five runs under optimal reaction conditions.The PEHA-HPW could still be separated and recovered well after five times of recycling. The FT-IR spectra and XRD pattern of PEHA-HPW did not change significantly before and after use, indicating that the catalyst structure remains stable (Fig. S12, Fig. S13). In addition, no signal of W was found in the reaction solution detected by ICP.Leaching experiments were carried out during the ODS reaction.After PEHA-HPW was removed from the reaction system at 15 min,the desulfurization rate remained unchanged,indicating that there was no leaching of active components from the catalyst (Fig. S14).
Several studies have demonstrated that a high desulfurization rate and exceptional desulfurization could be achieved with H2O2as oxidants and different kinds of HPW-based composites as catalysts [23]. The oxidative desulfurization performance of some HPW-based catalysts was compared in Table 1. Although PEHAHPW lacked porous support to improve mass transfer, its own higher specific surface area and larger pores still provide enough reaction sites, resulting in strong catalytic oxidation desulfurization activity. Moreover, compared to the complicated preparation process of other catalysts, the preparation method of PEHA-HPW was simpler and more environmentally friendly.
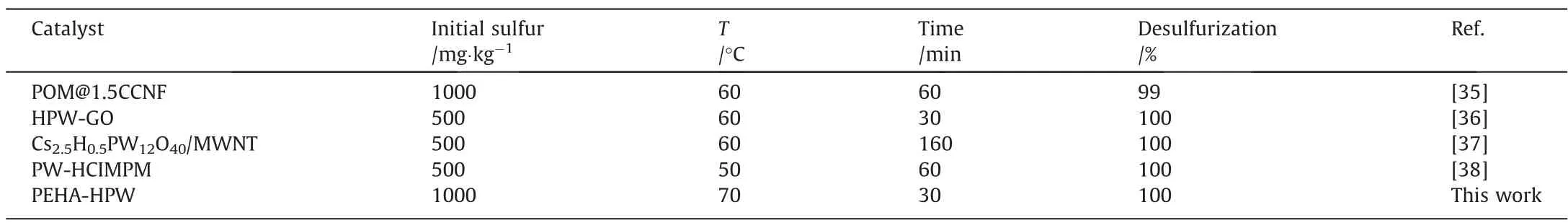
Table 1Comparison of performances for desulfurization processes containing HPW heterogeneous catalysts
3.3. Mechanism of oxidative desulfurization by PEHA-HPW
The systematic study of different desulfurization systems on DBT removal was displayed in Fig. 3(a). In the absence of MeCN,the sulfur removal can only reach around 4%. This was reasonable since H2O2was insoluble inn-octane, and it was difficult to form effective active oxygen in it,which inhibited the activity of the catalyst. Regardless of the presence of catalyst, the final desulfurization rate of the system was the same as that of MeCN extraction if H2O2was not provided as an oxidant.Finally,the role of catalysts in the system with MeCN and H2O2was investigated, and it was found that only PEHA or only HPW had no oxidative desulfurization activity. Only the reaction system utilizing PEHA-HPW composite material as a catalyst was capable of achieving complete desulfurization. Therefore, it is speculated that there was a synergistic effect between PEHA and HPW,which improved the activity of the catalyst.Analyzing the H2O2decomposition over various catalysts (Fig. S9), PEHA-HPW exhibited a stronger decomposition of H2O2compared to HPW, which is consistent with the catalytic activity. Overall, MeCN, H2O2, and catalyst were all essential components in the extraction-oxidative desulfurization and PEHAHPW was a catalyst with excellent oxidative desulfurization activity.
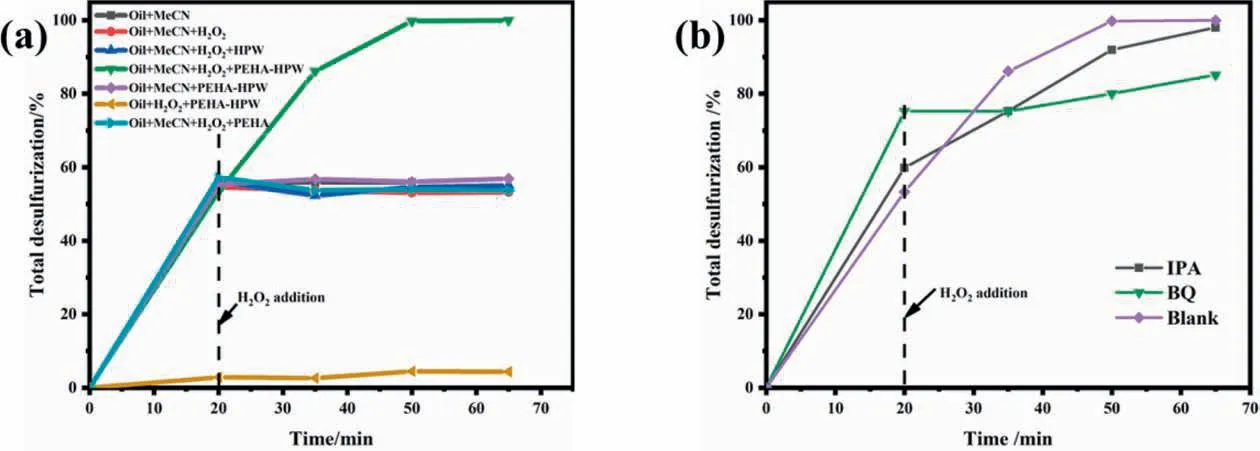
Fig. 3. (a) Sulfur removal from model oil in different desulfurization systems, (b) effects of trapping agents on the removal of DBT.
It is generally believed that the oxidative desulfurization with the participation of hydrogen peroxide is a free radical reaction,which would decompose into hydroxyl radicals (∙OH) or superoxide radicalsunder the action of a catalyst [33]. Isopropanol(IPA) and benzoquinone (BQ) as trapping agents quickly form corresponding stable substances with ∙OH and, respectively,thereby inhibiting the progress of the reaction[24,39].As indicated in Fig.3(b),add 5 times equivalents of IPA and BQ into the reaction system before the reaction starts, and the extraction rate was also increased due to the solubilizing effect of IPA and BQ. After the reaction started, the effect of adding IPA was relatively weak, and the removal rate of DBT could still be gradually increased to 98%.It is found that the desulfurization rate of the system becomes slower with BQ added, and the removal rate of DBT was about 82% after 30 min of reaction, which was only 4% higher than the extraction rate. It indicated thatwas the chief active species in the oxidative desulfurization reaction, with ∙OH playing a secondary role.
In addition,DFT calculations are employed to study the adsorption energies between polyamines and DBT and H2O2.The model of PEHA has been constructed, and the adsorption energy of PEHA with DBT and H2O2was calculated,respectively.At the same time,the interaction ofn-hexadecane with DBT and H2O2was studied as a reference. As illustrated in Fig. 4 and Fig. S10, the adsorption energies ofn-hexadecane with DBT and H2O2are -1.22 kJ∙mol-1and -8.69 kJ∙mol-1, respectively. While the adsorption energies of PEHA with DBT and H2O2increased significantly, reaching-4.76 kJ∙mol-1and -49.9 kJ∙mol-1, respectively. The results confirm that the amino group on PEHA does have a stronger interaction with DBT and H2O2.
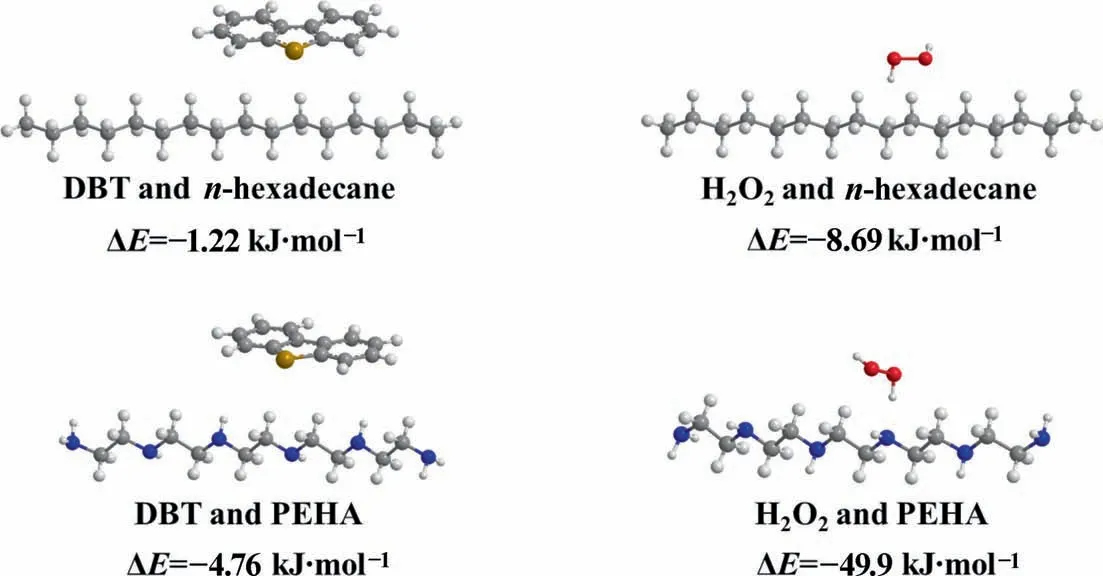
Fig. 4. Optimized models of DBT and H2O2 interaction with n-hexadecane and PEHA.
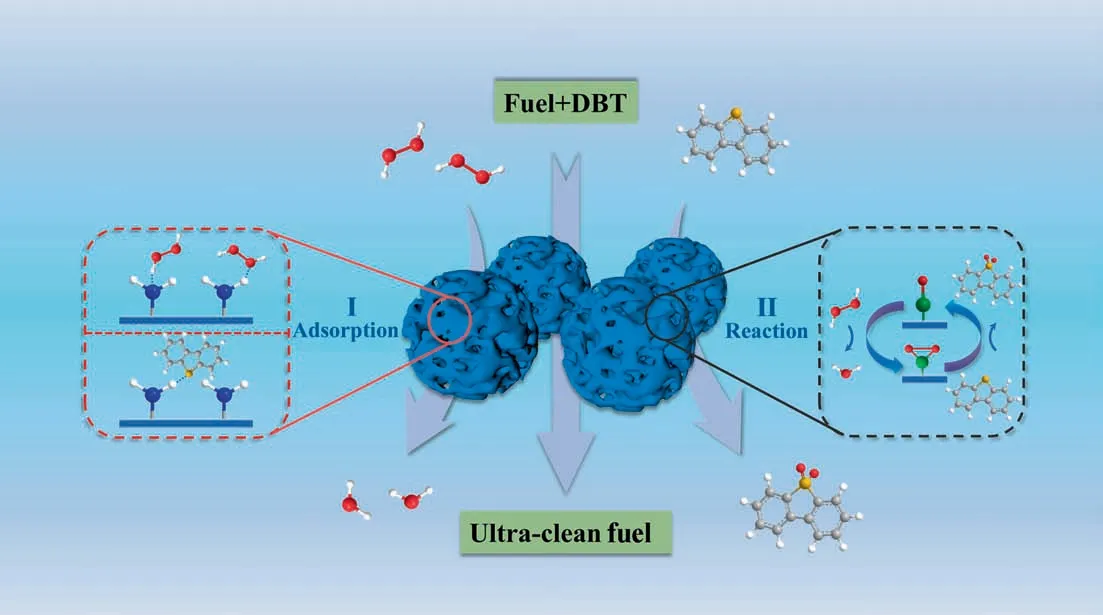
Fig. 5. Mechanism of oxidative desulfurization catalyzed by PEHA-HPW.
For the typical liquid–liquid–solid three-phase reaction system of extractive oxidative desulfurization,DBT was first extracted into the MeCN phase from the oil phase and then converted into highly polar dibenzothiophene sulfoxide/sulfone under the action of catalyst and oxidant. Based on the above results, a possible mechanism of ODS on PEHA-HPW catalysts was proposed below(Fig.5).The reaction seemed to occur on the surface of the catalyst,and the microenvironment riching in amino functional groups on the surface played an important role. The hydrogen bonding between H2O2and amino groups increased the concentration of H2O2on the surface, which aided its decomposition into ∙O2-radicals and attacked W-Odto form intermediate peroxide W-O2[27,40]. On the other hand, DBT in the oil phase diffused rapidly into the polar MeCN phase, and the interaction between DBT and the amino group also helped to adsorb DBT [20–22], increasing the possibility of DBT contacting with W-O2and oxidizing DBT to corresponding DBTO, and W-O2changed back to W-Odto participate in the next round of reaction. The continuous oxidation of DBTO to the corresponding DBTO2could also be catalyzed in a similar way. This has also been demonstrated in other similar HPWbased catalysts [12,35]. Finally, PEHA-HPW exhibits efficient oxidative desulfurization ability.
4. Conclusions
In summary,we proposed a facile synthesis method by modifying HPW with PEHA for preparing an HPW-based heterogeneous catalyst for oxidative desulfurization.Several structural characterizations confirmed that PEHA-HPW has been successfully synthesized with a large specific surface area and mesoporous structure. Under the optimal conditions, a 100% removal rate of DBT could be achieved within 30 min with an initial sulfur content of 1000 mg∙kg-1. Moreover, PEHA-HPW could be easily recovered and reused at least 5 runs without losing catalytic activity. Experiments and DFT calculations confirmed that the high activity of PEHA-HPW is related to the microenvironment of abundant amino functional groups on the catalyst surface. The amino groups interact with H2O2to help increase the contact between HPW and H2O2and further convert W-Odto W-O2.On the other hand,the adsorption of DBT by amino groups assists to increase the possibility of W-O2reacting with DBT. Taking into account the advantages of facile synthesis,reliable recyclability,low cost, and ecological sustainability of PEHA-HPW, it provides new optional material for ultra-deep desulfurization.
Data Availability
No data was used for the research described in the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22378065, 22278077) and the Fujian Province Department of Science & Technology, China (2019YZ017001).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.004.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
