An internal circulation iron–carbon micro-electrolysis reactor for aniline wastewater treatment: Parameter optimization, degradation pathways and mechanism
Yanhe Han, Han Xu Lei Zhang2, Xuejiao Ma, Yang Man, Zhimin Su Jing Wang
1 Department of Environmental Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617, China
2 SUEZ Environmental Technology (Beijing) Company Limited, Beijing 100026, China
3 Beijing Ecological Environment Assessment and Complaint Center, Beijing 100006, China
Keywords: Aniline Iron–carbon micro-electrolysis Circulating fluidized bed Waste water Degradation
ABSTRACT Aniline is a vital industrial raw material. However, highly–toxic aniline wastewater usually deteriorated effluent quality, posed a threat to human health and ecosystem safety. Therefore, this study reported a novel internal circulation iron–carbon micro-electrolysis (ICE) reactor to treat aniline wastewater. The effects of reaction time, pH, aeration rate and iron–carbon (Fe/C) ratio on the removal rate of aniline and the chemical oxygen demand were investigated using single-factor experiments.This process exhibited high aniline degradation performance of approximately 99.86% under optimal operating conditions(reaction time = 20 min, pH = 3, aeration rate = 0.5 m3∙h-1, and Fe/C = 1:2). Based on the experimental results, the response surface method was applied to optimize the aniline removal rate. The Box–Behnken method was used to obtain the interaction effects of three main factors.The result showed that the reaction time had a dominant effect on the removal rate of aniline. The highest aniline removal rate was obtained at pH of 2, aeration rate of 0.5 m3∙h-1 and reaction time of 30 min. Under optional experimental conditions, the aniline content of effluent was reduced to 3 mg∙L–1 and the removal rate was as high as 98.24%,within the 95%confidence interval(97.84%–99.32%)of the predicted values.The solution was treated and the reaction intermediates were identified by high–performance liquid chromatography,ultraviolet–visible spectroscopy, Fourier–transform infrared spectroscopy, gas chromatography–mass spectrometry, and ion chromatography. The main intermediates were phenol, benzoquinone, and carboxylic acid.These were used to propose the potential mechanism of aniline degradation in the ICE reactor. The results obtained in this study provide optimized conditions for the treatment of industrial wastewater containing aniline and can strengthen the understanding of the degradation mechanism of iron–carbon micro-electrolysis.
1. Introduction
As an important organic chemical intermediate, aniline has been widely used in rubbers [1], dyes [2], pharmaceuticals [3],and pesticides[4].Therefore,industrial wastewater often contains aniline substances. Aniline is a toxic compound that is carcinogenic, teratogenic, and mutagenic [5], with long-term residual characteristics in the environment,which can cause the severe pollution to the aquatic system[6,7].Therefore,aniline has been listed as a priority pollutant by the United States Environmental Protection Agency (US EPA) [8], which is one of the crucial monitoring and governance objects. Continued development of the chemical industry increases the demand for aniline, resulting in increasing amounts of aniline entering the environment and greater levels of environmental pollution. Thus, it is necessary and inevitable to develop an effective treatment method for the removal of aniline.
The treatment methods for organic matter usually include physical (represented by adsorption technology [9–12]), chemical(represented by advanced oxidation process [13–15]), and biological (represented by activated sludge) methods. Aniline, as an intermediate of pesticides [16], is mainly treated using physical,chemical, and biological methods. Physical and chemical methods can effectively remove aniline and reduce its risk,but these methods are generally more expensive to operate and lead to secondary contamination [17–19]. In addition, most conventional biological methods are less effective in treating high concentration aniline wastewater [20]. Currently, the iron–carbon micro-electrolysis(IE)process has been widely used for toxic and refractory wastewater pretreatment due to its low cost,simple operation and no secondary contamination [21–25].
The IE is the mixing of iron filings and activated carbon,and the active iron and inactive carbon can form a primary battery to achieve the purpose of treating wastewater. The degradation mechanism of the IE process is as follows[26].Under acidic conditions, the elemental iron is corroded to produce Fe2+and active reduced hydrogen (reaction (1) and reaction (3)). Under aeration conditions, oxygen acts as an electron acceptor to form H2O2,which can cause a Fenton reaction with Fe2+(reaction (4)) [27].Therefore, macromolecular organics were effectively decomposed into low-molecular-weight compounds, resulting in an improvement in the biodegradability of wastewater. In addition, Fe2+is easily oxidized to form Fe3+,eventually forming a hydroxide colloid(reaction (2)) that exhibits excellent flocculation performance.
Long-term experiments and industrial applications have revealed that the surface of the iron electrode is easily oxidized to form oxide film, which facilitates passivation of the surface of the filler and results in hardening of the bed and poor treatment[28]. Therefore, the industrial applications of the IE process are greatly limited. To overcome these shortcomings, Yang [29]replaced the fixed bed with a bubbling bed;however,aeration only delayed the hardening and passivation time. Some scholars have added mechanical stirring devices in the IE reactor to solve the problem of passivation of the iron–carbon filler plate junction[30]. However, although good results can be achieved, the cost of equipment is increased [31].
In order to solve the above problems, an internal circulation iron–carbon micro-electrolysis (ICE) reactor was developed by combining IE technology with an airlift internal circulation reactor[28].The working principle of the reactor is to concentrate aeration in the riser of the reactor, which induces a pressure difference between the inside and outside of the riser, causing the iron–carbon filler to move upward in the riser.The surface of the iron–carbon filler is cleaned by the rubbing action between the filler and the bubbles and within the filler itself.The cleaned iron–carbon fillers then enter the outer space of the riser and move downward.As a result,the fillers are always in motion,thus solving the hardening and passivation problems[32].In addition,due to the properties of the ICE reactor structure, oxidation and reduction can be achieved simultaneously [33]. These advantages have been confirmed by experimental and hydrodynamic numerical simulations [26].Although the reaction mechanism of micro-electrolysis technology is relatively clear, the degradation process of organic matter (e.g.aniline) in the ICE reactor remains unclear [34].
In this study, the IE was combined with airlift inner circulation reactor for the first time. The ICE reactor was developed, which could solve the hardening and passivation of the iron–carbon filler.The effects of reaction time, initial pH, aeration rate, and the ironcarbon(Fe/C)ratio on the degradation of aniline were investigated for wastewater treatment using an ICE reactor.The major intermediate products of aniline degradation were analyzed using ultraviolet–visible spectrophotometry, high-performance liquid chromatography, ion chromatography, Fourier–transform infrared spectroscopy, and gas chromatography–mass spectrometry.Finally,the pathways of aniline degradation in the ICE reactor were proposed.
2. Materials and Methods
2.1. Reagents and materials
Aniline (analytical grade) and other chemicals (e.g., HCl and NaOH) were purchased from Tianjin Chemical Industry Co. Ltd.(China). Sodium carbonate and sodium bicarbonate were obtained from Macklin Biochemical Co. Ltd. (China). Methanol (chromatographic grade) was obtained from ANPEL Laboratory Technologies(China). Iron scraps were obtained from the metalworking facilities of our institution. Commercial–grade granular activated carbon(GAC,Hebei Pengcheng Activated Carbon Co.,Ltd,China)with a particle size of approximately 2 mm was used in this study. Activated carbon was easy to form a galvanic cell with iron and then was selected as one of the fillers.In addition,the strong adsorption characteristics of activated carbon could effectively adsorb pollutants,resulting in the enhancement of the degradation effect of IE. Iron scurf with a mean diameter of approximately 1 mm was obtained from waste cast iron materials in a school metal processing workshop.The sulfuric acid and sodium hydroxide used in this study were of analytical grade. The cleaned GAC was submerged in a nitrobenzene solution until saturation was reached to remove the influence of absorption during the micro-electrolysis degradation process.
2.2. Material pretreatment
Iron scurf and activated carbon pretreatment was conducted in accordance with the method described in our previous study [23].First,iron scurf was screened with a sieve to a selected particle size of approximately 1 mm.Then,impurities and oil contamination on the surface of the iron scurf were removed with tap water, 5%sodium hydroxide solution for 12 h, and 3% sulfuric acid solution for 30 min in sequence. Finally, the iron scurf was cleaned with tap water prior to use.The GAC was washed with deionized water four to five times to remove ash and impurities,then soaked in the aniline wastewater for more than three days to eliminate adsorption during the ICE process.
2.3. Apparatus and procedure
The ICE reactor consists of three parts: an aeration system, a water intake system, and the reactor main body. According to the flow direction of the Fe/C filler,the reactor can be divided into two zones:a riser zone and a down–comer zone,as shown in Fig.1.The pretreated iron scurf and GAC were mixed in a set proportion and 460 ml of mixed filler was packed into the ICE reactor.460 mL of aniline wastewater,with the pH adjusted using 10%H2SO4solution, was pumped into the ICE reactor. Aniline was degraded at 20 °C for a set duration. The pH of the effluent was adjusted to 8–9 using 10% NaOH solution. The supernatant after precipitation was subsequently used for water quality analysis.
2.4. Analytical methods
The concentration of aniline solution was determined by high–performance liquid chromatography (HPLC, Waters 1525, Waters Corporation, USA) with a C18 reverse phase column (4.6 mm× 150 mm, 5 μm). The mobile phase was a mixture of methanol and ultrapure water(4:6)at a flow rate of 1 ml∙min-1.A detection wavelength of 280 nm was set for the detection of aniline.Ammonia nitrogen was determined using standard Nessler’s reagent spectrophotometry. The combination of solid-phase extraction and gas chromatography–mass spectrometry (GC–MS) has attracted great interest among researchers [35]. Therefore, the degradation intermediates of aniline were identified by GC–MS using GC Model (7890A) and NS Model (5975C) in this study. The GC–MS program was as follows: the temperature was raised from room temperature to 100 °C at 20 °C∙min-1, then to 280 °C at 10°C∙min-1and held for approximately 3 min.The time of solvent delay was set to 3 min and the total run time was 60 min.The mass scanning range was set from 20–700 m∙z–1[36].
2.5. Response surface experiment design
According to the results of the single-factor test, the aeration amount, initial pH value, and reaction time were selected as the three main factors influencing process optimization.With the aniline removal rate as a response variable, the Box–Behnken design of the response surface method was applied to optimize these parameters.Table 1 shows the Box–Behnken design of the experimental parameters.

Table 1Box–Behnken design factors and levels for response surface methodology
3. Results and Discussion
3.1. Key factors affecting aniline degradation by ICE
Fig. 2 shows the effect of operating conditions on aniline removal efficiency during the ICE process. According to Fig. 2(a),the concentration of aniline decreased significantly in the first 20 min, from 168 mg∙L–1to 3 mg∙L–1, with a sharp increase in the aniline removal rate. As the reaction time increased from 20–120 min, the concentration of aniline decreased slightly reduced and the removal rate increased slightly.This result could be attributed to the fact that,although the reaction site of IE did not change as the reaction proceeds,a decrease in the concentration of aniline caused the reaction rate to decrease and the removal rate to slow increase. After 120 min of reaction, the removal efficiency of aniline reached 99.8%. Considering the operating costs, the reaction time of subsequent experiments was set to 20 min.
As shown in Fig. 2(b), the ICE process achieved the highest removal rate of aniline at pH 3. When the pH was less than 3,the removal rate of aniline increased as pH increased, which contrasted with the IE degradation process of phenolic substances[37]. This result may be because aniline forms a cation at lower pH, which was detrimental to the adsorption and degradation of aniline on the surface of activated carbon.When the pH was higher than 3, the removal rate of aniline decreased as pH increased because the redox potential of the iron–carbon IE reaction under acidic conditions was higher than that under alkaline conditions[26],which was beneficial for the degradation of aniline.Therefore,aniline was subsequently treated at pH 3.
In the ICE process, aeration not only increased the dissolved oxygen in solution[30], but also eliminated the hardness and surface passivation of the Fe/C filler[28].An increase of dissolved oxygen is beneficial to the degradation of organic matter by IE with a Fe/C filler [38]. Therefore, the effect of aeration rate on aniline degradation was investigated, and the results are shown in Fig. 2(c). When the aeration rate was increased from 0.1 to 0.5 m3∙h-1,the removal rate of aniline increased from 97.7%–98.5%. When the aeration rate was increased further,the removal rate of aniline decreased and the rate of decrease increased. This was mainly a result of increased dissolved oxygen in the solution promoting the degradation of aniline. However, when the aeration rate was increased to a certain extent, iron and carbon were easily separated,thereby causing a decrease in the number of primary batteries, which was detrimental to the degradation of aniline [38].
The effects of the Fe/C ratio (1:3, 1:2, 1:1, 2:1, 3:1) on the removal efficiencies of aniline compounds were analyzed with fixed total dosages of iron scrap and GAC of 460 mL. In order to eliminate the influence of adsorption, GAC was immersed in 200 mg∙L–1of aniline solution for three days.The results are shown in Fig. 2(d). The concentration of aniline first decreased then increased,whereas the removal efficiency of aniline first increased then decreased. When the Fe/C ratio was 1:2, the aniline concentration decreased to 0.26 mg∙L–1and the removal efficiency reached 99.86%. This was because the particle size of GAC is twice that of iron scurf, and the maximum numbers of microscopic galvanic cells can be achieved at a Fe/C ratio of 1:2. In addition, Zhuet al. [39] indicated that effective contact between iron scrap and GAC can promote the formation of microscopic galvanic cells. In theory,a similar total contact area of iron scrap and GAC was beneficial for forming more microscopic galvanic cells in the Fe-C micro-electrolysis system [40]. Therefore, the optimum Fe/C ratio for the reaction was determined as 1:2.
3.2. Optimization of reaction conditions using response surface methodology
The results of the single-factor experiments showed that the aeration rate,initial pH,and reaction time had an important influence on aniline removal efficiency. However, single-factor experiments had some limitations because the possible interactions between parameters were not considered [41]. Therefore, multivariate statistical techniques,such as the response surface method,were developed for this purpose. The Box–Behnken method is a commonly used design for the response surface method,the experimental design and result of which are shown in Table 2.Multiple regression analysis of the resulting response yielded the following second-order polynomial equation, which explained the relationship and interaction of the response with the important factors:
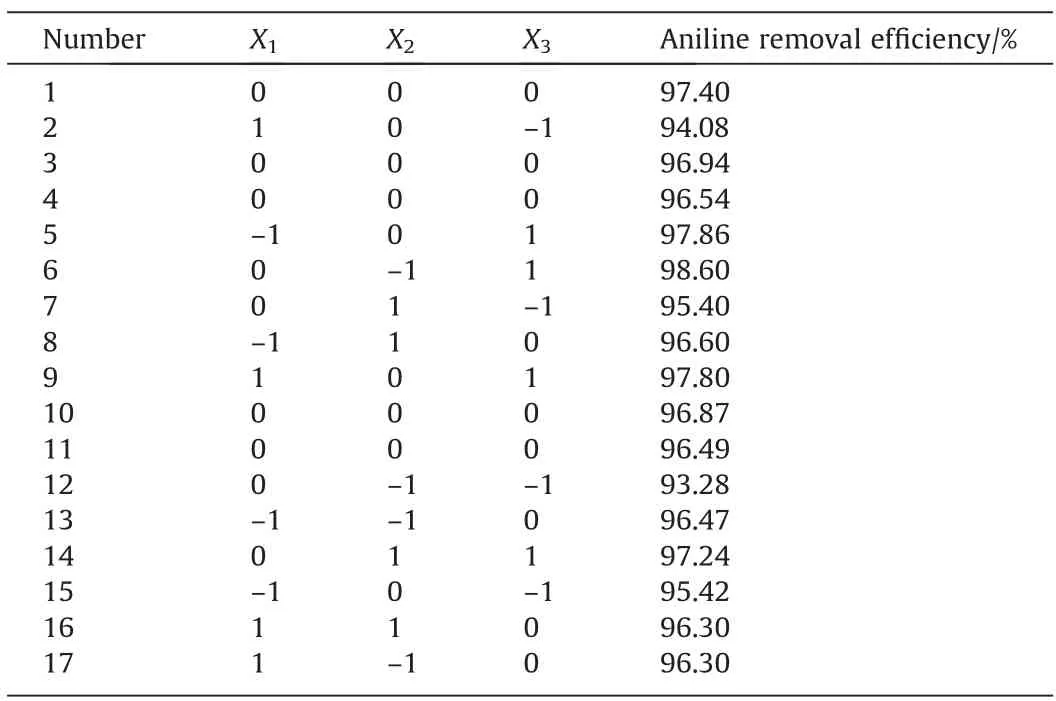
Table 2Design and results of experiments using the Box–Behnken design
whereYis the aniline removal efficiency andX1,X2, andX3represent the aeration rate, initial pH, and reaction time, respectively.The analysis of variances(ANOVA)was used to check the adequacy of the employed model.Table 3 shows the ANOVA of the regression model. TheP–value of the model was 0.0001, which indicated that the model was significant [42]. TheP–value of the lack of fit was 0.598, indicating that the regression was meaningful. The goodness-of-fit for the model was also evaluated using the adjusted regression coefficient obtained in this study,which indicated better correlation between the observed and predicted values. Hence, the response surface model proposed in this study was considered to have satisfactory representation.
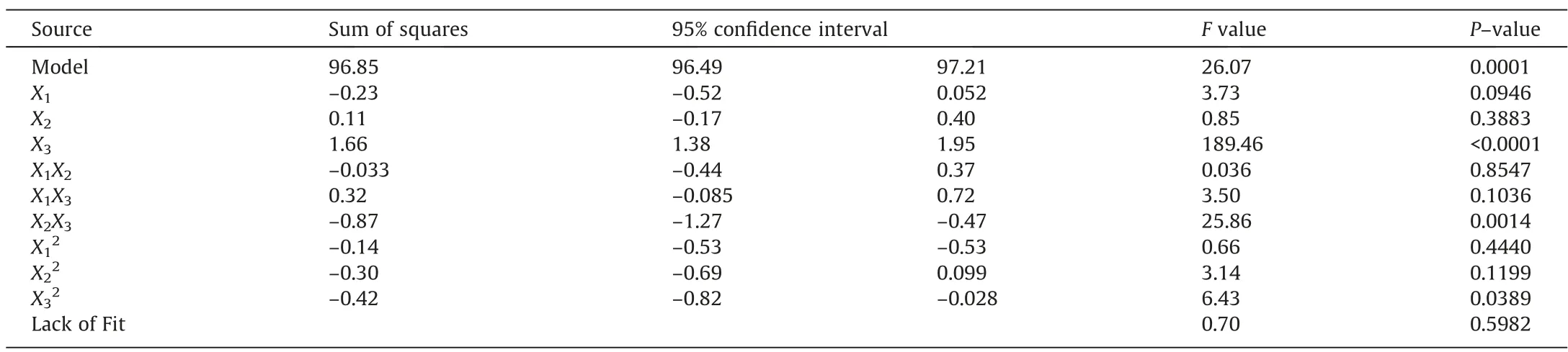
Table 3ANOVA results for the response surface quadratic model
The response surface and contour plots are shown in Fig.3.The effect of interaction between the aeration rate and pH on the aniline removal efficiency is illustrated in Fig.3(a).The contours wereparallel to the two axes, suggesting no significant interaction between the two parameters.Moreover,theP–value of the interaction between aeration rate and pH(X1X2)was 0.85,i.e.,greater than 0.05, also indicating no significant interaction between aeration rate and pH. Fig. 3(b) shows a similar trend between aeration rate and reaction time, indicating that the two parameters were relatively independent of each other.According to Fig.3(c),the aniline removal efficiency increased as the pH value increased within a short reaction time. However, when the reaction time was long,the aniline removal efficiency decreased as pH increased. This result indicated that there was a significant interaction between pH and reaction time, which was confirmed by theP–value(0.0014) of the interaction between pH and reaction time (X2X3),which was less than 0.05.
The optimum conditions obtained by the model are as follows:reaction time = 30 min, pH = 2, aeration rate = 0.588 m3∙h-1, and Fe—C ratio=1:2.Under optional experimental conditions,the aniline content of effluent was reduced to 3 mg∙L–1, and COD concentration was reduced to 25 mg∙L–1. To verify the reliability of the model, three repeated experiments were performed under these optimized conditions, which yielded an average aniline removal efficiency of 98.24%,i.e., within the 95% confidence interval(97.84%–99.32%) of the predicted values. Therefore, the model obtained by the Box–Behnken method was deemed effective for predicting the degradation efficiency of aniline by the ICE technique.
3.3. Comparison of aniline removal processes
In order to further investigate the effectiveness of ICE technology in removing aniline from wastewater, Table 4 compares various alternative treatments including physical, chemical,biological and coupling technologies, with regards to aniline removal rates, treatment mechanisms and characteristics.
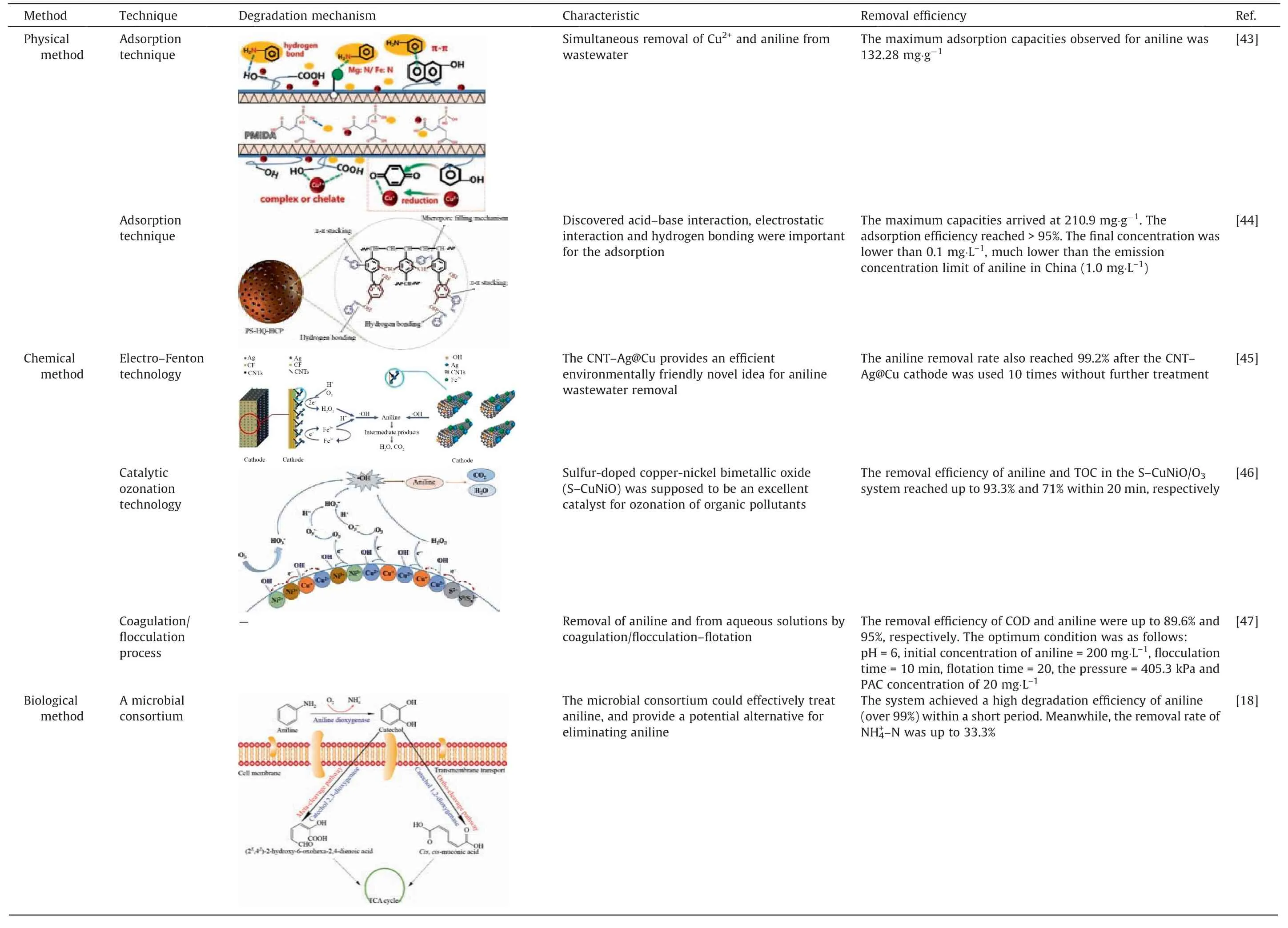
Table 4Comparison of aniline degradation performance with various aniline wastewater treatment methods
The physical method mainly involves adsorption technology,which uses porous solids(adsorbents)to adsorb aniline from sewage to achieve wastewater purification [43,44]. Although simple,this method is costly and unsuitable for treating high concentration organic wastewater, limiting its widespread applicability.Chemical methods mainly constitute of advanced oxidation processes and coagulating sedimentation. The advanced oxidation processes involve utilizing highly oxidizing free radicals to degrade aniline [45,46]. Advanced oxidation technologies often require strong oxidants and the use of catalysts. Although their reaction speed is fast and mineralization effect is good,these methods incur high treatment costs and often leave a residual amount of strong oxidants that might endanger the ecology or human health.Chemical coagulation precipitation is the formation of flocs through the addition of coagulants, which removes organic matter under the adsorption and net capture of the flocs [47]. It has the advantages of fast reaction speed and good processing effect,but the operation is complex and the cost is high. Biological methods rely on activated sludge that utilizes the metabolism of microorganisms to degrade aniline [5,18,48]. While cost-effective, the toxicity of aniline to microorganisms makes this method unsuitable for treating high concentration aniline wastewater. The combined process has been applied to the treatment of aniline wastewater, combining the advantages of each of the previous processes[49–51].Although highly effective in treatment, the combined process is relatively complex and requires substantial investment costs.
In comparison, IE technology has been shown to be effective in treating aniline wastewater with a low cost of waste treatment.Although requiring pH adjustment,it boasts many advantages such as high removal efficiency,long-lasting operation without the issue of hardening or passivation,and simple and safe operation.Therefore, compared to other processes, ICE technology is considered to be highly advantageous for treating aniline wastewater.
3.4. Reaction mechanism and possible pathways of the aniline degradation in the ICE system
Aromatic amines exhibit strong biological toxicity in the human body. Aniline, the simplest aromatic amine, is a typical methemoglobin inducer, which prevents red blood cells from carrying oxygen into tissues [37]. The amino group on the aniline ring is the main source of aniline toxicity; thus, the deamination process is an important method to reduce the toxicity of aniline [38].
Therefore, the degradation mechanism of aniline was analyzed by detecting the ammonia nitrogen content in water.According to Fig. 4, as the reaction proceeded, the aniline concentration decreased gradually,whereas the ammonia nitrogen concentration in water increased sharply in the first 30 s,where it reached a maximum of 3.66 mg∙L–1before decreasing gradually. This result indicated that ammonia nitrogen accumulated during the degradation of aniline. The decrease in ammonia nitrogen concentration with increasing reaction time could be due to two reasons. First, NH3+; therefore, as the OH–concentration increased during degradation by ICE,the ammonium ions in the water would be converted into ammonia molecules. These ammonia molecules volatilized from the water,reducing the concentration of ammonia nitrogen. Second, theremoved from the aniline benzene ring could become further oxidized toor N2[39].
Fig.5 shows the typical UV spectrum of aniline solution at reaction times of 2, 5, 10, 30, 60, 90, and 120 min. Aniline is a heterocyclic compound with a benzene ring and—NH2.The characteristic absorption band of the benzene ring was observed at 200 nm and 230 nm, and the absorption band related to amino content was observed at 280 nm. As shown in Fig. 5, the absorption intensity decreased significantly with increased processing time, and the peak intensity decreased significantly at 230 nm and 280 nm, but only slightly at 200 nm. These results showed that the ring structure of aniline was not completely destroyed during ICE treatment,but the amino structure of aniline was completely destroyed [40];thus,aniline was degraded.In this study,230 nm was chosen as the feature absorption wavelength of aniline by UV spectrum scanning.At this wavelength, the effects of different reaction times were determined by HPLC (Fig. 6).
The relationship between aniline concentration and peak area can be obtained from the standard curve in Section 3.2. In Fig. 6,in addition to the aniline peak (retention time,t= 3.8 min), a new peak appeared within 2.9 min, which may correspond to hydroquinone generated during aniline degradation. In order to confirm this hypothesis, the standard samples of hydroquinone and aniline were monitored by HPLC, and the results showed that the peak time was indeed the same as that of hydroquinone.Therefore, the peak at 2.9 min should be the characteristic peak of hydroquinone.
Fig. 7 presents the infrared spectrum of iron mud under the conditions of deionized water and aniline treatment for 2 min and 120 min. The absorption peak centered at 3400 cm-1corresponds to the O—H stretching of hydroxyl and the stretching vibration of alcohols, phenols and organic acids, such as N—H [41]. The absorption peaks observed at 1400 cm-1, 1500 cm-1, and 1650 cm-1were attributed to the stretching vibration of C—C in the benzene ring, whereas the absorption peak at 1100 cm-1was the stretching vibration of C—H. At 120 min, the absorption peak at 3200 cm-1was attributed to the tensile vibration of—OH in carboxylic acid [42]. These results revealed that the degradation of aniline produced carboxylic acid that accumulated over time.
Characteristic peaks related to the symmetric stretching of nitro—NO2were observed at 1400 cm-1and 1500 cm-1.At 120 min,the absorption peak of the nitro group increased, but the absorption peak of aniline decreased at 3450 cm-1,which indicated that nitrification of the amino group occurred on aniline. This confirmed that the nitrate observed by ion chromatography was formed by ammonia nitrogen oxidation and amino nitrification; therefore,the aromatic nitro compounds were another type of major intermediate product of the treatment process.During the ICE reaction,a new absorption peak appeared at 1250 cm-1in the infrared spectrum of iron mud after 120 min of treatment,which was related to the stretching vibration of C—O bond in carboxylic acid, further confirming that the degradation of aniline by the ICE reaction produced carboxylic acid.
During the aniline degradation process, phenol, benzoquinone,carboxylic acid, and other reaction intermediates were typically observed. Therefore, in order to explore the degradation of aniline by ICE, the intermediate aromatic compounds were analyzed by GC–MS and IC. Aniline, phenol, and benzoquinone were identified by GC–MS, whereas ions and organic small molecular acids were quantitatively detected by IC. Fig. 8 is an ion chromatogram of a mixed standard sample of small molecular acid,whose information is summarized in Table 5. The large response values of chlorideions easily masked the presence of other ions; therefore, the chloride ion was shielded in this study, resulting in the ion chromatogram shown in Fig. 9(a). Fig. 9(b)–(d) illustrates partially enlarged views of nitrate,formate,and oxalate.Clearly,oxalic acid,acetic acid, formic acid, and nitrate were produced during the degradation of aniline. Because there was no sulfate in the simulated wastewater, the discarded iron chips derived from factories may have contained some sulfate. Although the introduction of sulfate was a coincidence,it is still clear that the micro–electrolysis treatment achieved good sulfate ion removal efficiency, with the sulfate removal efficiency reaching 80%, which indicated the potential of micro-electrolysis for sulfate removal [52].
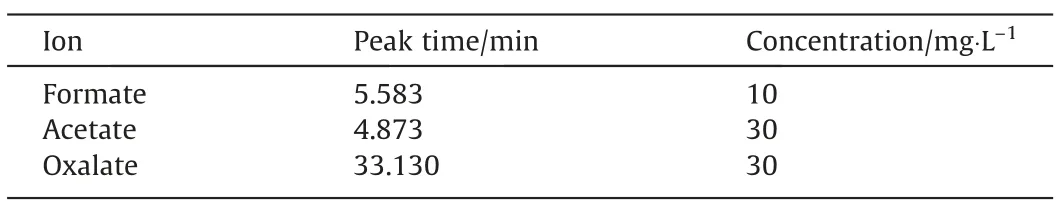
Table 5Standard solution of acid radicals
The results showed that the concentration of formate increased gradually with increasing reaction time, whereas the concentration of oxalate and acetate first increased then decreased. Aniline was degraded to low-molecular-weight carboxylic acid and finally mineralized by the ICE method. Nitrate was also detected during the degradation process. With increasing reaction time, the nitrate concentration first increased then decreased, indicating that the production of nitrate was synchronous with the deamination process. Typically, nitrite was difficult to detect after aeration because it was easily oxidized;therefore,it is suggested that ammonia nitrogen produced nitrite under oxidant action and then continuously oxidized to nitrate.Based on the above analysis, the hydroxyl radicals produced by Fe/C ICE play an important role in the degradation of aniline.The GC–MS spectrum in Fig. 10 shows the substituents of benzene rings and small hydrocarbons, which mainly included aniline, phenol, benzoquinone, and carboxylic acid.
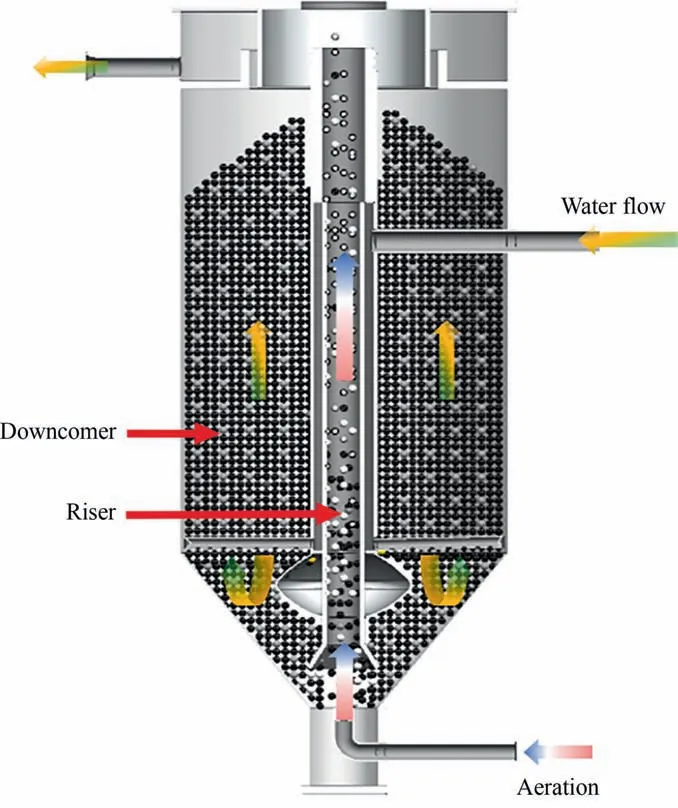
Fig. 1. The structure of the ICE reactor.
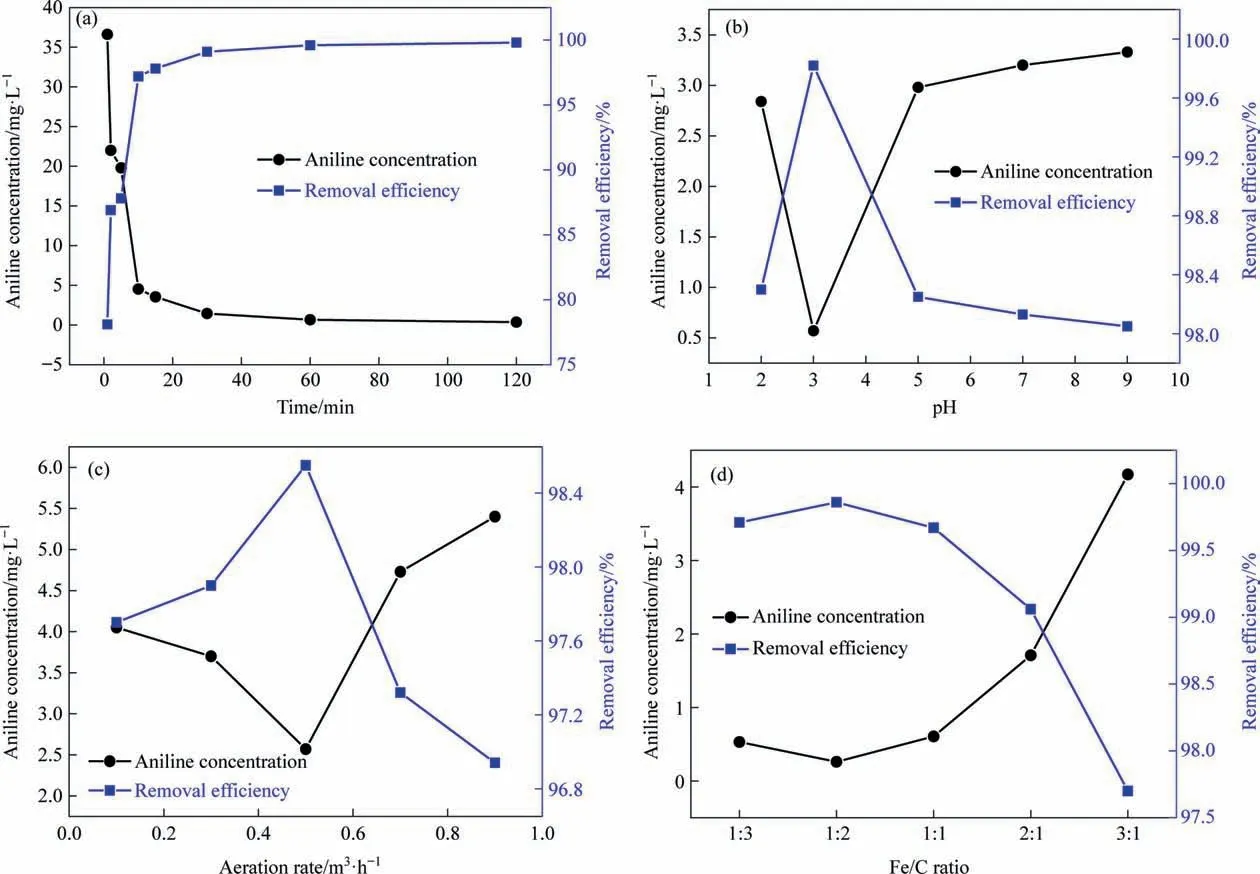
Fig. 2. Effect of operating conditions on aniline removal efficiency during the ICE process: (a) reaction time, (b) pH, (c) aeration rate, (d) Fe/C ratio.
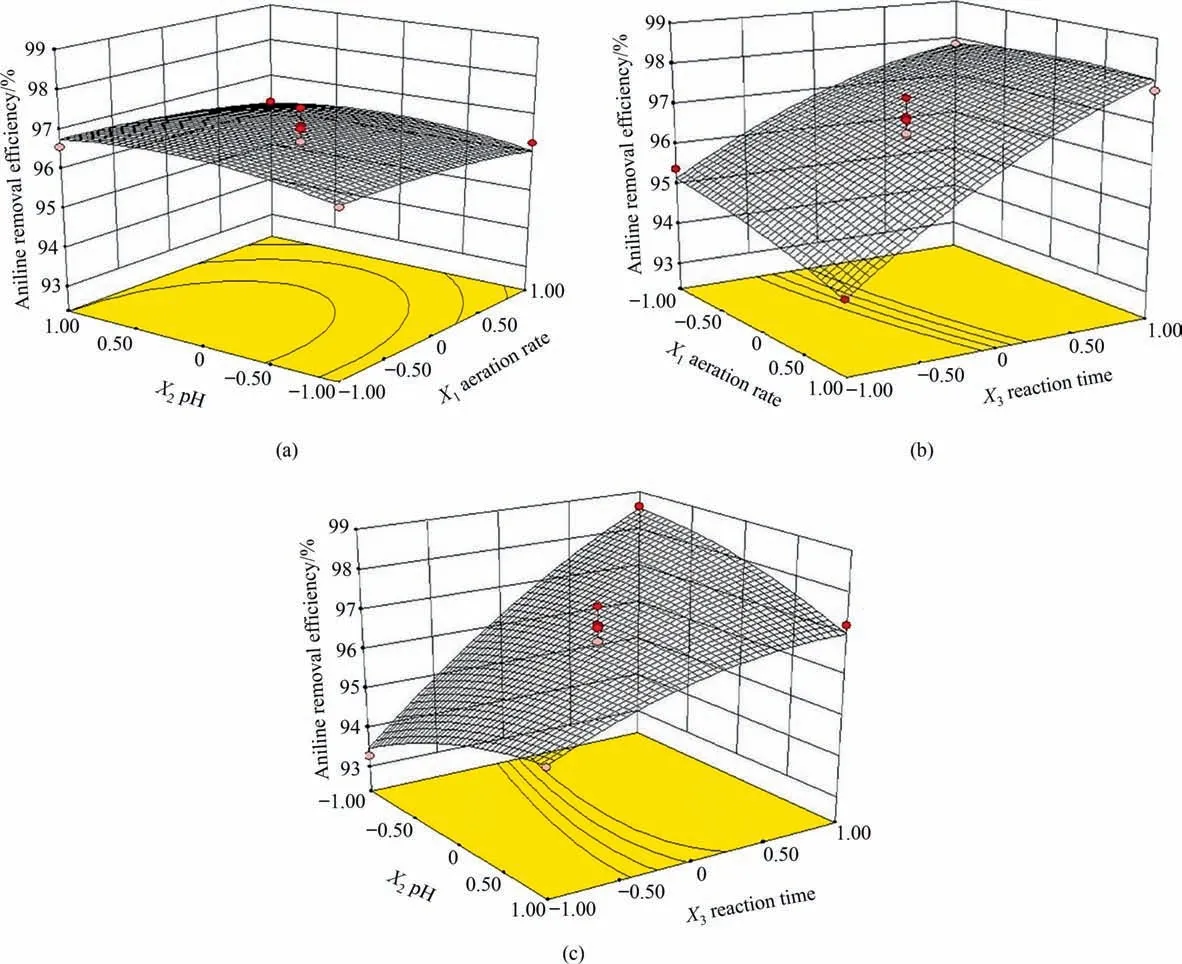
Fig. 3. Contour and three-dimensional surface plots of aniline removal efficiency showing the interaction effect between (a) aeration rate and pH, (b) aeration rate and reaction time, and (c) pH and reaction time.
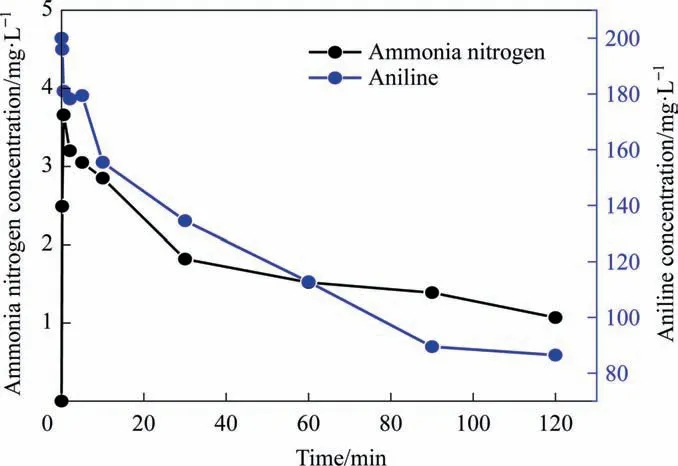
Fig. 4. Effect of reaction time on aniline and ammonia nitrogen removal.
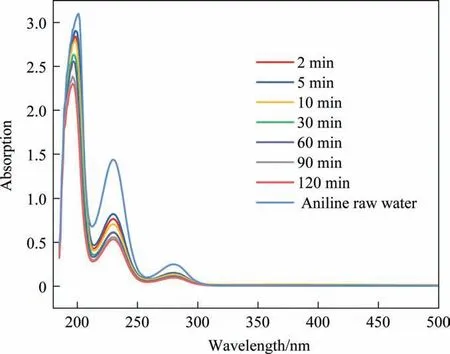
Fig.5. UV scanning spectra of simulated aniline wastewater at different treatment time.
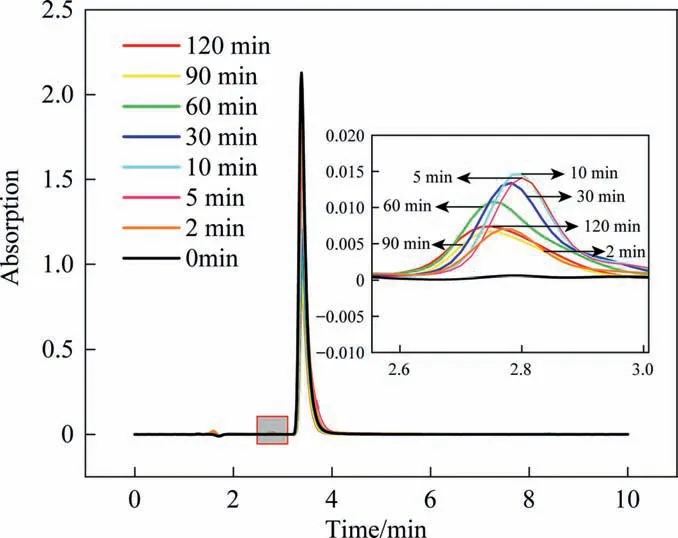
Fig. 6. Chromatographs of simulated aniline wastewater at different treatment time.
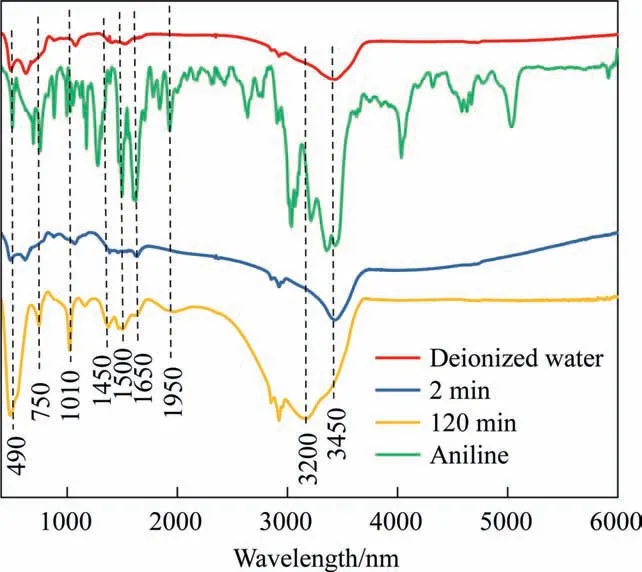
Fig. 7. FTIR spectra of simulated aniline wastewater at different treatment time.
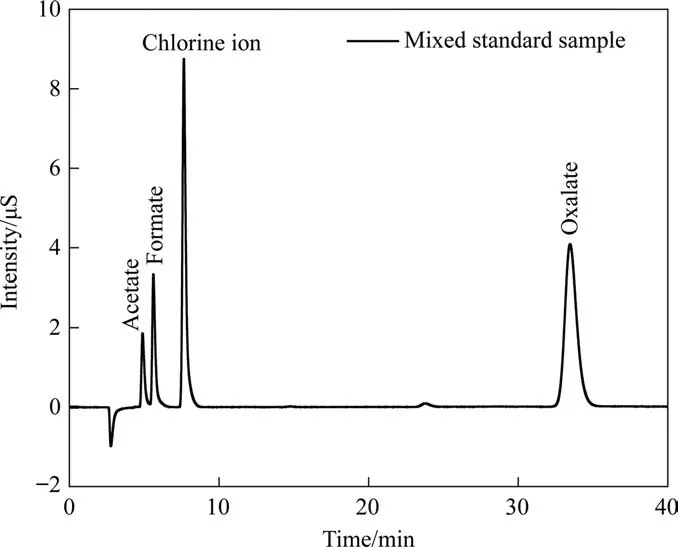
Fig. 8. Mixed standard ion chromatography results of small molecular acids.
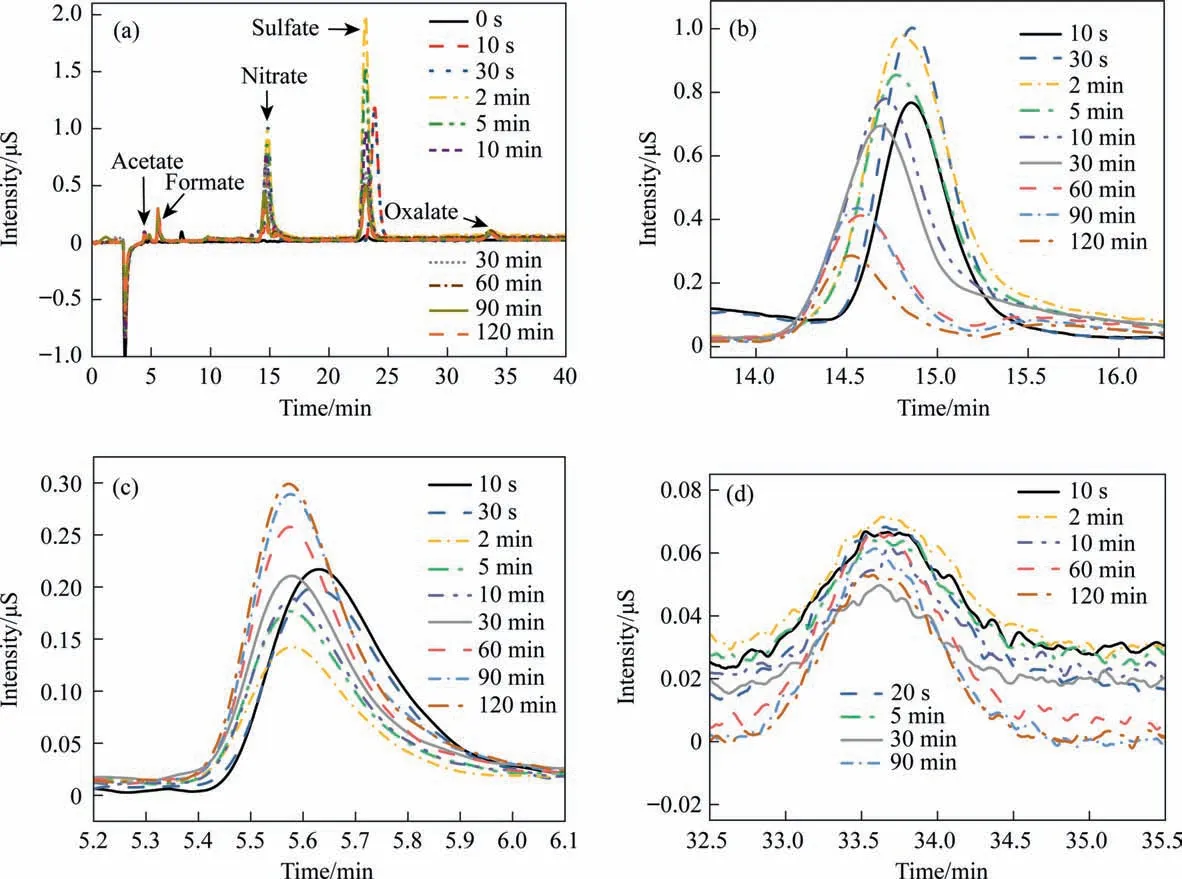
Fig. 9. Ion chromatography results at different treatment time (a). Local magnifications of nitrate ions (b), formate ions (c), and oxalate ions (d) from (a), respectively.
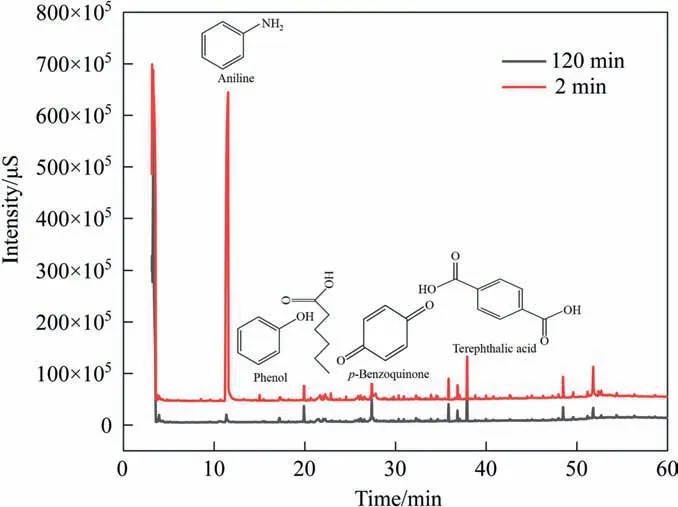
Fig. 10. GC–MS spectra of simulated aniline wastewater at different treatment time.
During the degradation of aniline by ICE,molecular hydrocarbons were produced. Considering that most of the aromatic organic compounds belong to refractory organic compounds,the amino group of aniline molecules had a strong activation effect. The hydroxyl radicals produced during the ICE reaction first undergo electrophilic substitution,then benzene ring opening oxidation. At the same time, a large number of small abundance absorption peaks were found at the end of the spectrum,corresponding to the products formed by benzene ring opening oxidation. The results showed that ICE treatment effectively destroyed the structure of the benzene ring, which was conducive to subsequent biological treatment of aniline wastewater.
Therefore,based on the results of the different analytical methods, the potential degradation pathway of aniline is illustrated in Fig. 11. Micro-electrolysis could achieve aniline degradationviatwo processes.1)Under acidic conditions,amino–bound hydrogen ions formed—NH3+then deaminated under the oxidation of hydroxyl radicals to produce phenol,further formingp–phenol;this process was very rapid. Phenol induced hydroxyl radicals to attack benzene rings and dehydrogenated them to formp–benzoquinone.However, benzoquinone was very unstable. When hydroxyl radicals or dissolved oxygen existed in the system, they could easily be opened to form small molecular acids, such as butene diacid and adipic acid, eventually forming CO2and H2O. 2) The presence of a nitro group was detected by ion chromatography and infrared spectroscopy.Therefore,during degradation,aniline might directly form nitrobenzene [53] under the action of the hydroxyl group,then further oxidize to phenol before participating in reaction (1).
4. Conclusions
In this study, a novel iron–carbon internal circulation microelectrolysis reactor was successfully established and achieved almost complete degradation of aniline. The effects and optimization of reaction time,initial pH,aeration rate and Fe/C ratio on the removal rate of aniline were investigated using single-factor experiments.The removal rate of aniline could be reduced to 0.26 mg∙L–1under the optimal conditions: a reaction time of 20 min, an initial pH of 3,an aeration rate of 0.5 m3∙h-1,and an Fe/C ratio of 1:2.An aniline removal rate of 98.24%was also obtained after the simulation of Box–Behnken method at the conditions: pH of 2, aeration rate of 0.5 m3∙h-1and reaction time of 30 min.
The probable degradation pathway of aniline was then proposed based on UV spectrophotometry,HPLC,ion chromatography,FTIR,and GC–MS analyses of the treated wastewater.Ammoniation occurred first,with a substantial amount of ammonia nitrogen produced during the first 30 s of the reaction. The maximum absorption wavelength of aniline determined by ultraviolet detection was 230 nm, and the amination process of the reaction was verified.Several intermediates were observed through HPLC analysis. Ion chromatography analysis indicated that the degradation products of aniline would eventually be converted into small molecular acids, including oxalic acid, acetic acid, and formic acid. The presence of nitrate was also detected, which proved that nitrogen was the product of aniline degradation.
In addition,FTIR analysis indicated that some intermediates and by-products in the wastewater were degraded by the ICE progress,and others in the iron-containing sludge (including aromatic hydrocarbons, carboxylic acids, ketones, nitro groups, and esters)were degraded by flocculation and co-precipitation.
The intermediates and degradation pathways of aniline degradation were determined by GC–MS.In pathway 1,ammonia nitrogen was first generated from aniline due to deamination. Then,phenol andp–phenol were formed through the oxidation of hydroxyl radicals and other oxidants. Phenolic hydroxyl groups in the benzene ring were easily oxidized into quinones and benzoquinone, which further degraded into carbon dioxide and water.In pathway 2, hydroxyl radical reacted directly with ammonium radicals to form nitroso, which was then oxidized to nitro groups.The nitro group on the benzene ring was then oxidized to phenolic hydroxyl, which was finally degraded to small molecule acids.Finally,a mechanism was proposed for ICE degradation and mineralization of aniline pollution in wastewater.
The IE technology has many advantages,such as environmental friendliness, low operating cost, convenient operation and management, wide application, and improved biodegradability of wastewater.Therefore,it has a broad prospect of application.However, IE technology encounters technical application problems,such as hardening and pH adjustment. The ICE technology used in this article can efficiently solve the hardening problem. Still, it necessitates adjusting the pH value, which may be an inherent defect that cannot be resolved for IE technology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China(21677018),the Joint Fund of the Beijing Municipal Natural Science Foundation and Beijing Municipal Education Commission (KZ201810017024) and the Cross–Disciplinary Science Foundation from Beijing Institute of Petrochemical Technology(BIPTCSF–22032205003/014).
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
