Photothermal-photocatalytic thin-layer flow system for synergistic treatment of wastewater
Zhongjiao Zha, Jun Wu, Shaoping Tong, Xuebo Cao
1 College of Chemical Engineering, State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang University of Technology, Hangzhou 310014, China
2 College of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, China
3 College of Advanced Materials Engineering, Jiaxing Nanhu University, Jiaxing 314001, China
Keywords: Wastewater disposal Solar-driven distill Thin-layer flow Clean water production Ternary film
ABSTRACT The integration of the photocatalytic effect into solar steam is highly desirable for addressing freshwater shortages and water pollution. Here, a ternary film structure for the adsorption and photothermal and photocatalytic treatment of wastewater was designed by combining the technique of self-assembled carbon nano paper with a nitrogen composite titanium dioxide(N-TiO2)deposited on the surface of carbon nanotubes (CNT) using polyvinylidene fluoride (PVDF) as a substrate. The photogeneration of reactive oxygen species can be promoted by rapid oxygen diffusion at the three-phase interface, whereas the interfacial photothermal effect promotes subsequent free radical reactions for the degradation of rhodamine B (93%). The freshwater evaporation rate is 1.35 kg∙m-2∙h-1 and the solar-to-water evaporation efficiency is 94%.Importantly, the N-TiO2/CNT/PVDF (N-TCP) film not only effectively resists mechanical damage from the environment and maintains structural integrity, but can also be made into a large film for outdoor experiments in a large solar energy conversion device to collect fresh water from polluted water and degrade organic dyes in source water simultaneously, opening the way for applications in energy conversion and storage.
1. Introduction
Due to the rapid development of industrialization, the global energy and environmental crisis has become increasingly serious[1]. Thus, extensive research is devoted to developing advanced materials [2,3] and technologies [4] to produce large quantities of fresh water from available brackish water,seawater,or wastewater.Among these developments,solar-driven interfacial desalination technology [5,6], which utilizes sustainable sunlight to produce fresh water from brine or sewage by heating water directly at the gas–liquid interface and driving its evaporation,appears to be a promising solution. Proper thermal management has been studied for various photothermal materials, such as metal-based nanoparticles [7,8], carbon-based materials [9,10],semiconductors[11],and porous polymers[12,13].Advanced photothermal materials have achieved efficiencies close to 100%.However, little attention has been paid to the fact that after the evaporation of contaminated water, nonvolatile impurities may remain or even enrich in the remaining water, thereby leading to more serious wastewater pollution.
Compared with the efficient solar interfacial evaporation[14],the semiconductor photocatalytic material TiO2has attracted extensive attention in water purification because of its safe, non-toxic, inexpensive, and superior photocatalytic ability [15–18].When sunlight irradiates the titanium dioxide catalyst,the titanium dioxide catalyst absorbs energy and generates electron–hole pairs to produce highly reactive oxygen radicalsand hydroxyl radicals (•OH). These radicals are highly oxidizing and can degrade the pollutants adsorbed on the titanium dioxide surface. However, in practical applications, they also suffer from narrow light absorption range,high photogenerated electron–hole complexation rate, and weak adsorption performance on organic matter [19,20]. Furthermore,the conspicuous photothermal effect and photocatalytic activity can be attributed to the proper oxygen vacancy density, which enhances the absorption of near-infrared light and promotes the separation of photogenerated electron–hole pairs[21–24].Therefore,the modification and incorporation of TiO2into various materials with excellent photothermal conversion functions to enhance their photocatalytic performance is the key to the industrialization of TiO2photocatalysts for wastewater treatment.
Currently, carbon nanotubes (CNT) are considered highly desirable photothermal material and catalyst carrier for the efficient evaporation of water because they have good thermal conductivity,large specific surface area, high thermal and chemical stability, and strong absorption of sunlight [25–28]. More importantly, the coupling between semiconductor photocatalysts and CNT has a synergistic catalytic effect [29,30]. The conductive structure of the CNT scaffold promotes the e–/h+separation at the CNT-TiO2interface,which improves the rate of thermal and photocatalytic oxidation greatly. On the other hand, the doping of non-metallic N extends the photoresponse range of TiO2to visible light while facilitating the separation of photogenerated electrons from holes and enhancing the photocatalytic ability. However, photocatalysts applied in general water treatment processes are powdered and difficult to separate and recover. Poly (vinylidene fluoride) (PVDF), a typical thermoplastic polymer, is easy to mold, lightmass, and has excellent corrosion resistance and mechanical properties [31–33]. Therefore,the introduction of PVDF solution that contains the pore-forming agent polyethylene glycol(PEG)not only has a wastewater transport channel but also enhances the mechanical properties and flexibility of the N-TiO2/CNT binary composite film material.
Herein, we designed a ternary composite catalyst system that combines adsorption,photothermal,and photocatalysis to achieve the efficient degradation of dyes and freshwater harvesting under ambient conditions. N-TiO2/PVDF (N-TP) films, CNT/PVDF (CP)films, and N-TiO2/CNT/PVDF (N-TCP) ternary films were prepared by simple deposition and self-assembly techniques. Among them,CNT absorbs light energy to generate thermal energy and then heats PVDF polymers and N-TiO2photocatalysts to provide enhanced photothermal-assisted freshwater yield. In addition,the N-element composite titanium dioxide promotes the separation of photogenerated electrons and holes and enhances the absorption of visible and UV light. The results showed that the ternary composite film exhibited significant synergistic photothermal/photocatalytic degradation of wastewater at room temperature under xenon lamp irradiation. Its catalytic activity was much higher than that of CNT for adsorption and thermal catalysis and TiO2for photocatalysis. Therefore, the introduction of pollutant removal technology in the water evaporation process can improve the efficiency and quality of collected water and inject new vitality into solar evaporation technology.
2. Experimental
2.1. Synthesis of ternary N-TCP composite films
The detailed experiment to prepare of N-TiO2, CNT and PVDF solutions are shown in the Supplemental Material. Self-supported N-TCP composite films were prepared in a mold(10 cm × 10 cm) with a wear-resistant copper foil (thickness ≈9 μm) as a substrate. In a typical procedure, 100 ml of N-TiO2solution (1.5 g∙L–1), 100 ml of CNT suspension (1.5 g∙L–1) were sonicated for 30 min. The N-TiO2solution was first poured onto the copper foil and dried in an oven at 100°C.Then the CNT suspension was spread on the deposited N-TiO2surface and dried again. The PVDF mixture solution is coated on carbon paper and dried. The separated N-TCP composite films were obtained by etching the copper foil with dilute H2SO4solution. Other binary N-TP films,binary CP films were prepared by the same method.Before characterization, the prepared films were stored in deionized water.
2.2. Characterization
Analyze the microstructure of samples and film materials by using a HITACHI S-4800 field emission scanning electron microscope (SEM). Transmission electron microscope (TEM) and highresolution TEM (HRTEM) images were taken with a FEI Tecnai G20 electron microscope.The crystalline properties of the samples were characterized by Panalytical X’Pert PRO powder diffractometer using Cu-Kα radiation. UV–Vis-NIR absorption and reflection were drawn with an Agilent Australia Carry-5000 spectrophotometer.Contact angles were determined using a dynamic contact angle meter (JC2000D1, Shanghai Zhongchen Digital Technology Equipment Co., Ltd., China). Fourier transform infrared spectra (FT-IR)were measured by a Thermo Nicolet AVATAR 320 instrument.
2.3. Catalytic activity test
Homemade devices for collecting fresh water and wastewater for recirculating flow degradation. The water supply flow is controlled using recirculating pumps. Thermal images were acquired by an infrared thermographic camera (FLIR ONE Pro). Methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) were selected as model pollutants to evaluate the photothermal photocatalytic activity of N-TCP ternary films.CP,N-TP binary films were also investigated as a control for comparison. A solar simulator(AM1.5, 100 mW∙cm-2) was used as the light source (CELHXF300-T3, China Education Au-light). The composite films were first left to stand in the dark for about 1 h to avoid interference with the adsorption process in photocatalysis. The absorption spectra of the solutions were recorded every 20 min using a spectrometer (JDH-2000, Guangzhou Jing Yi Photoelectric Technology Co., Ltd., China). The oxidation efficiency of the three dyes was determined by monitoring the absorbance peaks at 664 (MB),557 (RhB) and 506 nm (MO). Binary CP films, N-TP were tested using the same method.
3. Results and Discussion
3.1. Formation of N-TCP self-supporting films
Through the design of materials and systems,the parallel development of synergistic photothermal and photocatalytic strategies can further improve the overall efficiency of solar water purification. The N-TCP ternary film was prepared by a simple selfassembly method and the synthesis is shown in Fig. 1(a).The prepared Na2Ti3O7precursor[34]was placed into an acidic solution of hexamethylenetetramine, stirred for 30 min, and subjected to hydrothermal reaction and high-temperature calcination to obtain N-TiO2to broaden the light absorption range of TiO2. Stable CNT and PVDF solutions were obtained by ultrasonic vibration and magnetic stirring, respectively. Fig. 1(b) shows the photographs of N-TiO2nanocrystals and unmodified TiO2nanocrystals after tube furnace calcination. The color of the TiO2powder changed from white to gray black,indicating that hexamethylenetetramine may be supported on the surface of TiO2. Based on the selfassembly technology of functionalized carbon nanotubes with high thermal conductivity and large surface area on metal surfaces and the synergistic catalysis between TiO2and CNT, N-TiO2/CNT nanopaper was prepared on copper foil, and the active material containing pore-forming agent PVDF was added to increase the strength of the film. The N-TP and CP composite film (Fig. 1(c))was prepared by using the same method.The CP film is very black and is staggered carbon nanotubes. The photothermal conversion of carbon nanotubes mainly originates from the optical transition in the π-band. When carbon nanotubes are exposed to sunlight, a small amount of energy is required for the large number of conjugated π bands to excite loosely held electrons,followed by electron relaxationviaelectron–electron and electron–phonon scattering[35]. Therefore, the thermal vibration of the molecules generates heat,thereby increasing the macroscopic temperature of the material.The N-TCP film is lighter in color because the surface is loaded with N-TiO2, which may have a weak effect on light absorption.These prepared films can be easily etched from copper foils and have excellent flexibility but stable mechanical properties. As shown in Fig. S1 in Supplementary Material, it can be cut into any shape on demand, and no obvious damage is observed even when the film is bent by 180°.
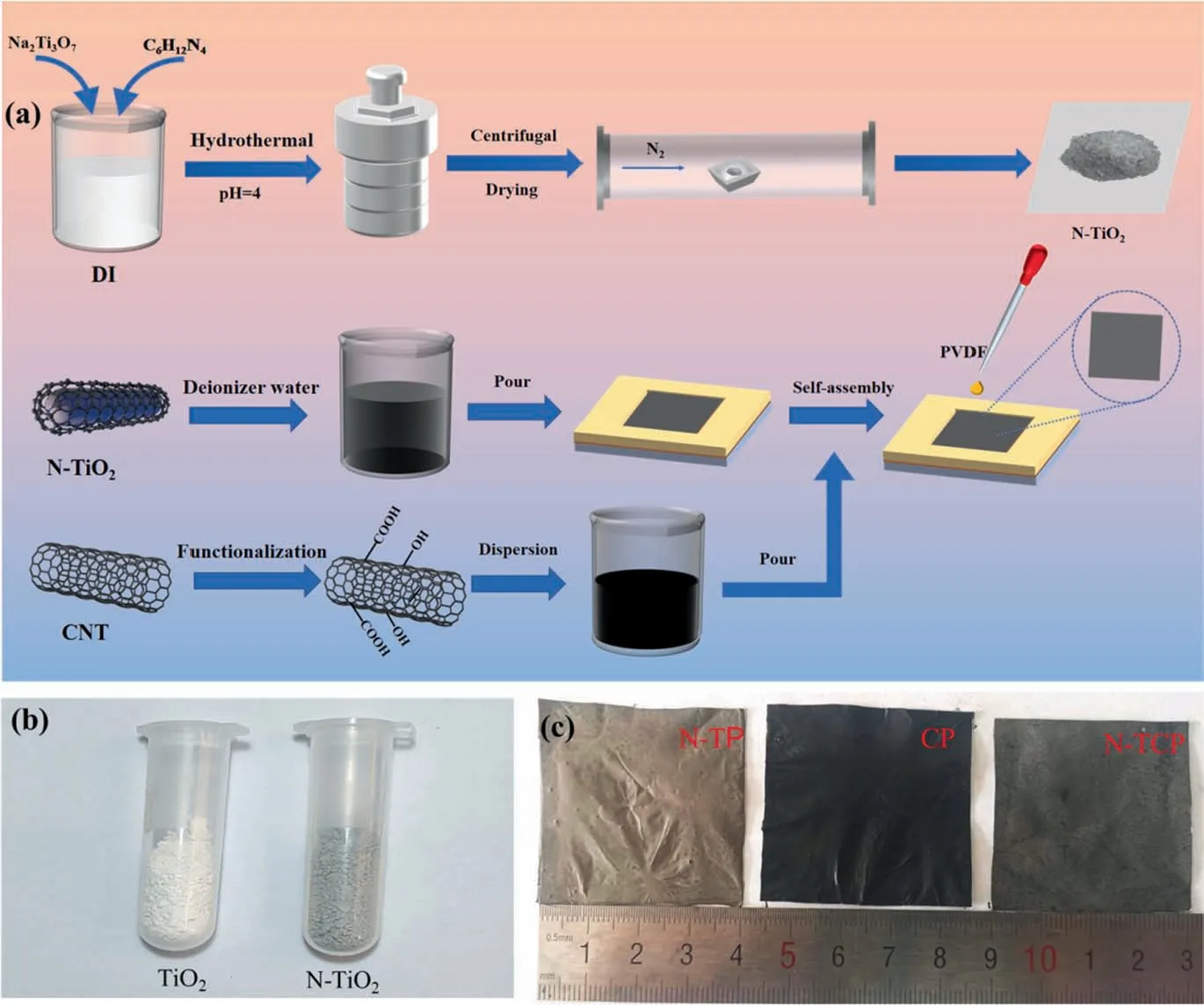
Fig.1. (a)Schematic of the preparation of N-TCP composite film;(b)a photograph comparing unmodified white and N-coated gray-black TiO2 nanocrystals;(c)photographs of N-TP, CP, and N-TCP.
3.2. Morphology and structure
In this study, the microscopic morphologies of TiO2, N-TiO2,CNT, and N-TCP composite films were characterized by SEM.Fig.2(a)shows that the TiO2synthesized by Na2Ti3O7after calcination in a tube furnace exhibits a regular rod-like structure with a smooth surface. Unlike pure TiO2, the surface of the N composite TiO2is covered with a layer of obvious square particles (Fig. 2(b)), which represents the successful loading of hexamethylenetetramine(C6H12N4).Fig.2(c)shows the surface morphology of acidified carbon nanotubes, which shows that most of the CNT are lying flat rather than upright, and the film surface is quite flat(Fig. 1(c)), which means that the CNT are organized in the film in an orderly and smooth manner. Fig. 2(d1)–(d3) show the morphological characteristics of the N-TCP film.Many pores on the surface of the underlying PVDF provide channels for wastewater transport.Microscopic images taken on the upper surface (Fig. 2d2) and cross-section (Fig. 2(d3)) showed the coexistence of CNT and NTiO2in the film, and the two were staggered, indicating that the N-TiO2did not cover the surface of the film completely.The structure is stable,and the synergistic effect of the two is played better.
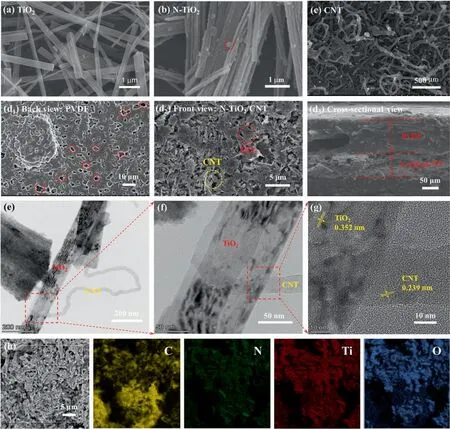
Fig.2. SEM images of the TiO2 nanofibers(a),N-TiO2 nanofibers(b),and NWCNT nanofibers(c);(d)Structure characterizations of the N-TCP film:SEM images of back view(d1), front view (d2), and cross-sectional view (d3). TEM images (e–f), HRTEM image (g) and corresponding EDX elemental mapping images (h) of N-TCP film.
TEM images were presented to show the internal structure of the composite. As shown in Fig. 2(e), carbon nanotubes are wounded on the surface of the titanium dioxide, and hexamethylenetetramine are distributed on the surface of the titanium dioxide. The HRTEM images of the N-TCP film (Fig. 2(f) and (g))show a clear interface between CNT and TiO2or N-TiO2. Fig. 2(g)shows that the lattice fringe spacing of 0.162 and 0.239 nm correspond to the (1 0 5) plane of anatase TiO2and the (1 1 1) plane of CNT, respectively, thereby confirming the coexistence of TiO2and CNT. Element mapping (Fig. 2(h)) shows that C, N, Ti, and O elements are uniformly distributed, hence verifying that hexamethylenetetramine are successfully compounded on TiO2and uniformly distributed on the CNT surface. The loading of hexamethylenetetramine plays an important role in enhancing the photocatalytic activity and is the key to broadening the usable spectrum of photocatalysts.
Fig.3(a)and(b)study the X-ray diffraction patterns of the crystal forms of CP,N-TP,and N-TCP catalysts.The results show that in N-TP and N-TCP catalysts, diffraction peaks exist at 2θ = 25.4°,37.9°, 48.1°, 54.0° and 55.1°, which correspond to the (1 0 1),(0 0 4),(2 0 0),(1 0 5),and(2 1 1)crystal planes of anatase titanium dioxide (JCPDS No. 01-084-1286), respectively [36,37]. Besides,additional diffraction patterns,which may be due to the new phase produced by hexamethylenetetramine composite titanium dioxide in N-TP and N-TCP nanocomposites,were observed in the samples.Compared with TP,we find that the XRD peaks of N-TP are slightly shifted, which may be due to the disorder induced lattice (Fig. S2)caused by oxygen vacancy. However, no corresponding diffraction peaks of carbon nanotubes are found in the X-ray diffraction patterns of N-TCP samples. This phenomenon may be because the overlap of the strong (0 0 2) diffraction peak of CNT at 25.5° and the (1 0 1) diffraction peak of anatase TiO2at 25.6° shields the appearance of any final CNT peak [38,39].
The nitrogen adsorption–desorption isotherms and pore size distributions of CP, N-TP, and N-TCP films are shown in Fig. 3(c)and(d).CP,N-TP,and N-TCP films have a clear hysteresis loop that belongs to type IV,H3 type hysteresis loop in the pressure range of 0.4–1.0P/P0in the high-pressure region, indicating that the materials have a mesoporous structure. The surface areas of the CP, NTCP, and N-TP samples were 153.7, 91.4 and 73.6 m2∙g-1, respectively. Compared with the N-TP film, the surface properties of the N-TCP ternary film were improved, thereby confirming that the addition of CNT increased their specific surface area. The pore size distribution curves of the samples showed that the three films all show a mesoporous structure of 5–40 nm.This finding suggests that the hierarchical porous structure of N-TCP will promote the diffusion and transport of reactants and products, which is favorable for photocatalytic–photothermal evaporation.
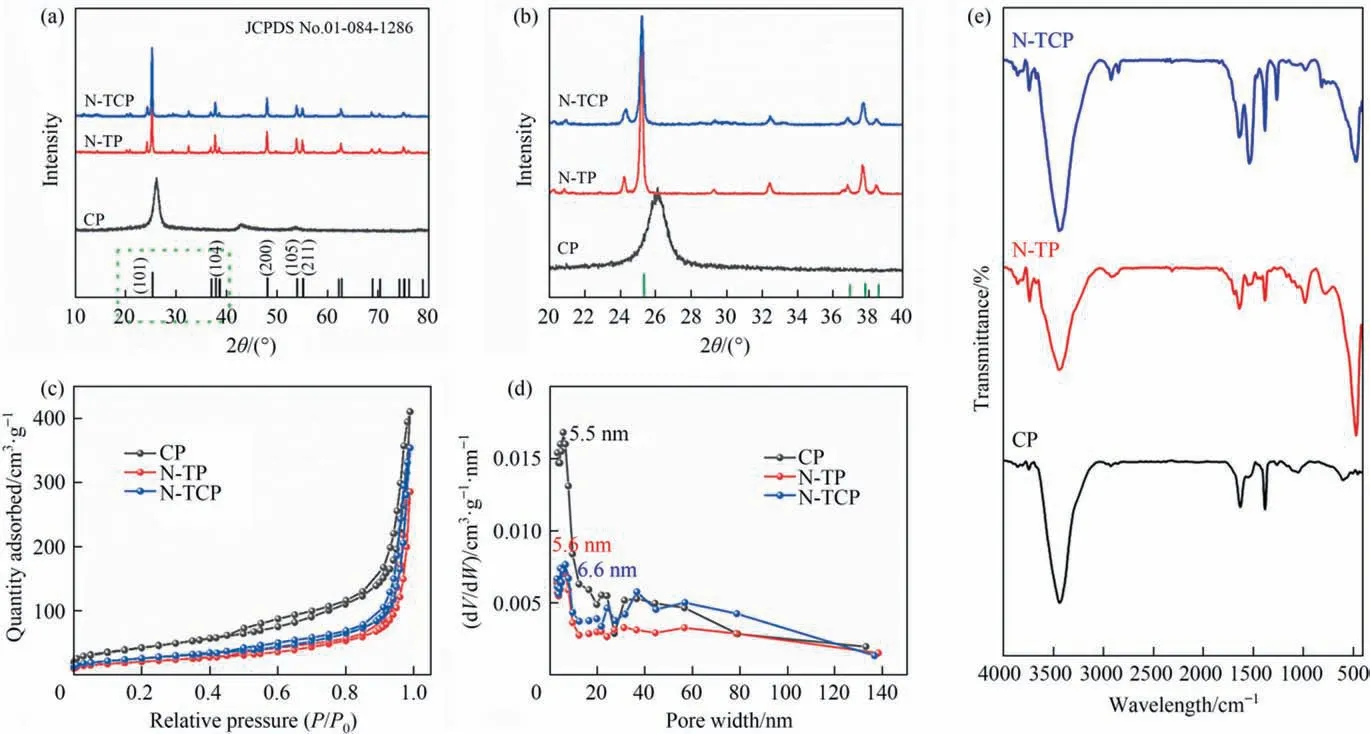
Fig. 3. XRD patterns (a, b), nitrogen adsorption–desorption isotherms (c), pore-size distribution (d) and FT-IR spectra (e) of CP, N-TP, N-TCP.
The chemical structure of the N-TCP film was analyzed by X-ray photoelectron spectroscopy (XPS). The Ti, O, N, and C elements shown in the full spectrum (Fig. S3(a)) are consistent with the EDX analysis element, which proves the successful recombination of N element. The C 1s (Fig. S3(b)) can be deconvoluted into four peaks at 284.8, 285.8, 286.9, and 289.3 eV, which come from C—C,C—O,C=O,and O=C—O bonds,respectively.It is divided into three different peaks in the O 1s spectrum(Fig.S3(c)).The peak at 530.7 eV is located on the Ti—O bond of anatase titanium dioxide,indicating that lattice oxygen is the main state of oxygen in titanium dioxide [40]. Ti—O—C and C—O bonds were observed at 531.7 and 532.9 eV,respectively.The newly formed Ti—O—C bond can be attributed to the covalent bond formed between TiO2and CNT surface defects [41]. The high-resolution XPS spectra of Ti 2p in Fig. S3(d) show that the peaks at 465.1 and 459.5 eV belong to Ti 2p1/2and Ti 2p3/2, respectively, which is slightly offset from the pure anatase phase.The segmentation between the two bands is 5.6 eV, which is consistent with the state of Ti4+in the anatase phase. Similarly, the N 1s (Fig. S3(e)) near 400.3 eV is due to the existence of nitrogen oxide in the form of Ti—O—N bond as interstitial nitrogen, and the higher binding energy at 402.2 eV is allocated to NOxspecies [42]. XPS spectra and element fitting curves not only show that N-composite titanium dioxide reduces the band gap and improves the photocatalytic efficiency, but also confirms the interface interaction between CNT and N-TiO2.
Three different film materials, namely, CP, N-TP, and N-TCP,were measured by FT-IR(Fig.3(e)).The results show that the peaks at 3442 cm-1and 1384 cm-1are attributed to the strong—OH and—COOH stretching vibrations, which are symbolic of the presence of hydroxyl groups on the surface of titanium dioxide and oxygen-containing groups from nitrate functionalization, respectively. The peaks in the range of 370–700 cm-1for N-TP and NTCP are attributed to the stretching vibrations of Ti—O bonds. In contrast, the absorption bandwidth of TP is broad and flat(Fig.S4).The quantum effect of size suggests that the fine structure of the IR absorption band disappears because of small particle size,which results in a broad spectral band [43]. Therefore, the FT-IR spectral analysis results are consistent with the XRD test results,that is, the tube furnace calcination transforms the crystal structure of TiO2from amorphous to anatase type, and the grain size decreases, which in turn leads to the tight integration of C6H12N4,TiO2, and CNT into a single unit. Moreover, the signal presented at 1257 cm-1should be attributed to the newly formed Ti—O—C bond. This covalent bond formed during in situ growth can promote the electron–hole separation to enhance the photocatalytic activity of the material [44], which is beneficial for pollutant absorption and photocatalytic applications.
3.3. Photothermal clean water production
Light absorption is a key feature affecting solar vapor production. To investigate the light absorption properties of CP, N-TP and N-TCP films, the materials were further wetted and analyzed by UV–Vis-NIR spectroscopy(UV–Vis NIR, Fig. 4(a)). CP has a light absorption that is higher than 93% in the whole band of 200–2000 nm. N-TP has the worst light absorption of 87%, whereas the ternary composite film N-TCP has a light absorption capacity that reach 92%between CP and N-TP.In addition,the light absorption of N-TP film photocatalysts is significantly higher than that of pure TP film (Fig. S5). The doping of N element absorbs titanium dioxide in the visible region(>380 nm),that is,it can absorb ultraviolet and visible light to excite electron transfer between its valence band (VB) and conduction band (CB). The solar thermal properties of CP, N-TP, and N-TCP films were studied systematically.The surface temperatures of CP,N-TP,and N-TCP films under primary sunlight were recorded by infrared thermal imager. The temperature change went through two stages: the rising section and the equilibrium section in Fig. 4(b). Under dry conditions,the surface temperature increased rapidly from room temperature to the equilibrium temperature. Finally, the surface temperatures of the N-TP, N-TCP, and CP evaporators increased to 63.2 °C,68.4°C,and 74.3°C,respectively.The surface equilibrium temperature and heating rate of the wet state are lower than those of the dry state because most of the thermal energy of photothermal conversion is converted into latent heat of evaporation. For example,the thermal infrared images show that the equilibrium temperature of the N-TCP mode in the wetted state is maintained at around 40.0 °C, and the heating rate is reduced to a lower value. The corresponding thermal IR images of different film materials in the initial and equilibrium states are shown in Fig. 4(c), indicating that the photothermal materials are uniformly heated under solar illumination.
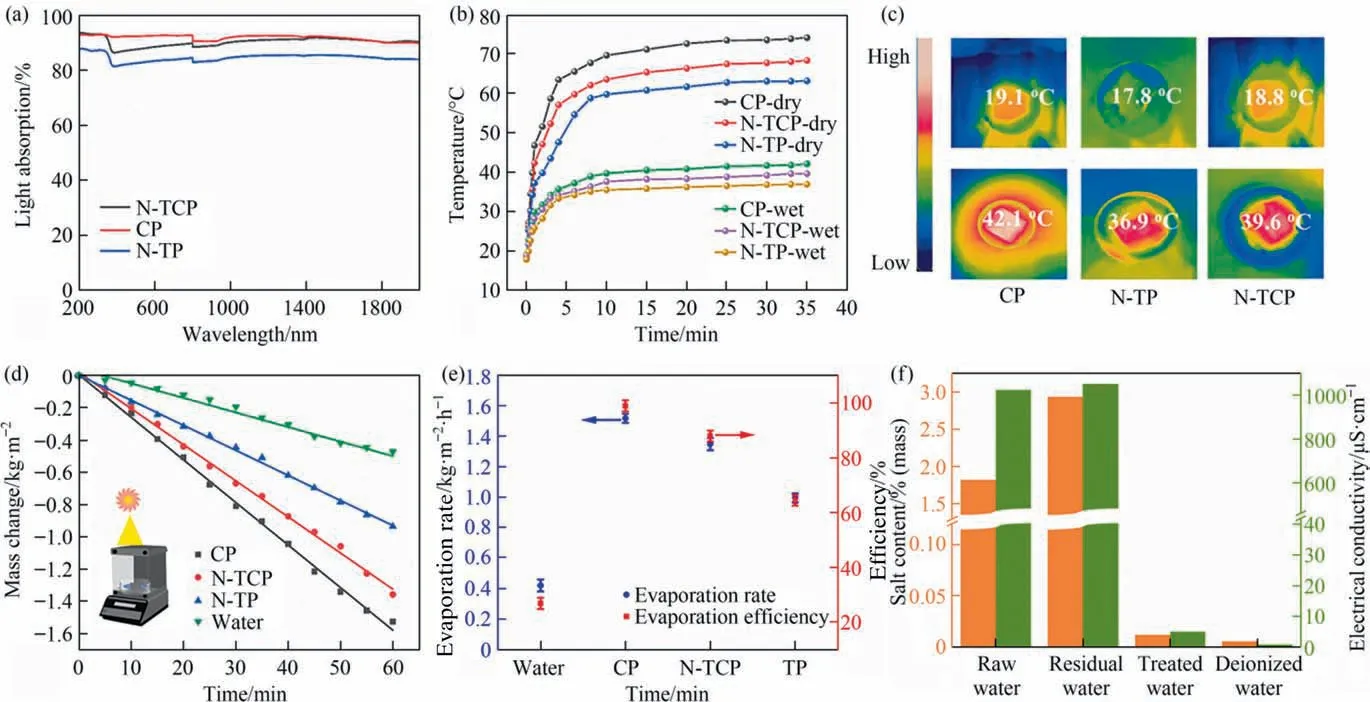
Fig. 4. (a) UV–Vis-NIR absorption spectra of CP, N-TP, and N-TCP. (b) Photothermal response of CP, N-TP, and N-TCP under one sun irradiation. (c) Temperature changes of different surface coatings recorded by an IR camera.(d)Evaporation rate under different film materials.The inset displays the schematic of the experimental setup for water evaporation (e) Evaporation rate (blue, left) and evaporation efficiency (red, right) of different evaporation substrates under solar light. (f) Water samples before and after membrane distillation.
Photothermal conversion efficiency is another important factor that limits solar vapor production. As shown in Fig. 4(d), the solar steam generation performance of different film materials was evaluated by monitoring the mass loss of dye wastewater with time under one solar illumination by electronic balance, and the curve of the linear variation of the sample quality with time was obtained. The corresponding water evaporation rate is calculated from the slope of the linear curve. Compared with other evaporators, the CP film has the highest water evaporation rate, close to 100%, because of its good photothermal effect. The evaporation rate of N-TCP ternary composite film is 1.35 kg∙m-2∙h-1, which is between CP and N-TP film. Despite this, the evaporation rate of N-TCP film is three times higher than that of pure water. This excellent water evaporation is closely related to the efficient photothermal conversion, water and vapor transport, and thermal management. The evaporation rates in this work were obtained by subtracting the evaporation rate under dark conditions from the evaporation rate measured under solar illumination and calculating the solar vapor generation efficiency without considering reflections and surface radiation losses.Therefore,the solar–water evaporation efficiency can be calculated from the solar thermal efficiency (η) [45,46]:wheremis the evaporation rate of water (kg∙m-2∙h-1),HLVis the total enthalpy that leads to the change in water from the liquid phase to the gas phase (kJ∙kg-1),Hpcis the enthalpy of evaporation(kJ∙kg-1) , andCoptP0is the total solar input (P0= 1 kW∙m-2,Coptis the optical concentration).Cis the specific heat capacity of water(4.2 kJ∙kg-1∙K-1).ΔTis the temperature difference between ambient and steam. Figs. 4(e) and S6 show that the solar water evaporation efficiency under solar light is ~94%,which is similar to the recently reported solar film evaporator water evaporation rate[47].Salt precipitation has a certain influence on the catalytic activity of membranes in wastewater treatment. Fig. 4(f) analyzes the water samples before and after membrane distillation. The conductivity of the collected pure water was 5.012 μS∙cm-1, which was close to 0.875 μS∙cm-1of deionized water, and was lower than that of the initial water.This thin layer flow system ensures the continuous flow of wastewater and reduces heat loss, thereby avoiding salt deposition on the surface of the N-TCP film [48], indicating its potential in water purification production.
In addition,in the device shown in Fig.S7(a),we simultaneously examined the solar evaporation rate and chemical oxygen demand(COD)values of the N-TCP thin film evaporator after degradation in different dye effluents. From Fig. S7(b), the N-TCP film exhibited a stable evaporation rate in both water and dye solutions, which indicated that the effect of the dye species on the evaporation rate was negligible. The COD before and after dye degradation was measured by a water quality analyzer. The COD content and removal efficiency of different dye-contaminated water are shown in Fig. S7(c) and (d). After 4 h of degradation, the COD removal rates of MO, MB, and RhB reached 84%, 85% and 87%, respectively.The dyes were all made of the original turbid color changed to clear and transparent, indicating that the self-assembled N-TCP membrane evaporator has strong practicability and is universal for treating water polluted by various dyes.
Fig.S8 shows the static contact angles of each component were measured. The results show that the PVDF surface is extremely hydrophobic with a static contact angle of approximately 90.3°.The modified PVDF has many more pores to transport water. On the other hand, the upper surface of the N-TCP film has strong hydrophilicity and is significantly better than those of CP and NTP films.This phenomenon is attributed to the fact that the binary film is so thin that it cannot cover the PVDF surface completely.The ternary film has a porous structure and an obvious layered structure,which increases the utilization of light through multiple reflections and absorptions. Moreover, the energy utilization rate of photothermal conversion of water evaporation is sufficiently high. At the same time, this asymmetric wettability will facilitate water evaporation and pumping.
3.4. Photocatalytic wastewater treatment
In addition to the solar thermal evaporation of water, the prepared film materials are used for photothermal catalysis to degrade organic pollutants in water.Fig.5(a)presents a photothermal photocatalytic integrated closure device that is constructed using NTCP films to collect clean water through the evaporation and degradation of wastewater. Under solar light, a vapor mist rapidly formed on the inner wall of the spherical glass cover,and condensate droplets were formed within 30 min(Fig.S9).The condensate falls into the device recess and is collected in a beaker. MB, MO,and RhB (10 mg∙L-1) dye effluents slowly flow through the lower surface of the N-TCP film driven by a peristaltic pump and are rapidly transported to the upper surface by the porous channels of PVDF and carbon nanotube adsorption. Moreover, the sunlight that irradiates to the water-impregnated N-TiO2and CNT surfaces absorbs energy and converts light energy into heat energy to enhance photothermal and photocatalytic performance for wastewater degradation.
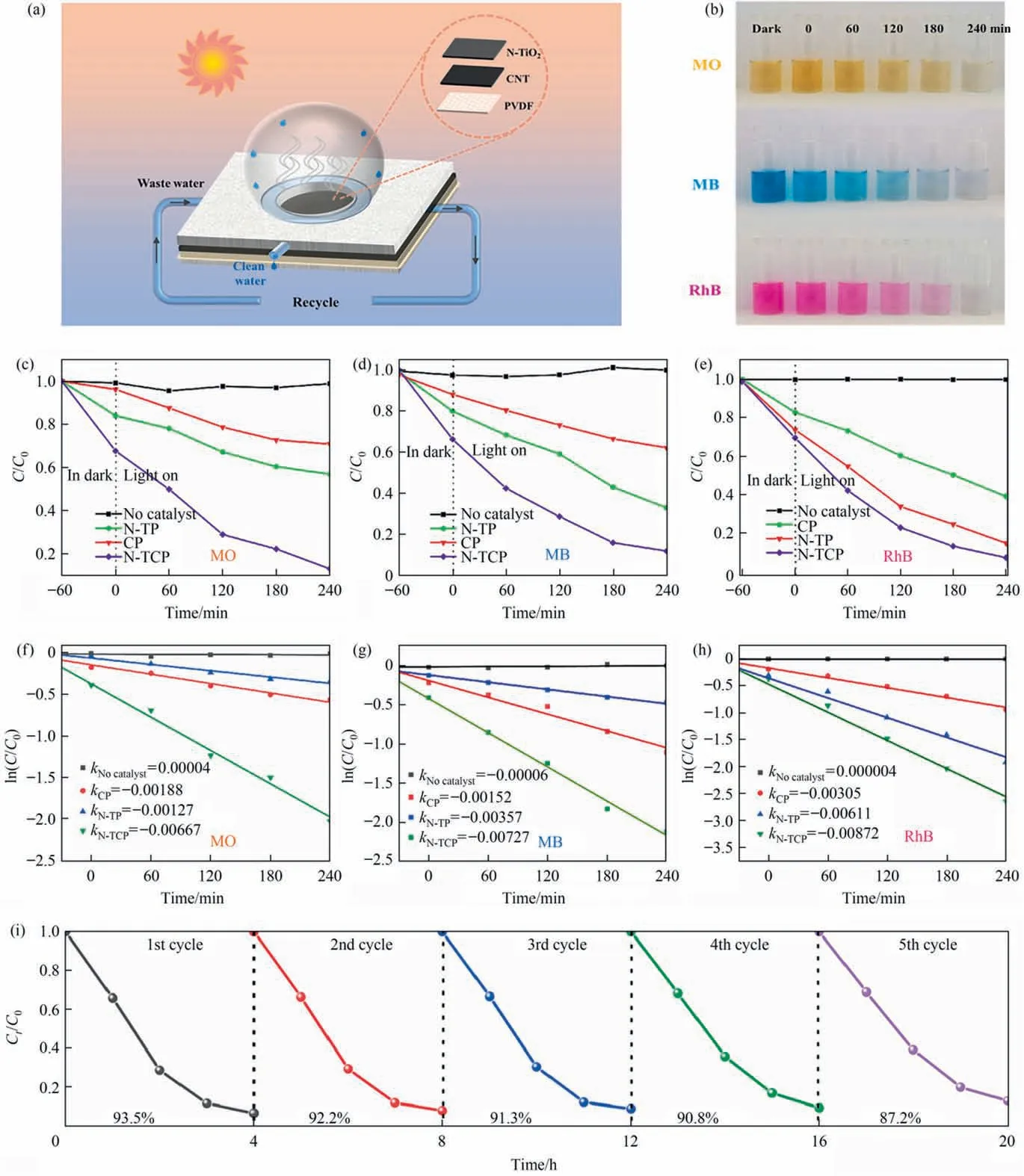
Fig.5. (a)Schematic of photothermal photocatalysis integrated device.(b)Photodegradation of MO,MB,and RhB by N-TCP within 4 h.(c)–(e)Photodegradation efficiency of MO, MB, and RhB (10 mg∙L-1). Kinetic curves of photodegradation of (f) MO, (g) MB and (h) RhB (10 mg∙L-1) by the CP, N-TP and N-TCP catalysts. (i) Cyclic stability of photocatalytic degradation of MB by N-TCP.
Fig.5(b)exhibits the actual degradation effect of the N-TCP film on the three dye solutions.After 4 h the dyes faded,and the effect was obvious. Fig. 5(c)–(e) analyze the photodegradation efficiency of CP,N-TP,and N-TCP films and blank catalysts on the above MB,MO, and RhB from the perspective of UV decolorization. The dye solution that contained the film material was left in the dark for one hour to reach the adsorption–desorption equilibrium of the catalyst dye molecules before illumination. The degradation rates of MO (87%), MB (88%), and RhB (93%) by N-TCP film after 4 h of light exposure were higher than those of CP and N-TP films. The substantial increase in the photocatalytic activity of N-TCP film was attributed to the synergistic effect of good photothermal effect and large specific surface area of CNT with the wide light absorption range of N-TiO2. Table 1 compared the degradation efficiency of N-TCP film with that of photocatalyst reported recently and showed that the photocatalytic performance of N-TCP film was at a higher level. Fig. 5(f)–(h) shows the kinetic curves for the photodegradation of MB, MO, and RhB by CP, N-TP, and N-TCP films.The N-TCP film reflects the largest reaction rate constant, which corresponds to the highest catalytic activity. Practical applications require material stability. After five cycles, the photocatalytic efficiency of N-TCP film for RhB can still reach more than 87% (Fig. 5(i)).The XRD analysis of the N-TCP film after repeated cyclic degradation of dyes (Fig. S10) shows that the characteristic peak does not change significantly, which further prove that the photocatalytic performance of N-TCP film has excellent cyclic stability.
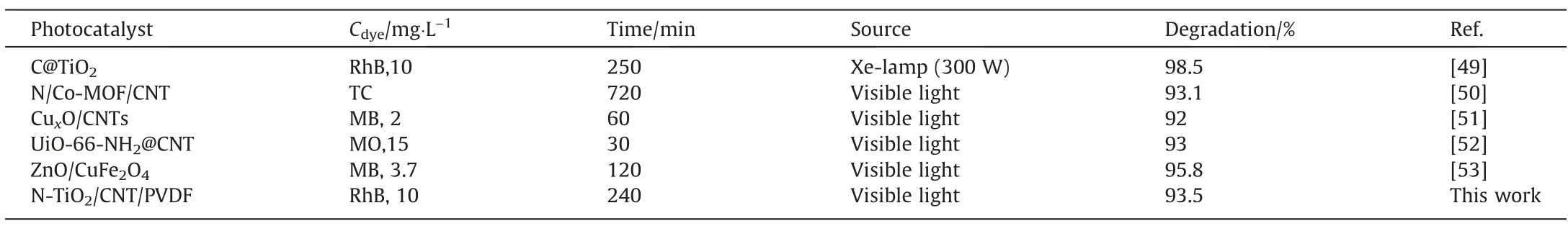
Table 1Comparisons of photocatalytic degradation efficiency between N-TCP and other recently reported photocatalysts
Electron spin resonance (ESR) experiments were performed using DMPO as radical scavengers to detect the presence of ROS generated by the N-TCP ternary membrane during the degradation of dye wastewater.As shown in(Fig.S11),the characteristic peaks of superoxide anion radicalsand hydroxyl radicals (•OH)were observed after the N-TCP film was irradiated under sunlight,but not under dark conditions.The results fully confirmed the existence of•OH,free radicals and their participation in the photocatalytic reaction[54].A photothermal–photocatalytic mechanism that could degrade dyes in visible light using N-TCP composites for freshwater collection was proposed.The hydroxylated carbon nanotube surface possesses a large number of oxygen-containing groups, allowing CNT nanowires and TiO2nanorods to be chemically bonded effectively, which can act as photoexcited electron receivers for titanium dioxide and improve charge separation and mass transfer significantly. Thus, under visible light irradiation,the titanium dioxide nanowires on the surface of the N-TCP film generate electrons and holes.Photogenerated electrons are excited from the valence band (VB) to the conduction band (CB) of TiO2and can move rapidly to the CB of CNT. The strong lightabsorbing CNT and its good electrical conductivity make it easier to transfer photogenerated electrons to the adsorbed oxygen to form superoxide anion radicaland the hole left in the valence band reacts with H2O to form hydroxyl radical (•OH). The strong redox effect of,, and•OH decomposes organic dyes into CO2and H2O and inorganic salts. In addition, the photocatalytic activity of the N-TCP film is significantly increased under visible light irradiation, which may be attributed to the nitrogen compound narrowing the band gap energy of TiO2, thereby resulting in a lower excitation energy and extending the light absorption edge from the UV to the visible region. Thus, this N compound and the strong chemical bonding of CNT with TiO2allow extensive solar energy absorption and effective electron–hole pair separation, thereby greatly improving the photocatalytic activity for TiO2photocatalyst applications.
4. Conclusions
A multifunctional system for the solar photothermal photocatalytic treatment of wastewater while collecting fresh water was designed on the basis of N-TCP ternary composite films prepared by a simple self-assembly method.The synergistic effect of N doping and CNT with superior photothermal effect can rapidly increase the surface temperature of the catalyst under light, promote the separation of photogenerated electron–hole pairs, reduce charge complexation of the photocatalyst,and improve the photocatalytic activity.The results showed that the N-TCP film catalyst exhibited better synergistic photothermal catalysis and photocatalytic degradation of dyes, and its catalytic activity was much higher than that of single N-TP and CP films, and the degradation efficiency of Rh-B reached 93%. Meanwhile, the solar-driven water evaporation using this multifunctional N-TCP film to produce clean water by solar distillation obtained a water evaporation rate of 1.35 kg∙m-2∙h-1and a solar-to-water evaporation efficiency of 94%, providing a new strategy to overcome the energy efficiency limitation of photocatalytic water treatment.In summary,the high catalytic activity, good stability and recyclability, and excellent mechanical properties of the film show great potential in addressing desalination, wastewater treatment, freshwater production,and solar energy utilization.
CRediT Authorship Contribution Statement
Zhongjiao Zha: Conceptualization, Methodology, Investigation,Writing – original draft. Jun Wu: Conceptualization, Methodology,Supervision,Writing–review&editing.Shaoping Tong:Investigation, Validation, Supervision. Xuebo Cao: Supervision, Resources,Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Scientific Research Fund of Zhejiang Provincial Education Department (Y202250501), and SRT Research Project of Jiaxing Nanhu University, which are gratefully acknowledged.
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.021.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
