Effect of modified MgAl-LDH coating on corrosion resistance and friction properties of aluminum alloy
Zuokai Wang,Zhuangzhuang Xiong,Xinxin Li,Di Wang,Yuelin Wang,Shangcheng Wu,Lixia Ying,Zhideng Wang,, Guixiang Wang,
1 College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China
2 College of Mechanical and Electrical Engineering, Harbin Engineering University, Harbin 150001, China
3 College of Materials Science and Chemical Engineering, Yantai Research Institute of Harbin Engineering University, Yantai 265503, China
Keywords: Anodizing Layered double hydroxide Superhydrophobic Corrosion resistance Tribological properties
ABSTRACT The in-situ growing approach was utilized in this article to construct the magnesium–aluminum layered double hydroxide(MgAl-LDH)film on the surface of a 1060 aluminum anodized film.To improve the corrosion resistance and friction qualities of aluminum alloy,the MgAl-LDH coating was treated using stearic acid (SA) and thiourea (TU). The aluminum substrate and anodized aluminum film layer corroded to varying degrees after 24 h of immersion in 3.5% (mass) NaCl solution, while the modified hydrotalcite film layer continued to exhibit the same microscopic morphology even after being immersed for 7 d.The results show that the synergistic action of thiourea and stearic acid can effectively improve the corrosion resistance of the MgAl-LDH substrate. The tribological testing reveals that the hydrotalcite film layer and the modified film layer lowered the friction coefficient of the anodized aluminum surface substantially. The results of the simulations and experiments demonstrate that SA forms the dense LDH-TU interlayer film layer by exchanging ions between TU layers on the one hand and the LDH-SA film layer by adsorption on the surface of LDH on the other. Together, these two processes create LDH-TUSA, which can significantly increase the substrate’s corrosion resistance. This synergistically modified superhydrophobic and retardant hydrotalcite film layer offers a novel approach to the investigation of wear reduction and corrosion protection on the surface of aluminum and its alloys.
1. Introduction
Aluminum and its alloys due to their abundant content in the crust,low density,high specific strength,good thermal and electrical conductivity,good plasticity and formability,and other properties, have been widely applied in the field of aircraft and shipbuilding industries, aerospace, electric transportation, automobiles, and electronic products [1–3]. At the same time, because of aluminum’s negative standard electrode potential, its chemical characteristics are more active, and its collective corrosion resistance cannot match the demands of a more complicated technical environment.Furthermore,the surface hardness of aluminum and its alloys is low,and their wear resistance is weak,making it difficult to fulfill the requirements of tribological qualities and metal material strength properties under unique circumstances [4–6].Therefore, surface treatment is needed to improve the wear and corrosion resistance of aluminum and its alloys to enhance their reliability and extend their service lives during the process of use.At present,the methods widely used to improve the corrosion resistance of aluminum alloys in the world mainly include electroplating[7],organic coating[8],anodic oxidation[9–11],micro-arc oxidation [12,13], chemical conversion coating [14], magnetron sputtering [15],etc. In addition to the addition of Si, Mg, Cu, Fe,and other elements[16,17],anodic oxidation and micro-arc oxidation are also commonly used to improve the mechanical strength and corrosion resistance of aluminum alloys [18,19]. The usual remedy, however, is to add PTFE, MoS2, WS2, iodide, etc. to the electrolyte in order to lessen the issue of increased film friction following anodic oxidation and micro-arc oxidation [20,21].
Hydrotalcite is an anionic layered compound, which can be expressed by the general formula∙mH2O,where M2+and M3+are metal cations, and An–is an anion that can exist stably in alkaline solution [22,23]. Hydrotalcite has vast application potential in the sectors of catalysis,adsorption,anticorrosion,drug delivery,and so on due to its unique structure of interlayer anion exchangeability and interlayer cation match ability[24–27].
Much study has been undertaken in recent years on the use of hydrotalcite as an anti-corrosion coating, and the primary processes for preparing hydrotalcite film layers include hydrothermal synthesis and electrodeposition [28,29]. Magnesium hydroxide film can be directly prepared on the metal substrate. Zhouet al.[30] produced Zn-Al layered double hydroxide (Zn-Al-LDH) films on magnesium alloy substrates using a hydrothermal approach,implanted vanadate ions between LDH layersviaan anion exchange process, and demonstrated that the obtained films successfully increased corrosion resistance of magnesium alloy by testing. Zhanget al. [31] prepared Li-Al hydrotalcite films on Al-Li alloy using thein-situgrowth method and modified the prepared hydrotalcite films with 1H, 1H, 2H, 2H-perfluorinated to prepare superhydrophobic hydrotalcite films with contact angle measurements greater than 164°, which exhibited good hydrophobicity.Wuet al. [32] prepared Zn-Al-LDHs films on AZ91D magnesium alloy substrate by electrochemical deposition method. The test results show that LDHs coated Mg alloy has better corrosion resistance than Mg alloy matrix. The results show that the presence of cerium nitrate improves the corrosion resistance of the coating.
Due to the poor adhesion of LDHs directly synthesized on the metal substrate, there is a high risk of coating detachment or blistering, as well as limitations on reaction conditions and high production costs. Therefore, an increasing number of studies have focused on the pre-deposition of a coating layer on the metal substrate prior to LDHs growth. The pre-coating methods can include plasma electrolysis oxidation (PEO) [33–35], microarc oxidation(MAO) [36], porous anodic oxide (PAO) [37],etc.Kaseemet al.[38]discussed the growth mechanism of coral reef-like structured LDHs on the surface of AZ31 magnesium alloy with plasma electrolytic(PE)pre-treatment.The results demonstrated that the coral reef structure effectively delayed the corrosion of magnesium alloy matrix and exhibited excellent corrosion resistance. Kuznetsovet al. [39] used nitrate hydrotalcite and vanadate to seal the AA2024 anodic oxide layer,which demonstrated greater corrosion resistance than standard hydrothermal sealing.
Anodizing has a wider range of applications compared to microarc oxidation and can be used for surface treatment of most metals and alloys.The process is simple,cost-effective,easy to implement and control, and results in a uniform oxide layer across the entire metal surface. The porous anodic oxide film composed of a mixed oxide of Mg and Al generated by anodization is used as an internal provider of Mg2+and Al3+for the subsequentin-situgrowth of LDHs film without the addition of extra metal salts [40,41]. However, LDHs grow vertically on the substrate, providing a pathway for corrosive media and promoting cathodic reactions [42]. Furthermore, prolonged ion exchange without modification results in the accumulation of large amounts of Cl–in the interlayers of LDHs, leading to coating damage and the formation of pits and voids[43].Thus, it is crucial to modify the generated LDHs to promote the growth of nanosheets in any non-oriented direction,effectively enhancing the corrosion resistance of LDH coatings,and to change the traditional vertical growth status of original LDHs perpendicular to the substrate. Additionally, modified coatings significantly reduce the friction coefficient on their surfaces,endowing them with excellent hydrophobicity and abrasion resistance.
To date,superhydrophobic films have been prepared by modifying surfaces with a large number of low surface energy reagents,such as myristic acid [44], sodium laurate [45],etc. However,research on the tribological properties of LDHs has been limited to their use as lubricant additives to improve the lubrication performance of oils[46,47].According to a review of current research reports in this area, the synergistic effect of a hydrophobic layer and an organic corrosion inhibitor to improve the substrate protection of a hydrotalcite film and the collective corrosion resistance of an alloy is rare, and related papers and research progress are scarce. Simultaneously, there are few publications on the use ofin-situsynthetic hydrotalcite coating to reduce the surface friction coefficient. Sulfur-containing molecules such as thiourea exhibit superior permeability and adsorption properties in modifying LDH surface due to their smaller molecular size and polarity.Compared with other surfactants, thiourea is non-toxic, environmentally friendly, and easy to synthesize, which makes it an ideal candidate as an LDH surface modifier.Through chemisorption onto the LDH surface and forming chemical bonds with metal ions,thiourea can alter the surface properties of LDH films and achieve surface modification. Additionally, thiourea can improve the thermal stability and corrosion resistance of LDH films. The ability of stearic acid to create superhydrophobic surfaces on magnesium alloys has been demonstrated [48].
Herein, thein-situgrowth method was used to prepare a layered magnesium aluminum dihydroxide film on the surface of 1060 aluminum anodized film, and the MgAl-LDH film was comodified with stearic acid and thiourea to enhance the corrosion resistance and friction properties of aluminum alloy. This paper offers a fresh perspective on the study and use of functional composite coatings for wear resistance, friction reduction, and corrosion protection on aluminum surfaces. It also paves the way for future research in a field that will be crucial to the practical use of the extensive capabilities of coatings modified with hydrotalcite.
2. Experimental
2.1. Experimental materials and reagents
The main material used in this study is 1060 aluminum plate.The main components of the aluminum plate are as follows: Al 99.6%, Si 0.25%, Cu 0.05%, Mg 0.03%, Zn 0.05%, Mn 0.03%, Ti 0.03%, V 0.05%, and Fe 0.35%. The sample size is 2 cm×4 cm×0.3 cm.All the chemical reagents used in this work are of analytical grade.
2.2. Preparation of LDH film
Aluminum flakes were oxidized for 40 min at a current density of 1.8 A∙dm-2and a temperature control of 16–18°C in mixed solution of 95 g∙L–1sulfuric acid and 10 g∙L–1oxalic acid(C2H2O4∙2H2O).Then, using distilled water to clean the prepared anodic alumina sample, it was treated with ultrasonics in distilled water for 30 s,then rinsed with distilled water, and the anodic alumina was blown dry at room temperature. 2.564 g Mg(NO3)2∙6H2O, 4.32 ml NH3∙H2O and 3.75 ml HNO3were mixed and diluted into 100 ml of mixed solution with distilled water.The pH value of the solution was adjusted to 9 with ammonia water, and the solution was transferred to the reactor. Then the anodic alumina pattern was immersed in solution, the reactor was put into the oven, and the hydrothermal reaction was carried out at 90 °C for 18 h. Finally,take out the sample, clean it with distilled water again, and let it dry at room temperature.
2.3. Surface modification of LDH film
The LDH film prepared in the previous step was first soaked in thiourea solution of 0.1 mol∙L–1, pH = 5 for 5 min, then washed with distilled water and soaked in ethanol solution of stearic acid of 0.1 mol∙L–1for 2 min, both at 45 °C.
2.4. Sample characterization
Scanning electron microscopy(SEM,APREO S LOVAC)was used to analyze the microstructure of the coating and obtain the crosssectional image of the sample.Simultaneously,the surface composition of the pattern film was analyzed by X-ray energy spectrometer (EDS). The chemical bond between LDH coating and the composite coating was analyzed by Fourier transform infrared spectroscopy (FT-IR, Nicolet 380, Thermo Electron Corporation,USA) in the wave number range of 4000–450 cm-1. X-ray diffractometer(XRD,XPER Pro)was used to characterize the crystal structure of the sample with a scanning range of 5°–70°and a scanning speed of (°)∙min-1.
The SFT-2M friction and wear tester(Zhongke-Kaihua,Lanzhou,China) were used to test the tribological properties of the coating when the experimental parameter load was 1 N and the rotational speed was 200 r∙min-1.The friction pair is a GCR15 steel ball with a diameter of 5.0 mm,the radius of the friction ring is set at 5.0 mm,and the test time is 10 min.
Finally, the coating’s corrosion resistance was evaluated using an electrochemical approach. The electrochemical testing uses the Electrochemical Workstation (CHI750E) with a threeelectrode, two-loop test system. The solution is sodium chloride solution at 3.5% (mass) concentration. The auxiliary electrode is platinum, while the reference electrode is highly saturated calomel. The working area of the working electrode is 1 cm2, and the test temperature is room temperature. The scanning rate of Tafel is 5 mV∙s-1, and the scanning range is ± 0.3 V relative to the open circuit potential. Electrochemical impedance spectroscopy (EIS)measured the potential of open circuit, a sine wave potential amplitude of 5 mV, and a test frequency of 0.01 Hz–105Hz.
2.5. Molecular optimization calculations
All calculations in this paper were performed using Materials Studio 2020. We do geometric optimization and look for low energy minima using the DMOL3 classical simulation engine.Additionally, we carried out population analyses, electrostatics, and electron density calculations. The functional was changed to GGA and BLYP under the ‘‘Setup” tab, and the quality was fine. The DFT was chosen as the primary therapy under the‘‘Electronic”tab.
2.6. Crystal morphology simulation of LDH
Computational model data is retrieved from the Materials Project for Mg4Al2H12CO18(mp-1222250) from database version v2021.11.10. To make the cell data acceptable for the micromorphological cells of MgAl-LDH (intercalated with NO3, TU, and SA)created in this article, this paper improves on the data presented in the previous paper.MgAl-LDH crystallizes in the P1 space group,with the unit cell parametersa=b= 5.32542 Å (1 Å=0.1 nm, and the software uses Å as the unit of measurement),c= 23.3535 Å,α = β = 90.00°,γ = 120.00°.
2.7. Surface adsorption model and construction of the solid–liquid interface
Change A(1 0 0)to(2 1 0),where γ changes from 120°to 90°and the cell’s hexagonal structure to a rectangular ‘‘box” in the ‘‘redefine lattice” tab. The supercell operation was performed so that the final box size was 36.893796 Å × 47.51064 Å × 31.5249 Å.The molecular force field chosen was COMPASSII, the van der Waals forces were calculated using the atom-based method, the Coulomb forces (electrostatic forces) were calculated using the Ewald method,and the annealing was simulated using the Adsorption Locater module with medium accuracy and a cutoff radius of 12.5 Å. Currently, the simulation program has three cycles of 15000 steps each.
3. Results and Discussion
3.1. Characterization of the prepared coating
The infrared characterization result of the hydrotalcite film is shown in Fig. 1(a). The spectra shows that the broad and strong absorption peak at 3479.20 cm-1is the stretching vibration peak of O—H associated with hydroxyl groups on the laminate and interlaminate water molecules. The characteristic absorption peak at 1631.12 cm-1represents the O—H bending vibration peak of interlayer water molecules; the characteristic peak at 1383.99 cm-1represents the stretching vibration peak ofbetween layers;and the peak at 833.92 cm-1and 591.17 cm-1represents the lattice vibration of Al—O and Mg—O bonds between laminates. The unique infrared peaks determine the nature and structure of aluminum hydroxide films and demonstrate that the aluminum hydroxide generated on the anodic aluminum oxide surface isintercalated aluminum hydroxide.
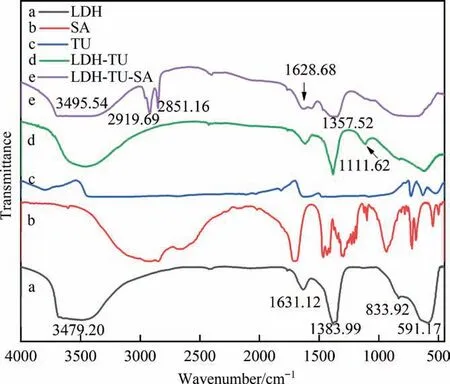
Fig. 1. The infrared spectra of samples with different coating.
When comparing Fig. 1(a) with (d), it is obvious that the IR spectra of the modified hydrotalcite film layer and the original hydrotalcite film layer differ significantly in the low wavelength region. The modified film layer also has an absorption spectrum peak at 1111.62 cm-1that is attributable to the C—N absorption peak, which is formed from the thiourea molecule. In comparison to the hydrotalcite film layer, the IR spectra of the synergistically modified thiourea and stearic acid film layers in Fig.1(e)show significant absorption peaks near 2919.79 cm-1and 2851.16 cm-1,which correspond to the symmetric and asymmetric stretching vibrations of —CH2and —CH3in stearic acid, respectively. In the vicinity of 3495.54 cm-1, the stretching vibration peaks of the hydroxyl groups of stearic acid and the N—H stretching vibration peaks of thiourea overlap with the stretching vibration peaks of the hydroxyl groups in the film of hydrotalcite, so the peak becomes wider than that of hydrotalcite. Combined with the unique IR peak of XRD(Fig.2),the nature and structure of the aluminum hydroxide film were determined and it was demonstrated that the aluminum hydroxide generated on the anodic aluminum oxide surface wasintercalated aluminum hydroxide.
The XRD characterization results of porous anodic alumina[49](PAO),the prepared LDH coating,and the modified LDH coating are shown in Fig. 2. Fig. 2(a) is the characteristic XRD pattern of an anodic alumina film, in which the diffraction peak at 65.07° is the characteristic diffraction peak of the PAO/Al substrate. Fig. 2(b) shows the characteristic XRD pattern of MgAl-LDH. The two low-angle diffraction peaks (0 0 3) and (0 0 6) at 10.91° and 21.91° are typical characteristic diffraction peaks of LDH, indicating that the film grownin situon an anodic alumina surface has a typical structure of LDH [50,51].
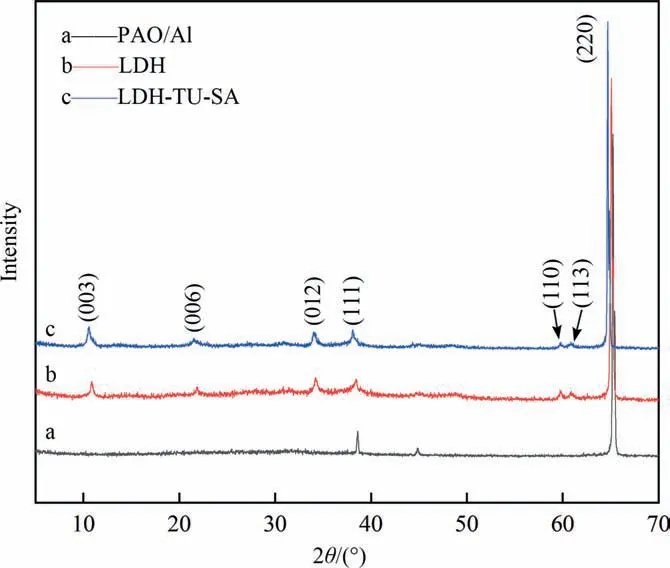
Fig. 2. XRD spectra of samples with different coating preparation.
As shown in Fig.2(c),after the modification treatment,the characteristic peaks of (0 0 3) and (0 0 6) weakened and shifted to 10.61° and 21.53°, respectively, indicating that some of the interlayer ions were replaced, causing the layer spacing to change,and compared with Fig. 2(b), the modified film layer still had a good crystallinity. Studies have shown thattends to peak at around 10.5°–10.7°, but the process of preparing LDH in this paper uses almost no substances containing carbonates, the results showed that the LDH coating, comodified by stearic acid and thiourea, was successfully prepared on the anodic alumina surface by thein-situgrowth method.
Fig. 3(a) shows the cross-section of a Mg-Al hydrotalcite film generatedin situon an anodic alumina surface. The crosssectional thickness of the Mg-Al hydrotalcite film prepared by thein-situgrowth method is about 3.55 μm. It can be seen from the figure that the growth of LDH film on the anodized film surface is more uniform and the thickness is average. The adhesion strength is a crucial factor limiting the widespread use of LDH films. Fig. 3(b) shows the optical image of the LDH film after the grid test. The results indicate that there was no grid detachment after the knife passed through, and from the micrograph of the cross-sectional morphology in Fig. 3(a), it can be observed that there was no lifting or peeling of the film layer after cutting. This suggests that thein-situgrown LDH film has good adhesion to the anodized aluminum surface.
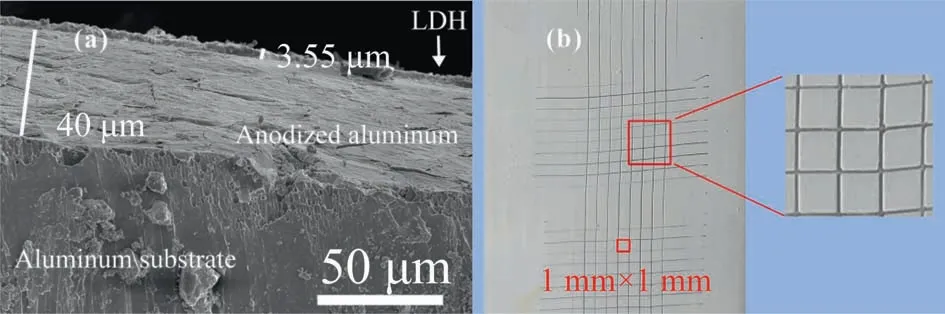
Fig. 3. The preparation of LDH of sample cross-section SEM image and the adhesion test optical figure: (a) SEM image; (b) the adhesion test optical figure.
Fig. 4 shows the SEM image and EDS energy spectrum of the modified sample.As can be seen from Fig.4(a),the prepared lamellar LDH microcrystals are interlaced with each other and are uniformly covered on the surface of the anodic oxidation film along the direction perpendicular to the substrate material. The overall growth state of the nanosheets is compact, uniform, and flat. The vertical crossover micro-nano structure is consistent with the research and discussion of Linet al. [52].The surface micro-nano structure of this material has the characteristic of sheet roughness[53,54],which creates the conditions for enhancing the hydrophobic property of the hydrotalcite film surface.
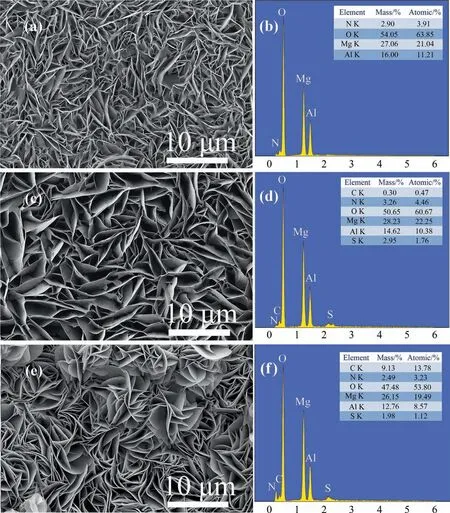
Fig. 4. SEM and EDS images of (a, b) LDH; (c, d) LDH-TU; (e, f) LDH-TU-SA.
As can be seen from Fig. 4(c) and (e), the microstructure of the film after modification does not change significantly compared with that before modification,and the vertically intersecting micro and nano structures are still retained.To further confirm the modification effect, EDS analysis was performed on the film layers before and after the modification. As shown in Fig. 4(b), the main elements in the film before the modification are N, O, Mg, and Al,which are consistent with the elemental composition of MgAl-LDH. The C element and S element were added into the modified hydrotalcite film,which further proved that the thiourea and stearic acid modified hydrotalcite film was successfully prepared.
XPS characterization was used to undertake extensive chemical analysis of the components in the thiourea and stearic acid modified hydrotalcite film layers, and the findings are displayed in Fig. 5. According to the observed XPS complete spectrum, the film layer is mostly formed of the components C,N,O,S,Mg,and Al.The Al 2p spectra in Fig.5(b)shows just one peak at 74.73 eV,confirming the existence of aluminum-containing hydroxides. The Mg 1s spectrum in Fig. 5(c) exhibits just one peak at 1304.13 eV, which is ascribed to the bonding energy of Mg—O,showing the existence of Mg hydroxide in the film layer.Fig.5(d)depicts the C 1s spectra;the fitted peaks at 284.8 eV, 285.5 eV, and 288.6 eV are attributed to the —CH2, —CH3, and —COO— groups in stearic acid, respectively, and Fig. 5(e) depicts the O 1s spectra; 530.9 eV, 531.9 eV,and 532.6 eV are attributed to the M—O bond, the C=O bond,and H2O adsorbed in the film layer present in the metal hydroxide.Fig.5(f)depicts a narrow S 2p spectral peak with a binding energy of 169.06 eV, indicating the possible presence of thiourea molecules in the aluminum hydroxide film layer.
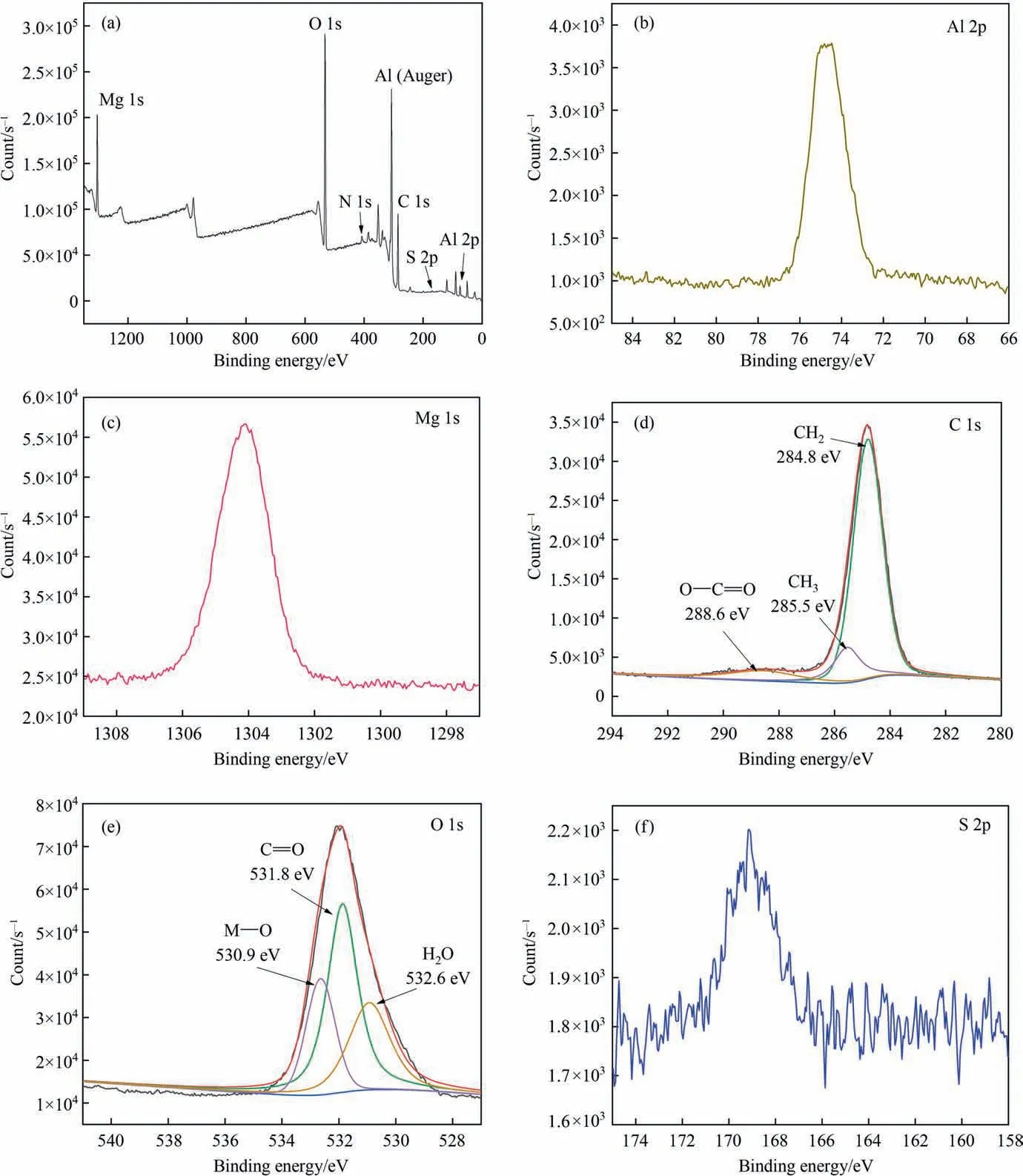
Fig. 5. XPS measurement spectra and XPS surface of LDH: (a) LDH surface high resolution spectrum; (b) Al 2p; (c) Mg 1 s; (d) C 1 s; (e) O 1 s; (f) S 2p.
3.2. Surface wettability
Contact angle (CA) is a characterization tool to measure the wettability of a material surface; when the contact angle is larger than 90°, the material surface is thought to have hydrophobic qualities,and when it is less than 90°,it is seen to have hydrophilic capabilities. The better the hydrophobic property of the prepared film surface, the less likely the corrosive medium will approach the film surface and penetrate into the substrate, the less likely the metal will corrode, and the stronger the barrier effect of the film on the corrosive medium. Therefore, the ease of corrosive medium penetration into the coating can also be used as an important indicator to judge the protective performance of the film on the substrate.
Photos of various sample surfaces’ contact angles are shown in Fig. 6. As shown in Fig. 6(a), the prepared hydrotalcite film layer’s water contact angle is only 4.1°,and the film layer exhibits a superhydrophilic state because of the material’s layered bimetallic structure, the presence of interlayer water molecules and hydroxyl groups on the laminate in its film layer, combined with the high roughness of the film surface. By comparison with Fig. 6(b)and (c), it can be seen that the contact angle value is 144.8° and 144.2°, respectively, and the contact angle increases significantly.This is because after the surface modification treatment, the LDH surface obtains a compact and uniform micro and nano structure,stearic acid can interact with its surface to produce the superhydrophobic surface.
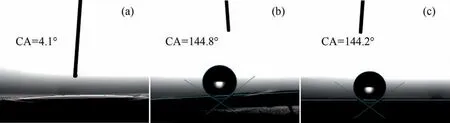
Fig. 6. Water droplets on the surface of the samples of different shapes and the corresponding water contact angle: (a) LDH; (b) LDH-SA; (c) LDH-TU-SA.
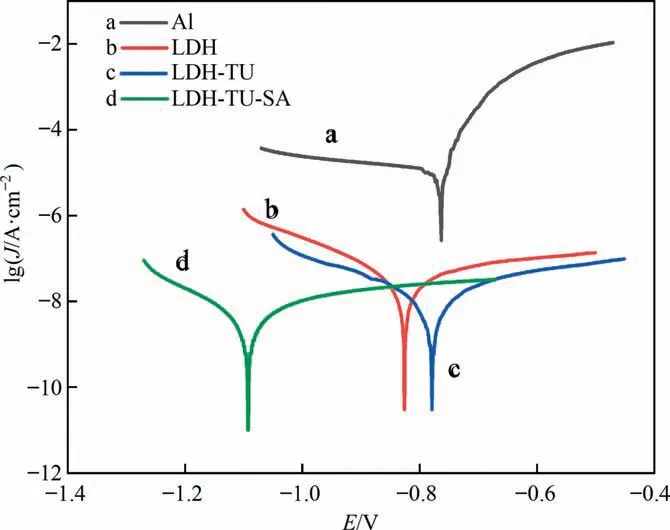
Fig.7. Polarization curves of different coated samples in 3.5%(mass)NaCl solution.
3.3. Corrosion resistance of the as-prepared coatings
Typically, an electrochemical test is used to assess a coating’s corrosion resistance. The dynamic polarization curves of the aluminum matrix, the LDH coating, and the modified LDH coating are shown in Fig. 7. Table 1 displays the corrosion potential (Ecorr)and corrosion current (Jcorr). In the corrosion current density test comparison, we can find that after modified with stearic acid, the corrosion current density relative to the aluminum substrate and LDH decreased significantly, and the corrosion current density of composite modified LDH is smaller than a single modified with stearic acid, which means that the corrosion inhibitor and hydrophobic layer composite modification,compared with a single modification,can further enhance the corrosion resistance,and has better corrosion resistance and inhibition effect on the film.
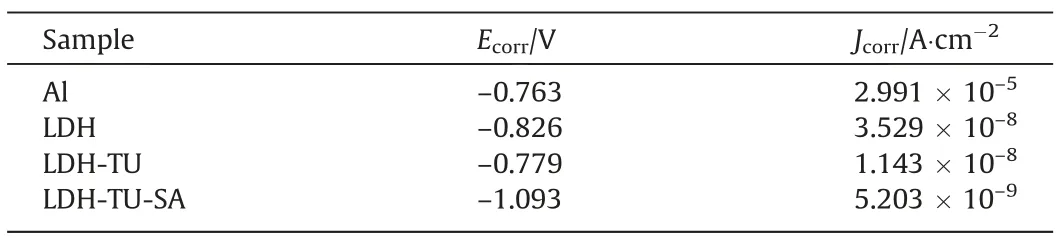
Table 1Tafel fitting results of dynamic potential polarization curve of different coatings
After 1 h immersion of the test piece,the corrosion of aluminum matrix, LDH coating, stearic acid modified LDH coating, and thiourea-stearic acid composite modified LDH coating in 3.5%(mass) sodium chloride solution was studied, respectively, by EIS measurement. The test results are shown in Fig. 8.
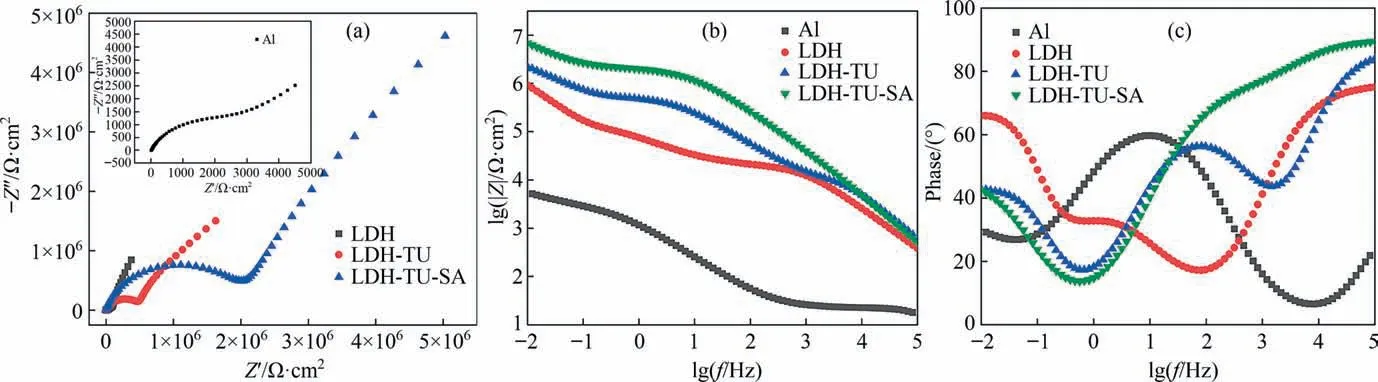
Fig. 8. Nyquist diagram of samples with different coating (a); modulus value diagram (b); phase diagram (c).
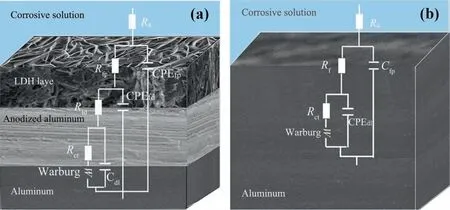
Fig. 9. Equivalent circuit is used for fitting the EIS data. (a) The preparation of film layer equivalent circuit; (b) aluminum matrix equivalent circuit.
It can be seen from the Bode diagram that there are two times constants, high frequency and low frequency, in the phase angle diagram of the aluminum system. The high-frequency time constant corresponds to the capacitance at the interface between the matrix and the solution, while the low-frequency time constant represents the corrosion process of aluminum in sodium chloride solution.After the preparation of the hydrotalcite film and its modification treatment, it is generally considered that the time constant in the high frequency region is the capacitance between the film and the solution interface,the time constant in the middle frequency region is the capacitance of the hydrotalcite layer, and the time constant in the low frequency region is from the alumina film.The fitting circuit is shown in Fig.9.Fig.9(a)shows the equivalent circuit models of LDH and composite modified LDH.Table 2 corresponds to the parameters in the equivalent circuit diagram.Rsis the solution resistance,Rfpis the outer resistance of the coating,Cfpis the capacitance at the interface between the substrate and the solution,Rfdis the inner resistance of the film,CPEfdis the inner capacitance of the film,Rctis the charge transfer resistance,double electric layer capacitance outside the interface ofCdl, andWis the diffusion resistance.Fig.9(b)shows the equivalent circuit model of an aluminum substrate, whereRsis solution resistance,Rfis substrate surface film resistance, CPEfpis capacitance at the substrate and interface,Rctis charge transfer resistance,and CPEdlis the corresponding capacitance.
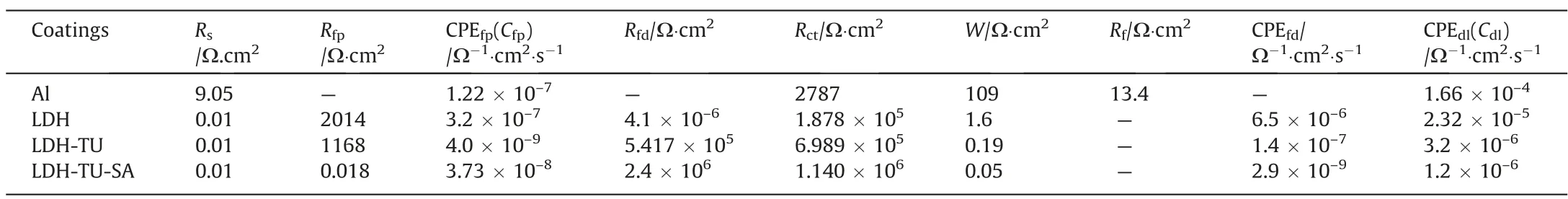
Table 2The parameters corresponding to the equivalent circuit
As shown in Fig. 8(b), the LDH-TU-SA film layer is plotted as a diagonal line in the high-frequency region lg|Z| to lgf, indicating that the film layer is equivalent to an isolated layer with a large resistance value and a small capacitance value at this time.This situation is caused by the hydrophobic effect of stearic acid on the surface of the hydrotalcite film layer after stearic acid modification,which isolates the corrosive medium.LDH-TU-SA film layer has the largest phase angle curve in the high-frequency area,showing that LDH-TU-SA has a high resistance value in comparison to LDH film layer and LDH-TU film layer. Film overall corrosion resistance can be estimated using the Bode diagram: (0.01 Hz) |Z| impedance modulus; the higher the modulus value, the higher the corrosion resistance [55]. As shown in Fig. 8, the low-frequency impedance modulus of the stearic acid-modified LDH coating and the thiourea-stearic acid-modified LDH coating samples are all higher than that of the aluminum matrix. The impedance modulus of LDH is two orders of magnitude greater than that of the Al substrate at 0.01 Hz. The low-frequency impedance modulus is enhanced by about 2 times after thiourea exchange compared to LDH and by about 6 times after compound modification by thiourea and stearic acid, showing that LDH has improved corrosion resistance after modification by thiourea and stearic acid.
In the Nyquist figure, the capacitive reactivity arc of the composite modified coating is the largest in the high-frequency region,indicating that the composite modified coating has the strongest blocking ability and the largest polarization resistance, so the corrosion resistance is the best.The above is attributed to the blocking effect of the film on the corrosive medium in the initial stage of corrosion. At low-frequency, the electrolyte solution is difficult to penetrate into the matrix due to the blocking effect of the thiourea inhibitor. The corrosion process is a slow step controlled by the mass transfer process of the particles involved in the interfacial corrosion reaction.
In conclusion, the modified hydrotalcite film has a good corrosion protection effect on aluminum matrix. On the one hand, the hydrotalcite surface after composite modification forms a hydrophobic layer between the corrosive medium and the film layer,which prevents the corrosion of corrosive ions on the matrix.On the other hand,the insertion of thiourea inhibitor between the layers of hydrotalcite prevents the adsorption of corrosive chloride ions.
3.4. Corrosion state after long time immersion
Fig. 10 shows the comparison of the microstructures of aluminum,anodic alumina,hydrotalcite film,and the modified hydrotalcite film after soaking in 3.5% (mass) sodium chloride solution.As shown in Fig. 10(a), after the immersion test, the surface of a pure aluminum sheet shows the aggregation of corrosion products.The reason is that Cl–in the solution reacts with the aluminum matrix to generate oxidation products attached to the surface of aluminum, namely, the corrosion phenomenon. As shown in Fig. 10(c), pitting corrosion appeared on the alumina film after being immersed in 3.5% (mass) sodium chloride solution for 24 h,and a large number of corrosion holes appeared on its surface.The nature of the pitting corrosion causes the corroded and uncorroded positions to constitute the activation battery, which causes the degree of the pitting corrosion to rapidly aggravate. Fig. 10(b)and (d) show the surface microscopic morphology of aluminum and anodized aluminum in 3.5% (mass) sodium chloride solution after 7 d of immersion,compared with 24 h of immersion,the aluminum surface is completely covered by corrosion products, and anodized aluminum also has a large amount of corrosion products attached to the surface after 7 d of immersion.As shown in Fig.10(e),(g),(f),and(h),no traces of corrosion were observed on the surface of the film layer after 48 h and 7 d of immersion, indicating that the LDH film layer and the modified LDH film layer provided effective protection to the substrate.
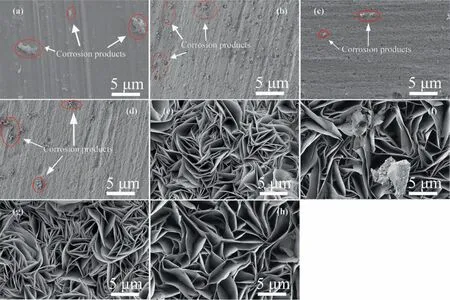
Fig.10. Different samples for different times of SEM images:(a)Al 24 h;(b)Al 7 d;(c)PAO/Al 24 h;(d)PAO/Al 7 d;(e)LDH 48 h;(f)LDH 7 d;(g)LDH-TU-SA 48 h;(h)LDH-TUSA 7 d.
The hydrophobic property of the film surface,i.e., whether the hydrophobic film layer is destroyed in the corrosive environment of the sample for a long period of time, determines whether the hydrophobic film layer can protect the substrate in the corrosive environment for a long time. The sample was immersed in 3.5%(mass) NaCl solution for 65 h to assess the modified coating’s long-term corrosion resistance in the corrosive medium. Fig. 11 depicts a snapshot of the contact angle.The wettability of the modified, treated coatings after immersion was nearly identical when compared to Fig. 6. The surface hydrophobicity test of the LDHTU-SA film layer after 1.5 and 3.5 months in 3.5%(mass)NaCl solution is depicted in Fig.12 using macroscopic optical photos.Fig.12(a) shows that after 1.5 months of immersion in 3.5% (mass) NaCl solution,the produced film layer exhibits a hydrophobic state,with water droplets still having a spherical shape and a contact angle greater than 90°. For the sample immersed for 3.5 months, as shown in Fig. 12(b), the created superhydrophobic surface was extremely stable because it did not exhibit the greater contact angle of Fig. 12(a) but also made it harder for the liquid to wet its surface.
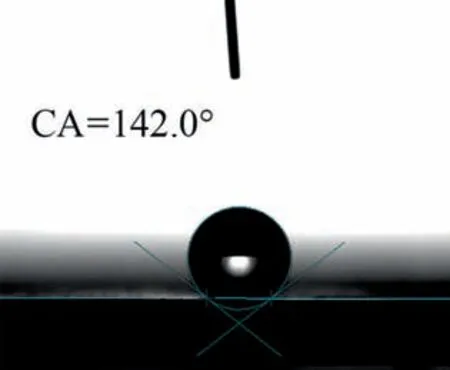
Fig. 11. In 3.5% (mass) NaCl solution for 65 h after LDH-TU-SA film layer contact angle of the photo.

Fig. 12. LDH-TU-SA samples in 3.5% (mass) NaCl solution soak for (a) 1.5 months and (b) 3.5 months of droplets optical images.
3.5. Corrosion resistance mechanism
Fig. 13 shows a schematic illustration of the corrosion protection mechanism of the modified LDH film. The schematic diagram of the corrosion prevention system shows five components: the corrosive medium in the environment,the air film formed between the corrosive medium and the long chain of stearic acid due to the hydrophobic effect of the stearic acid, the hydrotalcite film layer with the organic corrosion inhibitor thiourea inserted between the laminates, the anodized aluminum film layer, and the aluminum substrate. In conclusion, it can be concluded that the LDH film modified by stearic acid and thiourea has excellent corrosion resistance. The main reasons are as follows: First, the superhydrophobic layer formed by stearic acid on the surface of LDH and the corrosive solution form an ‘‘air film”, which can prevent the corrosive ions from being immersed in the matrix. Second, the hydrotalcite film layer acts as a protective barrier once more when a small number of corrosive ions penetrate the hydrophobic film and immerse into the substrate surface. The thiourea molecules between the layers can be exchanged with chloride ions,resulting in the presence of thiourea molecules between the hydrotalcite layers,or they can be exchanged with the hydrotalcite surface,preventing the immersion of chloride ions. Additionally, the thick layer of aluminum anodic oxide acts as a strong deterrent against corrosive ions attacking the metal substrate. In summary, this complex film structure plays an important role in the corrosion resistance of the substrate.
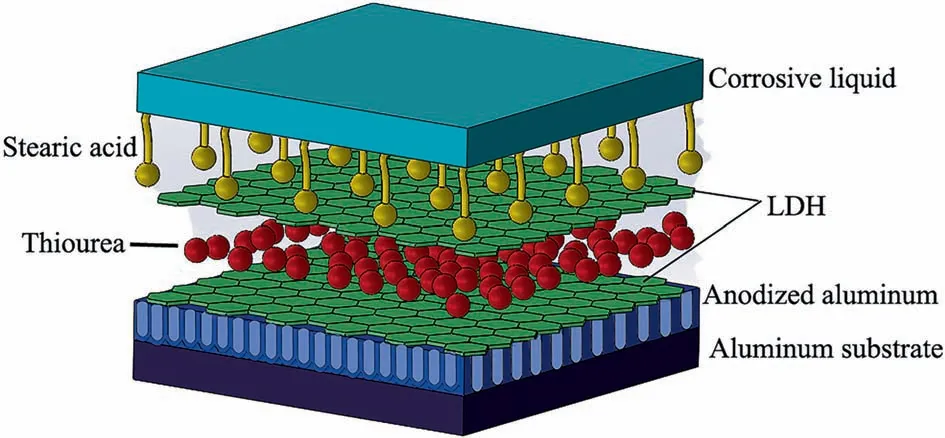
Fig. 13. LDH-TU-SA anticorrosion mechanism of the film.
The XPS and FT-IR results indicate that the mechanism for preparing the modified film mainly involves two steps. Firstly,the porous film formed after anodic oxidation provides sufficient Mg2+and Al3+for the synthesis of LDHs.The reaction can be represented by the following equation [37]:
The second step involves modifying the LDH film using low surface energy reagents (TU and SA), by the following reactions [56]:
Eq.(4)indicates that the modification of Mg-Al-LDHs film by SA is achieved through neutralization reaction with Mg(OH)2. There are polar atoms N in thiourea, which may bind to the empty orbitals of Mg or Al through the provided lone pairs of electrons from the polar atom (Eq. (5)). The thiourea molecule also contains a double-bonded sulfur atom. The carboxyl group in stearic acid can form a coordination bond with the sulfur atom in thiourea,causing the double bond to convert to a single bond,thereby allowing thiourea to bind with stearic acid (Eq. (6)). These reactions modify the Mg-Al-LDH, significantly reducing its surface energy,resulting in hydrophobicity and corrosion resistance.
3.6. Tribological properties
Fig. 14 shows the friction coefficient test diagram of aluminum matrix,anodized film,hydrotalcite film,and modified hydrotalcite film. As can be seen from Fig. 14(c) and (d), after the hydrotalcite film was generated on the surface of anodic alumina by theinsitugrowth method, the friction coefficient was significantly reduced compared with that of the aluminum matrix and anodic alumina film, as shown in Fig. 14(a) and (b), and the friction coefficient was more stable. By comparing curves c and d in Fig. 14, it can be seen that the friction coefficient of the modified hydrotalc film has no significant change compared with that of the unmodified hydrotalc film,indicating that the modified hydrotalc film still has good wear reduction performance.
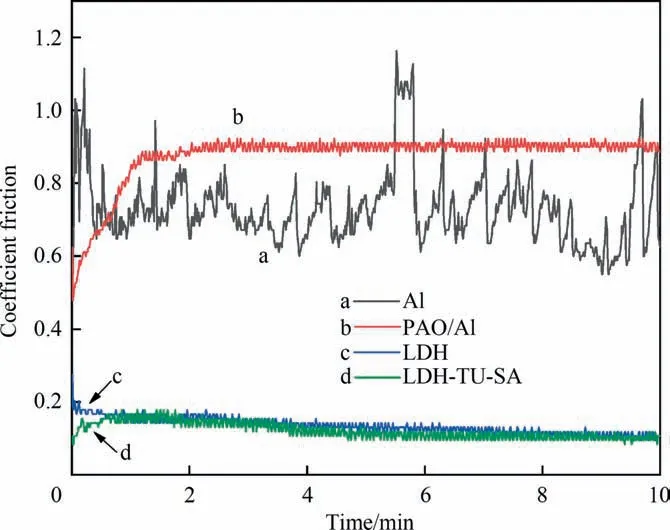
Fig. 14. The friction coefficient curve of different coating.
As shown in Fig.15(a),due to the low hardness of the aluminum matrix, the steel balls will go deep into the matrix during friction,resulting in deep and uneven abrasion and large fluctuations in the aluminum matrix. As can be seen from the SEM figure of anodic alumina in Fig. 15(b), the uneven ceramic structure on the anodic alumina surface improves the hardness of the material, and its uneven structure increases the friction between the surface and the steel ball when the friction test is conducted. On the other hand, the surface develops wide fissures as a result of pressure and friction interacting,which results in a high friction coefficient.As shown in Fig. 15(c), there are electrostatic forces, hydrogen bonds, and other forces between the lamellar structures of hydrotalcite. This soft phase material can form a lubricating film on the surface of the friction pair, resulting in interlamellar sliding of hydrotalcite under the action of shear force,thus reducing the friction coefficient and improving the friction performance of the film.
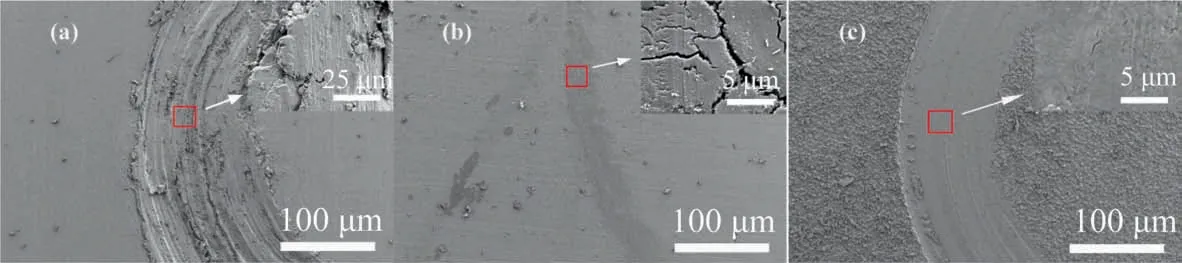
Fig. 15. Different coating grinding crack surface topography. (a) Al; (b) PAO/Al; (c) LDH.
3.7. Theoretical study
3.7.1. Molecular structure optimization
Stearic acid and thiourea molecules were mapped in this study,but as the small molecules may have very complicated potential energy surfaces with several local energy minima and an overall energy minimum,it is necessary to optimize the existing molecular structures. Fig. 16 depicts the charge and electrostatic potential(ESP) after molecular optimization. Fig. 16(a) depicts the stearic acid molecular model,while Fig.16(b)depicts the thiourea molecular model.The changing color of the ESP from blue to green to yellow to red indicates increasingly negative potentials at the corresponding positions. The SA charge’s relative negative region is concentrated on the O1 (–0.440) and O2 (–0.579) of —COOH,with two active sites. The TU charge’s relative negative region is concentrated on S (–0.545), N1 (–0.709), and N2 (–0.709). These active sites suggest that the molecule may rely on these atoms for adsorption (or ion exchange).
3.7.2. Surface model of LDH
Different angles of the constructed simulated structure are shown in Fig. 17. Fig. 17(a) shows an oblique view of MgAl-LDH,Fig. 17(b) shows a side view, and Fig. 17(c) shows a top view.The structure is two-dimensional and consists of one NO3cluster;three O2clusters; and two Mg2Al(HO)6sheets oriented in the (0 0 1) direction. In the NO3cluster, N is joined to three equivalent O atoms in a trigono-planar configuration. The average N—O bond length is 1.283 Å. O is joined to one C atom by a single bond. In each O2cluster, O is bound to atoms in a 2-coordinate geometry.Mg is bound to six O atoms to produce MgO6octahedra, which share edges with three equivalent MgO6octahedra and three equivalent AlO6octahedra in each Mg2Al(HO)6sheet.Two different O sites are present. O is bound in a 4-coordinate geometry to two equivalent Mg, one Al, and one H atoms in the first O site. O is bound in a 4-coordinate geometry to two equivalent Mg, one Al,and one H atoms in the second O site.
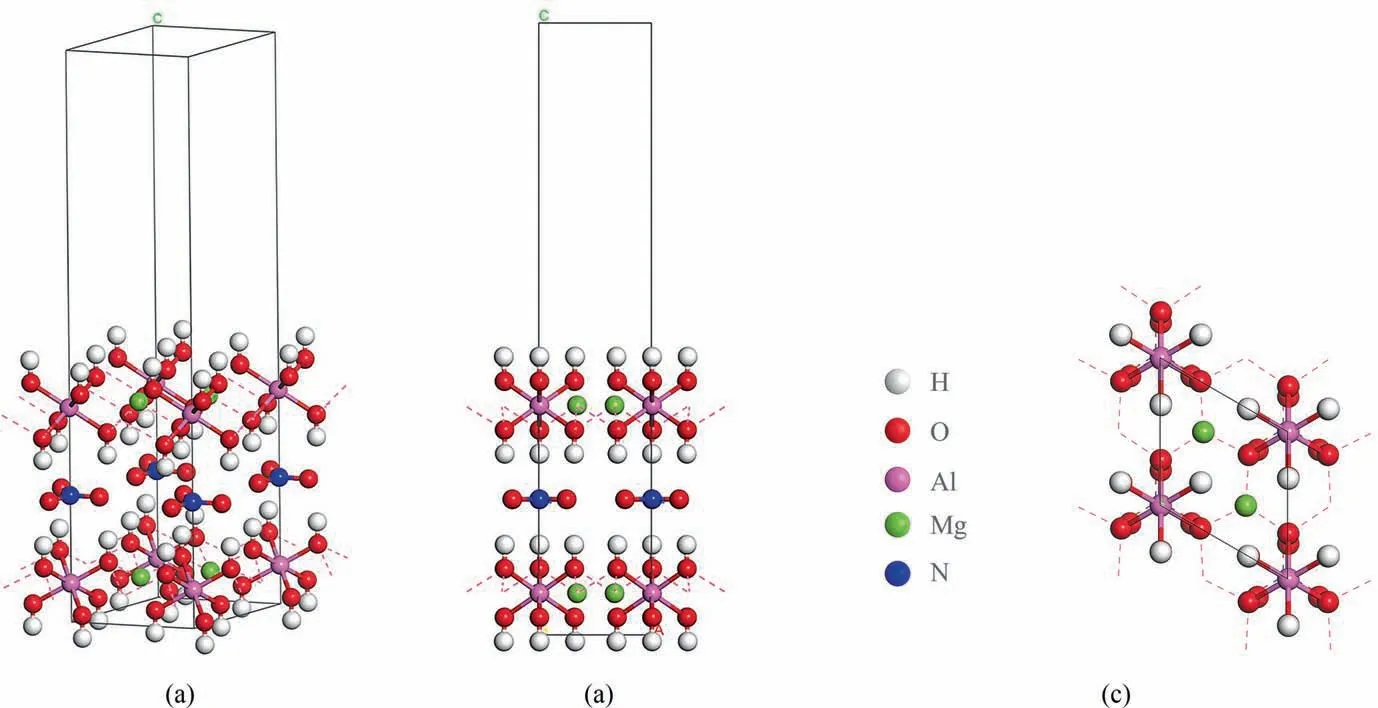
Fig. 17. Different angles of the constructed simulated structure: (a) oblique view of MgAl-LDH; (b) side view; and (c) top view.
3.7.3. Surface adsorption and solid–liquid model construction
In order to further understand the mechanism of action of the MgAl-LDH enhanced anodic oxide film of aluminum, a simulation model of SA adsorption (because the SA molecule is too lengthy to travel through the gap of the Mg-Al-O–H surface system,adsorption on the LDH surface is the mechanism of action, which is compatible with the experiment) on the LDH surface and TU(TU molecules are tiny enough to pass through the voids and enter the interlayerviaion exchange)substitution of interlayer NO3was produced, and 3.5% (mass) NaCl solution system was built in this article. The optimized SA molecules were adsorbed on the LDH (0 0 1)surface using the Adsorption Locator module,and Monte Carlo simulations were run. Fig. 18(a) depicts the box used for adsorption simulation after the initial LDH cell was optimized, and Fig. 18(b), (c), and (d) depict the front, top, and perspective views of individual SA molecules adsorbed on the LDH surface box,respectively.
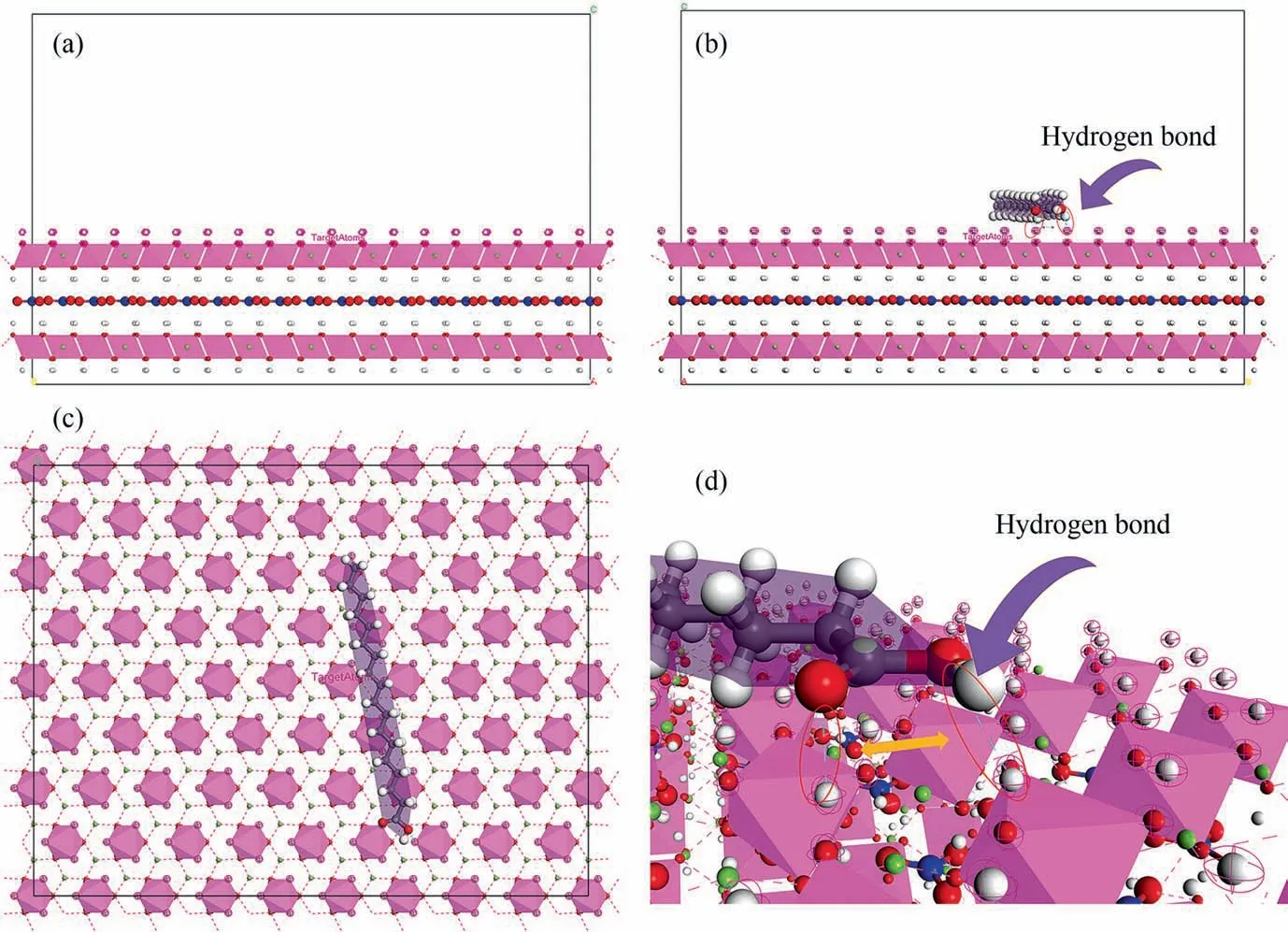
Fig. 18. The optimized LDH (a) surface box with the adsorbed SA from the (b) front; (c) top; and (d) perspective.
The SA molecule is in its lowest energy conformation when it is lying sideways on the LDH surface, as depicted in the image. SA is almost parallel on the LDH surface due to the close potential of the two active sites and the nearly equal forces of the two oxygens on the hydrogen. SA depends on the double-bonded oxygen in—COOH (O1) and oxygen in —OH (O2) to adsorb on the surface hydrogen of LDH and form hydrogen bonds(Fig.18(d)).This is consistent with the ESP analysis.Additionally,despite the fact that O2 has a greater negative potential,the SA molecule is actually slightly inclined toward O1 thanks to the effect of the H of —OH (Fig. 18(b)).
The solution system (which contains 600 water molecules, 16 sodium ions, 16 chloride ions, 1 SA, and 8 TU) was created as depicted in Fig. 19 in order to effectively and graphically depict the modification mechanism of LDH.
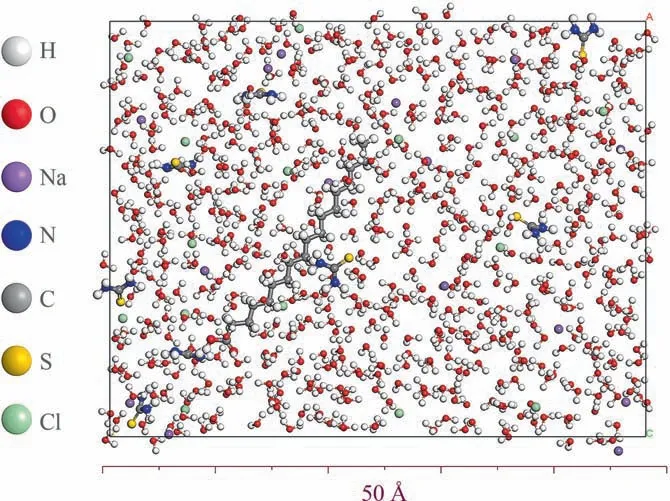
Fig. 19. Solution system box.
The LDH surface is employed as the bottom layer, the solution system as the second layer, and the solution system and LDH surface models are finally integrated into a solid–liquid interface, as illustrated in Fig. 20. Front, oblique, and perspective views of the solid–liquid interface are shown in Fig. 20 (a), (b), and (c), respectively.In addition to simulating LDH exposure to 3.5%(mass)NaCl solution, the full solid–liquid interface also illustrates how SA and TU affect LDH’s surface and therefore explains the mechanism of corrosion prevention.
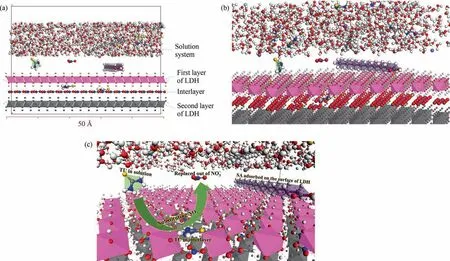
Fig. 20. Solid-liquid interface of LDH form the (a) front view; (b) oblique view and (c) partial perspective view.
The LDH interlayer is composed of multiple,with TU entering and remaining in the interlayer (viaion exchange) in the gap between Al and Mg, while SA molecules (molecules too long to pass through the gap) are adsorbed on the LDH surface. Some of the Al atoms are hidden for observational purposes, as shown in Fig. 20(b), (c). Fig. 20(c) depicts a partial perspective view indicating that TU in solution replaces an interlayerand thus remains in the interlayer to form a dense LDH-TU film layer, while SA is firmly adsorbed on the LDH surface to form an LDH-SA film layer,and the two together form an LDH-TU-SA protective layer to prevent Cl–from passing through the substrate surface and preventing substrate corrosion.
4. Conclusions
In this study,we investigated and assessed the effect of the synergistic action of stearic acid and thiourea on the corrosion and wear resistance of aluminum matrix followingin-situgeneration of hydrotalcite on anodic alumina surface. The findings revealed that:
(1) The stearic acid and thiourea modified LDH film were effectively formed on the surface of anodic alumina through XRD,FT-IR, and EDS characterization.
(2) The morphology of the LDH coating after modification was the same as that without modification, according to SEM,which demonstrated that the hydrotalcum coating developed evenly on the surface of anodic alumina.
(3) Modified LDH films outperformed the aluminum substrate and unmodified LDH films in electrochemical tests,and after immersion in 3.5%(mass)NaCl solution,they still possessed superior hydrophobic characteristics.
(4) After friction experiments, it was shown that the friction coefficient of the anodic alumina surface was significantly reduced due to the growth of the LDH film layer on the alumina surface, which did not change significantly after the synergistic modification of stearic acid and thiourea.
(5) According to theoretical studies, SA adsorbed on the LDH surface, and TU replacedbetween the layersviaion exchange, resulting in the LDH-TU-SA film layer.
This study extends the all-encompassing performance of LDH film layers and offers suggestions for investigating materials with corrosion and wear resistance under unique operating situations.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (51971071 and 52075112) and Fundamental Research Projects of Science & Technology Innovation and development Plan in Yantai City (2022JCYJ023).
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
