States of graphene oxide and surface functional groups amid adsorption of dyes and heavy metal ions
Zhaoyang Han, Ling Sun,2,, Yingying Chu, Jing Wang, Chenyu Wei, Qianlei Jiang, Changbao Han,Hui Yan, Xuemei Song,
1 Institute of Advanced Energy Materials and Devices, Faculty of Materials and Manufacturing, Key Laboratory of Advanced Functional Materials of Education Ministry of China,Beijing University of Technology, Beijing 100124, China
2 Beijing Guyue New Materials Research Institute, Beijing University of Technology, Beijing 100124, China
Keywords: Stock graphene oxide Surface functional groups Existence state Adsorbates Enhanced adsorption Dyes
ABSTRACT Water pollution regarding dyes and heavy metal ions is crucial facing the world. How to effectively separate these contaminants from water has been a key issue. Graphene oxide (GO) promises the greenwater world as a long-lasting spotlight adsorbent material and therefore, harnessing GO has been the research hotspot for over a decade. The state of GO as well as its surface functional groups plays an important role in adsorption.And the way of preparation and structural modification matters to the performance of GO. In this review, the significance of the state of existence of stock GO and surface functional groups is explored in terms of preparation, structural modification, and adsorption. Besides,various adsorbates for GO adsorption are also involved, the discussion of which is rarely established elsewhere.
1. Introduction
Organic dyes and heavy metal ions discharged from the printing and dyeing industry have caused severe environmental problems,and some of the pollutants are highly toxic. Much effort has been paid to finding simple and effective ways to remove contaminants from water bodies. Common treatments include chemical precipitation [1], ion exchange [2], Adsorption [74] has become an effective means of separating organic dyes and heavy metals because of its simple, economical, and efficient characteristics. Common sorbents such as activated carbon and diatomaceous earth have been long used to remove contaminants [3]. Graphene oxide (GO) has emerged in recent years as an effective adsorbent candidate due to the reducing cost of preparation. GO can be obtained through chemical oxidation and exfoliation of the graphite powder by the Hummers method. Through this process, the surface of graphene oxide integrates a large number of oxygen containing functional groups such as epoxy, carbonyls, hydroxyls, and carboxyl groups[4]. These oxygen-containing groups can attract metal ions and organic matter through multiple interactions,such as coordination and electrostatic interactions [5]. Pristine GO and functionalized GO as the emerging carbon-base adsorbents have been extensively researched. However, current progress rarely discussed from the perspective of the existence states of GO itself and the oxygencontaining groups on the GO surface.These two aspects essentially correspond to a variation of the GO structures or some oxygencontaining groups.As a matter of fact,the transformation of specific group had played an essential role to enhance the adsorption of GO. Therefore, this mini review is conducted concerning these perspectives.
2. Existence States of Stock GO
As an adsorbent, stock GO can be of two states when added to the pollutant solution. One is wet GO (WGO), which is dispersed in solvents such as water,and the other is dry.The WGO is usually a solution containing mono-dispersed GO at a known concentration. The state of in-liquid dispersion prevents the structure of GO from restacking damage to some extent,and it retains the original single-layer form well. Noticeably, using such WGO usually meets these issues: First, intrinsically-metastable WGO sheets in solution possibly delaminate and precipitate after a long time,and the concentration of GO may vary. Second, the total volume and the initial concentration of adsorbate shall vary because of mixing the WGO and pollutant solutions. To ignore this impact and better quantify the sorption process, researchers often set the volume ratio of the GO solution to the pollutant solution infinitely close to zero. The WGO retains the most original structure of freshly-prepared GO when stored, but a long duration of standing leads to the precipitation of GO flakes,and the WGO turns out to be an inhomogeneous liquid which subsequently affects the adsorption. Dry GO (DGO) compensates for this deficiency.
DGO is achieved by drying the stock solution of GO.To date,dry GO, such as GO powder, bulk, and aerogel, has been the most reported for the utilization of adsorption. It is straightforward and convenient to use DGO.The exact mass of DGO can be weighed before GO is added to the pollutant solution. This convenience reduces the error occurrence in the adsorption results. Most researchers obtained the DGOs by simply drying them in a drying oven or a freeze-dryer [6–10]. However, these processes would bring irreversible changes to the structure of GO, leading to the deterioration of the performance of GO [11]. It is also an unavoidable issue in the preparation of DGO.To gain insight into the mechanism with WGO and DGO, various characterizations of WGO and DGO are the least requirements.
2.1. Wet GO
The Hummers method and those modified are the common way to prepare GO. Graphite is typically mixed with concentrated sulfuric acid, potassium permanganate, and sodium nitrate at low temperatures, following reaction steps at medium and high temperatures, and then purified with deionized water to yield GO[12]. Fresh GO nanosheets were dispersed in an aqueous solution to form a GO suspension. As mentioned, the WGO preserves the original structure of the GO. After various treatments,WGO is utilized for adsorption.
Zhanget al.[13] investigated the adsorption of methylene blue(MB) by WGO. The experiment found that the adsorption amount of GO became stable after only 5 minutes,indicative of fast adsorption. The adsorption capacity of GO to MB reached 1939 mg∙g-1.WGO diffused rapidly in the MB dye solution and reacted quickly with pollutants. The structure of GO allows it to adsorb dyes through hydrogen bonding, electrostatic interactions, and π-π interactions,resulting in efficient adsorption.The adsorption exhibits the excellence of mono-dispersed GO sheets and their effectiveness. Modification of WGO is an important research direction for advanced adsorption. To date, it has come to two typical advances. The first is to chemically alter the surface structure of GO, and the second is to have different lateral sizes of GO. For the former,two typical examples are summarized.First,laser technology is a practical technique for GO modification because laser irradiation can effectively modify the structure of GO. Paola Russoet al.[14] used a laser to irradiate the GO solution with different irradiation times to obtain the WGOs (GO, rGO-30, and rGO-90)which were used for MB adsorption. The concentration of carbon and oxygen-containing groups on GO was found to decrease after laser irradiation. The C—OH, C—O, C=O and —COOH groups in GO decreased from 23.4%, 35.9%, 9.5% and 6.1% to 17.7%, 24.1%, 7.8%and 6.0%,respectively.Besides,laser irradiation reduced the energy band of GO from 2 to 1.25 eV, indicating reduction of the GO, and X-ray photoelectron spectroscopy (XPS) confirmed this change.The laser-irradiated GO also had the good adsorption performance.Amongst the GO,rGO-30,and rGO-90,the rGO-30 had the maximal adsorption capacity, reaching 746 mg∙g-1. The proportion of oxygen-containing groups on the surface was not positively correlated with the adsorption capacity. That is, GO adsorption did not degrade with laser irradiation while GO roughness increased with increasing laser irradiation duration. Interestingly, the band gap widths of rGO-30 and rGO-90 were around 50 nm and 20 nm,respectively, which related to the variation in surface roughness of the GO. Subsequent irradiation (over 90 min) resulted in fragmentation of the pore network. Thus, the forming of macro pores enhanced the adsorption process (rGO-30), whereas the fragmentation of the porous network occurred at the prolonged irradiation(rGO-90)decreased in the adsorption capacity,resulting in reduced adsorption sites.
As noted above, proper laser irradiation can improve the adsorption performance of WGO(Table 1),but the laser technology requires stringent experimental conditions. By contrast, chemical modification is a common method, which can be achieved using only a variety of reducing agents and oxidizers [10,18]. For instance, GOs were oftenex-situtreated directly with reducing agents for adsorption towards aqueously removing some organic dyes[19].Theseex-situmethods have improved the GO adsorption to some extent,but still underutilize the adsorption sites of the GO structure. To this end, anin-situmethod was proposed with WGO as the adsorbent by Sunet al.[15], aiming to activate those inert sites of WGO for adsorption enhancement. Consequently, a multiple-equilibrium-route-base adsorption was proposed as new application model and implemented, which is distinct from the conventional single-equilibrium way (Fig. 1).
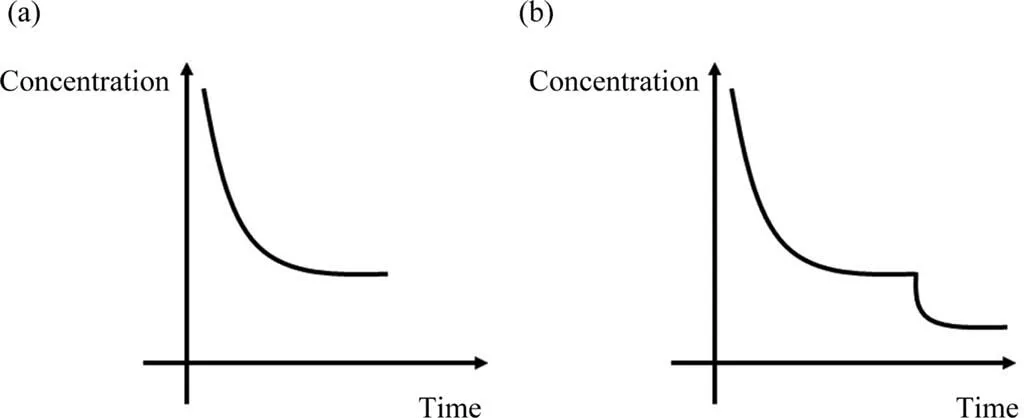
Fig. 1. Adsorption models via (a) single-equilibrium route and (b) multiple-equilibrium route [20]. They respectively represent the time-dependent changes of residual pollutant concentration after the addition of adsorbent.
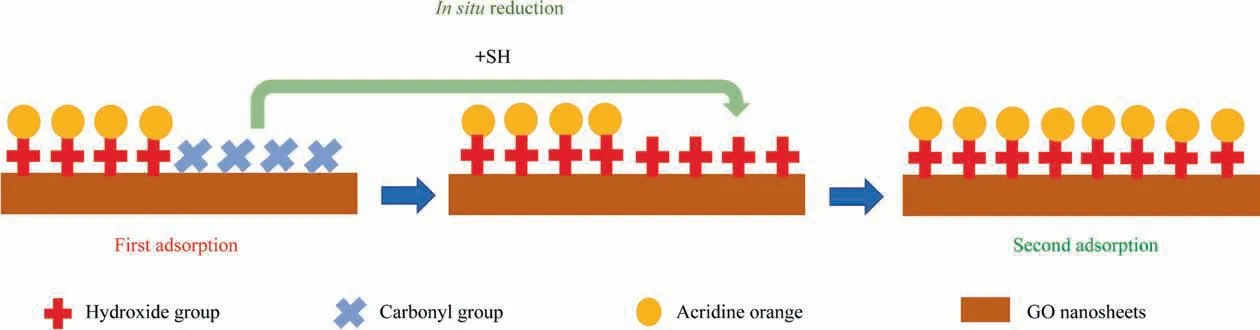
Fig.2. Schematic of the in-situ reduction-enhanced adsorption of GO for the removal of acridine orange.Two steps occur in the process:the first adsorption of GO completes through interactions between the hydroxyl groups and acridine orange molecules, and then a reducing agent is added, converting the original carbonyl groups of GO to hydroxyl groups. Then, these newly formed hydroxyl group interacts with more acridine orange to establish the second adsorption. SH: sodium hydrosulfite.
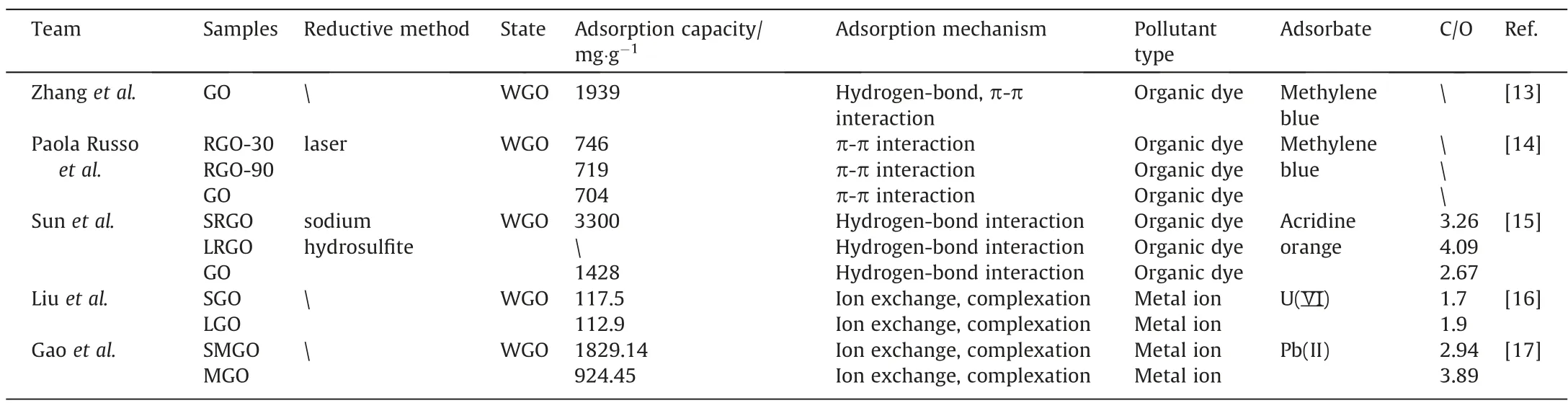
Table 1Various WGOs for adsorption
In this case, WGO droplets were added to acridine orange (AO)solution to approach the first adsorption equilibrium.During such,many visible GO-AO complexes(GOAO)formed inside the solution.After the addition of reducing agent (RA), massive active adsorption sites were brought in due to thein-situconversion of surface GO functionalities, facilitating second-stage adsorption. Subsequently, a second adsorption equilibrium appeared in the system of GOAO. The study was facilitated by the establishment of a control and experimental group.The control group included WGO and overnight-reduction WGO(LRGO),and the experimental group hadin-situreduction WGO (SRGO). The adsorption performance ofSRGO was the highest among the GO, SRGO, and LRGO. The C/O ratio of GO, SRGO, and LRGO was found to be 2.67, 3.26, and 4.09, respectively. As the reduction time increases, the oxygencontaining group on the surface of the GO decreased, but the amount of GO adsorption did not. And the theoretical maximum adsorption capacity to acridine orange could reach 3.3 g∙g-1(SRGO). The adsorption mechanism between acridine orange and WGO was mainly concluded to the hydrogen bonding the between hydroxyl groups on the GO surface and acridine orange.The LRGO achieved a high degree of reduction, that means the oxygenated groups were significantly reduced, but it was not conducive to the adsorption of targeted dye. Therefore, the maximum adsorption capacity was found for SRGO, attributing it to the abundant freshlyin-situformed hydroxyl groups that converted from the unused carbonyl group on the GO surface (Fig. 2).
Of note,the literature above studied the WGO and succeeded in promoting the site utilization ratio of GO, but it ignored the effect of size difference of GO on the oxygen-containing groups [21–23].The common methods to prepare GO of different sizes include ultrasonication [24], centrifugation [25], and graphite sourcespecific preparation [26]. The first two methods used external forces for size grading of GO. The latter uses graphite of different sizes as raw material. Liuet al.[16] prepared GO by the Hummers method and separated GO into different sizes by centrifugation.Centrifuged at 5000 rpm∙min-1for 10 min, GO was divided into two parts: the supernatant containing GO with a length of ~25 μm, and the bottom layer of GO sediment containing GO with a size of 200–300 nm.The average size of GO was calculated,where the GO from the supernatant(LGO)was ~4060 nm and the bottom (SGO) was ~282 nm. LGO and SGO were used for the removal of U(VI). The adsorption can be summarized as follows:(1) the ion exchange between H+and U(VI) ions; (2) strong complexation of U(VI) ions on the GO surface [31]. Their adsorption and desorption behaviors were investigated in the presence of varying pHs and humic acid. When the pH was 6, the adsorption capacity of LGO and SGO was the largest.SGO had a higher adsorption capacity than LGO. Besides, in the presence of Na2PO4, LGO and SGO had the highest adsorption rate and the lowest desorption rate.LGO has a higher adsorption capacity than SGO.SGO and LGO adsorption properties are affected by pH and ions. The adsorption capacity of SGO for U(VI)was 116 mg∙g-1at 323 K,higher than that of SGO.the content of the oxygen containing groups in the SGO(C/O 1.7)was higher than that in the LGO (C/O 1.9), which facilitated the adsorption of U(VI).Gaoet al.[17]synthesized WGO(the pure sols of MGO and SMGO) nanosheets using micron grade natural flake graphite(44 microns)and submicron grade natural flake graphite (0.5 microns) as carbon sources by a modified Hummers method.The WGO was stored in a dialysis bag for storage and then used to adsorb Pb(II) in water. Since the edge of flake graphite tends to preferentially oxidize to form carboxyl groups when GO is prepared by the modified Hummers method [28], under the same oxidation conditions, more edges derived from smaller submicron flake graphite would produce more carboxyl groups at the edges of SMGO. The C/O values for MGO and SMGO were 3.89 and 2.94, respectively, indicating that MGO has a lower proportion of oxygen containing groups compared to SMGO. The proportions of C=O and O—C=O of SMGO were 4.22% and 3.37%,respectively, which were higher than 3.04% and 2.04% of MGO.Some oxygen-containing groups on the GO surface, especially C=O and O—C=O, form strong surface complexes with heteroions such as rare earth ions and heavy metal ions,which can be used as adsorption active sites[9].Therefore,SMGO has higher adsorption capacity than MGO.At 318 K,SMGO and MGO have the maximum adsorption capacities, which are 1829.14 mg∙g-1and 1671.29 mg∙g-1, respectively. After five adsorption–desorption cycles,both SMGO and MGO were able to maintain high adsorption capacities of 700.35 mg∙g-1and 514.26 mg∙g-1.
The preparation of WGO is relatively simple without much post processing. WGO can be modified by size grading or chemical treatment, with changed oxygen-containing groups. Oxygencontaining groups are closely related to the adsorption performance of organic dyes and heavy metal ions,but the excess groups are not positively correlated with the adsorption capacity(Table 1).In addition to the oxygen-containing groups, other structures also affected the adsorption of WGO. For example, the relatively integral aromatic structure allows WGO to complete adsorption through π-π interaction. For metal ions, a different situation occurs. The more oxygen-containing groups on the surface of WGO, the greater the adsorption capacity. This phenomenon can be explained by the fact that the adsorption of metal ions mainly completes through ion exchange and complexation (Table 1). In addition,adsorption can also be affected by specific environmental factors,such as acid,base, ion, and temperature. Under the appropriate pH, the electrostatic interaction between the WGO and metal ions will dominate, thus increasing the adsorption capacity.
2.2. Dry GO
Since the WGO is a sort of adsorbent with high dispersibility and rapid reaction,it is inevitable that some difficult problems will exist.The instability of the solution state and the unique modification method of WGO set obstacles for the further development of novel GO materials.
Compared with WGO, dry GO (DGO) is a practical and chemically-stable adsorbent. Using DGO as a substrate, various GO derivatives can be prepared with excellent adsorption properties by a variety of methods. DGO is usually obtained by drying the GO solution. The way of drying can be roughly divided into two.One is drying without pretreatment,and the other is with pretreatment.Drying without pretreatment refers to drying through a commonly-used oven. The pretreatment refers to freezing, which means the sample is to be frozen in advance.Here,for convenience,the former is referred to as ordinary drying,the latter as the freezedrying. The state of such DGO is thus either a solid particle or an aerogel.It is more convenient for further modification and preparing composite materials. Thus, DGO has also been widely used in adsorption. However, with such state of existence, the previously dispersed GO sheets pile up again, which prolongs the adsorption to become an equilibrium.
2.2.1. Ordinary drying
As mentioned, ordinary drying refers to drying the GO solution into GO solids in a drying oven.Wernkeet al.[29]prepared DGO by the Hummers method and dried it in an oven for 12 h at 60 ℃.Then it was added to the antibiotic cephalexin (CFX) solution for the adsorption study,and the structural changes of GO before and after adsorption were compared. The FTIR spectra of the adsorbed and pre-adsorbed GO are similar, but when compared to the preadsorbed GO, the adsorbed GO show discrete changes at 1635 and 1615 cm-1and discrete bands appear at 1220 cm-1. These changes were due to the presence of CFX overlapped GO, which confirmed the adsorption of CFX by GO. The GO surface is rich in OH groups, and CFX is preferentially adsorbed by OH groups.Adsorption of CFX on adsorbents rich in surface OH groups (e.g.,GO)preferentially occurs and is more stable with horizontally distributed molecules because the amine group of CFX is more likely to interact with the adsorbent surface in this orientation [30,31].But the vertical orientation of the CFX molecule is negatively charged,so the proximity of the negatively charged carboxyl group creates repulsion. Since the surface of GO is negative-charged,there was naturally a tendency of repulsion between adsorbates and adsorbents. Therefore, under the right conditions, the adsorption of more CFX has become a key issue.As a result,when the pH value is close to 7,the adsorption capacity of CFX is 164.35 mg∙g-1(65.73%). The adsorption capacity at pH 4 and 10 is 110.55 and 134.92 mg∙g-1(44% and 54%), respectively. And the maximum adsorption capacity of GO at 318 K can reach 548.77 mg∙g-1. The adsorption ability of DGO is reduced compared to WGO. Although the adsorption ability of DGO is reduced,it is still superior to some carbonaceous materials.
The structural differences and adsorption properties of GO and other nanomaterials such as activated carbon(AC)and carbon nanotubes (CNTs) were compared in the previous studies. Their characteristic structures and electronic properties enable them to interact strongly with organic molecules through non-covalent forces such as hydrogen bonds, π-π stacking, electrostatic van der Waals forces, and hydrophobic interactions [27,32]. Besides,the nanostructure also gives it the advantage of a fast equilibrium rate and high adsorption capacity.Liet al.[24]investigated the difference in the adsorption of methylene blue (MB) by graphene oxide (DGO), activated carbon (AC), and carbon nanotubes (CNTs)after drying them at 100 °C. The Zeta potential of all adsorbents is ≤–17.2 mV in the pH 2.0–9.0, indicating that suspended particles are not easy to aggregate.The uniform dispersion of the adsorbent particles in the solution is beneficial to improve the adsorption efficiency of the adsorbent. The Zeta potential analysis further indicated that nitric acid oxidation introduced large oxygen-containing functional groups onto the surface of these adsorbents.From the isothermal adsorption study over the adsorption behavior of MB on GO,AC,and CNTs,the adsorption amount of methylene blue on AC was the largest (Table 2). The high normalized adsorption amount of MB in GO was due to the electron donor–acceptor interaction of GO with MB [34]. The electrons in the carbon–carbon double bond of MB interact with the benzene ring electrons on the GO surface through electron coupling[35,36].Compared with CNTs,GO has larger and smoother surface,which is conducive to the electron donor receptor interaction between MB molecules and GO, resulting in a higher MB adsorption capacity. DGO has the characteristics of stable structure. The ability of DGO prepared by prolonged drying time to maintain high adsorption properties remains to be verified.
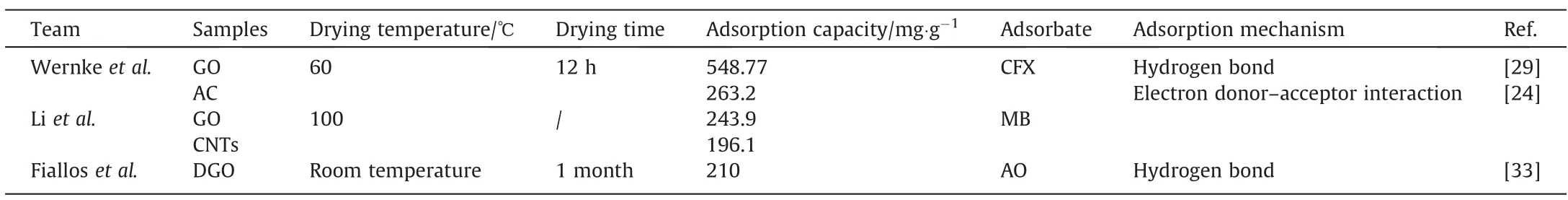
Table 2Various DGOs obtained by ordinary drying for adsorption
Fialloset al.[33]dried WGO at room temperature for a month in order to obtain DGO for the adsorption of acridine orange (AO) at room temperature. The intensity of IR spectra of GO after AO adsorption increased, which the electrostatic interaction between AO and COO groups may cause. With the rise in GO dosage, the adsorption capacity and removal rate gradually increased and eventually became stable. And the DGO obtained at a longpreparation time still maintained high performance.Investigations suggest that there reported various drying temperatures for DGO.The higher the drying temperature, the fewer oxygen-containing functional groups on the surface of DGO as dehydrated and condensed,and the lower the utilization rate of the groups[37].However, the reduction degree of DGO will also increase, and the surface aromatic structure can be repaired,which is also the reason why DGO still has good adsorption performance after drying at a high temperature(≥100°C)(Table 2).From this table,the adsorption process of organic matter by DGO results from the synergistic action of multiple forces. In addition to temperature, drying duration also affects the adsorption amount of DGO. As noted above,the long-term drying also dehydrates and condenses oxygencontaining groups on the surface of the DGO, and its adsorptioncapacity is significantly less than that by short-time drying at a low temperature. However, ordinary-drying DGO remains to have excellent adsorption performance (Table 2).
2.2.2. Freeze drying
Freeze-drying is an unconventional dry method used to prepare GO solid materials known as GO aerogels. Freeze-dried GO has a higher specific surface area than GO by conventional drying. A more porous structure in adsorption favors a better performance[38,39]. In 2020, Zhuet al.[39] prepared chitosan/GO aerogel,and the specific surface area of such aerogel reached 297.431 m2∙g-1, demonstrating significant adsorption for methyl orange (MO). Adsorption efficiency is relatively stable over the pH range 1–5. Under acidic conditions, MO has a Kun-type structure.The sulfate end of MO is negatively charged and can generate electrostatic adsorption with —NH3+on chitosan to achieve the purpose of removing MO.Adsorption efficiency drops rapidly once pH exceeds 5.The negative charge of methyl orange repels the oxygen containing functional groups of chitosan and graphene and its adsorption efficiency is poor.The adsorption efficiency of the composite aerogel to methyl orange was up to 97.2%,with the adsorption capacity was 48.6 mg∙g-1at pH 1.
For GO aerogel,the influence of size difference on its adsorption performance is also essential.Two typical cases were summarized with GO aerogel prepared by GO with different sizes. Shenet al.[40] prepared GO aerogel by a freeze-drying method using GO in three different sizes (small GO: 1 μm, big GO: 5–8 μm, and giant GO: 20 μm). GO was pre-frozen in a refrigerator and then transferred to a freeze-dryer. GO aerogel prepared from the giant GO sheet (3DGGO) showed the fastest adsorption rate for methylene blue and Cd(II). As the concentration of GO blocks decreases, the rate of adsorption increases, and decreasing the filling factor promotes diffusion of pollutant molecules; therefore, the relatively low preparation threshold concentration was an advantage of 3DGGO.The adsorption capacity of GO aerogel was further studied by selecting diesel as an adsorbent. 3DGGO5 (three-dimensional GO aerogel prepared from 0.5 mg∙ml-1Giant GO)had an excellent effect on diesel oil,with an adsorption capacity of up to 193 g∙g-1.According to XPS data, 3DGGO5 has the most oxygen-containing groups on its surface (2.55 mmol g-1—COOH, 30.7 mmol∙g-1—OH/—O—).It has more active adsorption sites because of the large number of oxygen-containing groups. The adsorption capacity of 3DGGO5 is better than that of other samples. Through BET and SEM, the porous structure and high specific surface area(56.7 m2∙g-1)of GO aerogel were confirmed key factors in increasing the adsorption of pollutants. 3D GO aerogels constructed from Gaint GO nanosheets have a larger single pore volume and more oxygen containing groups. Formation of a stable 3D GO aerogel structure requires the consumption of many active sites. The 3D GO aerogel composed of small-sized GO has few available active sites.Thus,3D GO aerogels built from Giant GO sheets can provide a higher adsorption capacity. Leeet al.[41] used the Hummers method to prepare the GO solution, and the freshly prepared GO solution was centrifuged at 500 r∙min-1for 10 min,and the supernatant was taken. The supernatant was lyophilized for 48 h, and the lyophilized products were sonicated for 0 h, 4 h, and 12 h,respectively, denoted as GO 0 h, GO 4 h, and GO 12 h. The results show that the smaller the volume of GO aerogel is, the faster the iron absorption rate is, and the maximum adsorption capacity of Fe(III) can reach 133.3 mg∙g-1. The GO aerogel surface’s hydroxyl groups increased with the increase of ultrasonic time. The adsorption rate of Fe(III)on GO aerogel rises in the order of GO 0 h,GO 4 h,and GO 12 h.According to the adsorption rate of Fe(III),the adsorption process of GO aerogel can be divided into two steps: (1) the chemical interaction between Fe(III)and GO functional groups(fast rate) and (2) the accumulation of Fe(III) ions on the surface of GO sheet (slow rate). The GO aerogel with a smaller particle size had a larger specific surface area and more active sites,and the adsorption was faster than others. That is, the smaller aerogel has more edge surface oxygen functional groups capable of interacting with Fe(III) ions, which can be seen in the rate at the beginning of the adsorption.Compared with previous studies,the material has even better adsorption performance [42–44].
In addition to controlling the particle size, chemical modification of GO aerogels is also the mainstream to improve the adsorption properties. Wuet al.[45] prepared RGO hydrogel by hydrothermal method. Oxalic acid and GO were mixed to obtain RGO hydrogels. The RGO hydrogel was freeze-dried. Oxalic acid was used as a reducing agent. Carbon dioxide was released and embedded into 3D RGO hydrogel (3D RGO) to make the hydrogel porous.The adsorption capacity for Hg(II)and F(I)is approximately 185 mg∙g-1and 31.3 mg∙g-1, respectively. The 3D RGO has better adsorption performance for Hg(II). The involved adsorption mechanism of 3D RGO aerogel was speculated: the hydroxyl group on the 3D RGO aerogel surface reacts with mercury ions: 2(—OH)+Hg2+⇌(—OHg)+2H+;in 3D RGO aerogel,delocalized π electrons(–Cπ)mainly affect the alkalinity of carbon atoms in the graphene plane, as the following chemical equilibrium formula is established in aqueous solution: –Cπ + 2H2O ⇌–Cπ–H3O++OH–;therefore,-Cπ–H3O+can attract fluorine ions by electrostatic force so that fluorine adsorption occurs. This reaction can be described by a formula: –Cπ-H3O++F–⇌–Cπ-H3OF. With this adsorption mechanism, the 3D RGO was unveiled regarding how the 3D RGO was easier to adsorb mercury ions than fluorine ions. Table 3 lists ever-reported GO-based aerogels. All the aerogels were prepared by freezing the precursors in liquid nitrogen and rendered with network structures, having high surface area. Consequently,the excellent adsorption performance of GO aerogel turned out.
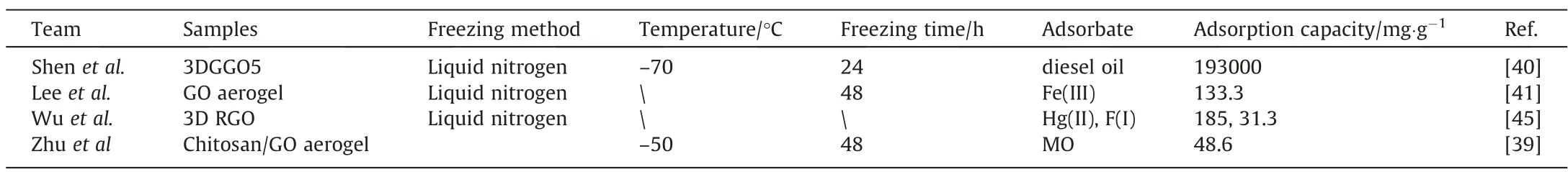
Table 3Various DGOs obtained by freeze-drying for adsorption
Compared with the ordinary drying, the adsorption capacity of the DGO preparedviafreeze-drying was reduced for organic dyes and metal ions,but the adsorption capacity of diesel oil could reach 193 g∙g-1. This phenomenon indicates that the GO material has a clear adsorption preference.
WGO generally has a better adsorption performance over the DGO (Fig. 3). The structure of DGO is stable, but the drying treatment reduces the oxygen-containing groups on the surface of DGO, leading to a decrease in the adsorption capacity of DGO. In the presence of organic dyes, both WGO and DGO tend to have strong adsorption.The aromatic structure of the organic dye allows it to bind to GO through π-π interactions. The oxygen-containing groups on the GO surface are bound to organic dyes through hydrogen bonding. These two mechanisms ensure the highadsorption capability of GO materials for organic dyes. However,the adsorption of metal ions is excessively dependent on the number of active oxygen-containing groups,but the number of oxygencontaining groups contained in GO materials is certain.
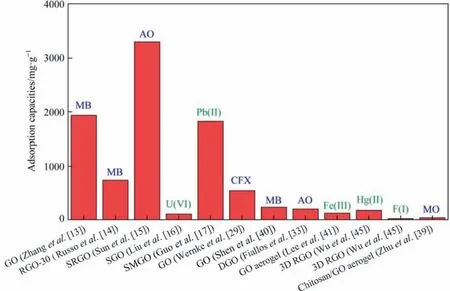
Fig. 3. Various WGOs (left five samples) and DGOs (right seven samples) for removal of organic dyes and metal ions.
3. Existing States of Functional Groups
The essence of studying these two states of GO is to study the changes in the structure of GO or the changes in the oxygencontaining groups of GO. GO featuring a two-dimensional nanostructure have both sides distributed with oxygen-containing groups such as hydroxyl, carboxyl, carbonyl, and epoxy groups[12].Regulating GO,such as surface groups,has become a method to improve the adsorption performance of graphene oxide. The states for these groups involved in adsorption can be roughly divided into two. One is their raw state on the pristine GO; the other is the changed state resulting from the structural modification of GO, employing chemical treatments of either reduction or oxidation. Therefore, three functional group states will be discussed: pristine GO, reduced GO, and oxidized GO.
3.1. On pristine GO
Current methods to make GO often employ different intercalating and oxidizing reagents, and even different graphite sources,thus causing differences in the structure of GO and subsequent adsorption performance. Bezerra de Araujoet al.[46] prepared GO using multiple approaches. The removal rates of GO showed noticeable differences for the adsorption of MB.The GO15 was prepared using KMnO4and ultrasonic bath, having the best performance, and the maximum adsorption capacity was 308 mg∙g-1.Oxygen-containing groups of GO played a vital role in the adsorption. The IR spectrogram of GO15 indicated a strong and broad band observed near 3400 cm-1attributed to O—H stretching vibrations and a weak band observed at 2352.62 cm-1due to C—H bond stretching vibrations.The spectra also showed the presence of carbonyl(C=O)at 1724.21 cm-1,C—OH group at 1409.36 cm-1,epoxy groups(C—O)at 1168.28 cm-1,alkoxy groups at 1039.07 cm-1and the vibration of unoxidized graphite base (C=C aromatic) at 1587.28 cm-1. According to the thermodynamic data (ΔG0value)provided by Araujoet al.[47],the process of GO to MB was physical adsorption.After four adsorption–desorption cycles,MB could still be released from GO and reused with a recovery of more than 90%[48].All of this suggests that in fact the adsorption is reversible and takes place due to physical interactions.
More work with pristine GO was summarized to investigate whether such a mechanism of GO is universal for all pollutants.Mollaet al.[49] investigated the adsorption of GO to MB, methyl orange (MO), and rhodamine B (RhB) experimentally. Compared with MO and RhB, GO has an apparent propensity to adsorb MB and RhB.A mutual repulsion is generated between the electronegative GO and MO, resulting in a reduction in the adsorption of GO to MO. The FTIR spectrum showed the peaks at 1736, 1595, and 1074 cm-1of the GO corresponding to C=O stretching (—COOH),C=C aromatic, and O—H bending. A reduction in the C=N band intensity of GO was observed after the adsorption,indicating good adsorption through the N—H linking, where electrostatic interaction occurred between the =N+H group (positive dipole from the dye molecule, MB) and the oxygen functional group of GO (negative dipole). As reported by Patilet al.[50], this interaction ends with physical adsorption. The adsorption of MB and RhB by GO reached 97% and 88%, respectively, within 15 min. The adsorption of GO showed differences to the three dyes.This phenomenon confirmed that GO has selective adsorption and is more inclined to adsorb cationic dyes.
With the research mentioned above, it was found via infrared spectroscopy that there were various oxygen-containing groups on the surface of GO, such as hydroxyl, carbonyl, and carboxyl groups.Due to the differences in preparation parameters,the peak in the infrared spectrum belonging to the same oxygen-containing group of the final raw GO appeared to have noticeable differences.
3.2. On reduced graphene oxide
Since the pristine GO does not meet the requirements for highperformance adsorbents,the modification based on the pristine GO becomes the best way to achieve high-performance adsorbents.Reduced graphene oxide (RGO) is the most common form in GObased materials. The adsorption of GO to a specific pollutant can be enhanced by selecting an appropriate reduced form of GO. As introduced above, the reduction methods involve two categories:applying reducing agents and introducing new functional groups onto GO.
3.2.1. By chemical reduction
Regarding reduction, hydrazine hydrate (HH) [51], vitamin C[10] (sodium ascorbate, VC), sodium hydrosulfite (SH) [52], and sodium borohydride (SB) [53] are widely acknowledged as effective reducing agents to regulate the microstructure of GO.
HH is likely the earliest reported reagent used to change GO functional groups. Rameshaet al.[54] prepared RGO by mixing exfoliated graphene oxide (EGO) with HH. Inspection of the infrared spectrogram showed that the intensity of the oxygen containing groups on the surface of the RGO was significantly decreased,which meant that oxygen containing groups on the surface of the RGO were considerably removed. The aromatic structure in RGO was recovered during the process.Therefore,RGO adsorbed orange G (OG) mainly by π-π interaction. Furthermore, due to the presence of residual oxygen containing groups in the RGO, adsorption between dye and adsorbent can be supplemented by electrostatic interactions. As a result, the adsorption capacity of RGO to OG reached 5.98 mg∙g-1.Guptaet al.[55]added HH to the GO solution,and washed and dried the product to obtain the GO powder(rGO).The rGO was then used to adsorb malachite green(MG).The significant contribution of C—C/C=C/C—H in the rGO, and significant decrease of the oxygen functionalities shifted the peak maxima of the C 1s X-ray photoelectron spectrum towards the lower binding energy at 284.4 eV. The chemical reduction broadened the tail of the C 1s spectrum of rGO and decreased the intensity ratio(ID/IG)of GO from 3.85 to 3.33.The removal of the oxygen functional during GO reduction increased the grain size of the sp2carbon domain.Therefore,the adsorption between the MG dyes and rGO was facilitated and the adsorption capacity of rGO reached as high as 476.2 mg∙g-1. As to the mechanism of MG adsorption by rGO, it was speculated as follows: First, the rGO became an enrichment source of π-electron cloud. Each molecule of MG has three aromatic rings and cationic centers (N+). Such π-π interactions took place between aromatic rings of MG dyes and graphite frameworks of rGO. Second, the electrostatic interactions also existed between cationic centers of MG molecules and π-electron domains/residual oxygen functional groups of rGO.Third,the defect and hole sites of RGO trap dye molecules [56].
SH is a white sandy crystal or light-yellow powder chemical with substantial reducing and oxidizing properties due to the intermediate valence of sulfur. SH has been used to treat GO due to its strong reducibility. Liet al.[57] used SH to reduce GO and prepared sulfhydryl-reduced GO aerogels (TrGOAs) by a one-pot hydrothermal method under atmospheric pressure. TrGOA-5 was prepared with the mass ratio of GO/SH at 1:5. The XPS characterization showed four subpeaks of the C=C,C—C,C—O/C—S,and C=O bonds at 284.6, 285.5, 286.6, and 288.3 eV in the C 1s peak of TrGOA-5, respectively. Compared with GO, the binding energy intensities of part oxidized groups of TrGOA-5 were significantly reduced. Most oxygen-containing groups were decreased by the SH during the chemical process. It is worth mentioning that the sulfur content was 5.13%, of which the thiol group accounted for 2.44%. It is beneficial to improve the adsorption capacity of TrGOA-5 for Cu2+, and the adsorption capacity for Cu2+reaches 421.21 mg∙g-1.At low pH values,a large amount of H+protonated the oxygen-containing groups on the surface of TrGOA-5,resulting in a low adsorption capacity(~62 mg∙g-1). These results also provide evidence that thiols and other oxygen containing groups play a key role in the Cu2+adsorption process.With such a large number of adsorption sites,TrGOA should have great potential as an adsorbent material for the effective adsorption of heavy metal ions.
Moreover, SB is also a typical GO-reducing chemical for RGObased adsorption.Wanget al.[58]one-step prepared RGO by mixing GO and SB under heating for helianthine adsorption. As compared the group changes of GO and RGO through the FTIR spectrum: for GO, the stretching vibrations of CO—H groups at 3390 cm-1and C=O at 1730 cm-1,skeletal vibration of unoxidized graphitic domains at 1620 cm-1,carboxy C—O at 1350 cm-1,epoxy C—O at 1220 cm-1, and alkoxy C—O at 1060 cm-1; for RGO: the O—H stretching at 3400 cm-1, the skeleton vibration of the graphite oxide domain at 1590 cm-1, and the polysilicate at 1080 cm-1, there was much weaker peak as to the O—H group.Therefore, most of the OH groups of GO were removed. Although most oxygen-containing groups have been removed from RGO,some groups like COOH and OH remained, due to which RGO was negatively charged and easy to adsorb cationic dyes. The adsorption of helianthine by RGO reached 55 mg∙g-1. In addition,sodium borohydride can also assist in the modification of GO.Weiet al.[59] reduced GO with SB and obtained rGO for MB adsorption. The oxygen-containing groups of rGO were effectively removed. Meanwhile, the rGO introduced the functional group containing the benzene ring. For GO, electrostatic attraction and π-π interaction dominated the removal of the cationic dyes; For rGO, the π-π interaction enhanced between rGO and MB. As a result, the amount of MB adsorbed by rGO reached 1250 mg∙g-1.
VC is a natural antioxidant essential to the human diet and is widely used as a food additive [60]. Compared with hydrazine hydrate, VC is harmless to the environment [61]. Tiwariet al.[62] dispersed GO in an aqueous solution and then ultrasonically dissolved VC in the solution.Reduced GO hydrogel(3D RGO hydrogel) was obtained after further filtration, washing, and drying.Methylene blue(MB)and rhodamine B(RhB)were used to investigate the adsorption properties of 3D RGO hydrogel. The specific surface area of the 3D RGO hydrogel approached 298.2 m2∙g-1,and the pores featured the mesopores and macropores. The C 1s bands in the XPS profiles of GO and 3D RGO hydrogel were deconvolved into four peaks,corresponding to C=C/C—C(284.5 eV),C—O(286.6 eV),C=O(287.8 eV),and COOH(289 eV),respectively.Compared with GO, the oxygen content of 3D RGO hydrogel decreased from 52.08% to 38.62%, and the carbon content increased from 47.92% to 61.38%. The C/O ratio of 3D RGO hydrogel rose from 0.92 to 1.59,with the oxygen-containing groups of 3D RGO hydrogel being markedly removed. Due to the strong π-π stacking and anion cation interaction, and the large specific surface area and mesoporous attribute, the 3D RGO hydrogel gained good removal performance toward MB and RhB (removal rate: 97%).
Table 4 shows typical chemically-reduced GOs and adsorption performance for pollutants. Either way, the reduction will significantly reduce the oxygen-containing groups of GO, which oftenincreases the carbon–oxygen ratio.As such reduction incompletely removes these groups from the GO, the dominant mechanism of the adsorption of RGO shifts to through π-π interaction or hydrogen bonding.
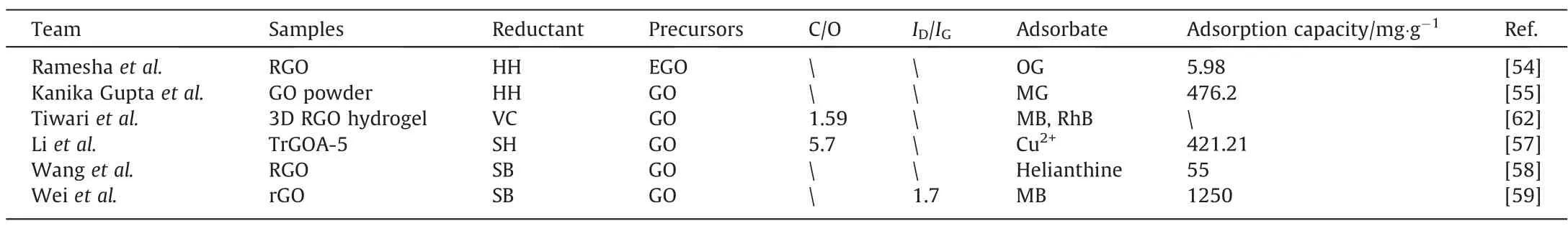
Table 4Chemically reduced GOs for adsorption
3.2.2. By grafting functional groups
GO grafted with specific functional groups is often attempted to enhance the adsorption in addition to chemical reduction.
Wuet al.[63] prepared a rhamnolipid functionalized graphene oxide (RL-GO) complex using a one-step ultrasonic method. GO was mixed with rhamnose,dimethylformamide,and a mixed solution ofN-(3-dimethylamino propyl-n-ethyl carbodiimide)hydrochloride and 4-(dimethylamino pyridine). As a result, the C/O ratio for GO was 5.88, while for RL-GO, this ratio decreased to 5.15. More oxygen-containing functional groups, such as C—OH and C=O, were introduced. Use RL-GO to perform MB adsorption.Because each carbon atom in the RL-GO sheet has a π electron orbital perpendicular to the surface,and MB molecules containing C=C double bonds and benzene rings with π electrons, π-π bonds formed between them.As was confirmed through the FTIR characterization, the C—N stretching for MB shifted from 1319 and 1389 cm-1to 1334 and 1392 cm-1. Therefore, the adsorption capacity of RL-GO was up to 581.40 mg∙g-1at 318 K.
In addition to cationic dye,functional group grafted GO can also adsorb anionic dyes.Buet al.[64]prepared n-phenylthiourea functionalized graphene oxide (GO-NPT) composites by the condensation reaction of GO with N-phenylthiourea. XPS results showed that GO-NPT generated a new N 1s peak(5.18%N atom)compared with GO.Also,from FTIR,the carboxyl group disappeared while the amide C=O bond, the N—H bond of amide, the N—H bond of Nphenylthiourea, and the C=S absorption peak of Nphenylthiourea appeared. The adsorption capacity of GO-NPT to MO came up to 141.467 mg∙g-1,benefiting from a growing number of hydrogen bonds, electrostatic attraction, and π-π interaction compared with the parent material GO (63.84 mg∙g-1). Besides,the GO-NPT had good reusability. Xiaoet al.[19] prepared the Lcysteine-reduced graphene oxide (RGO-Cys) by time-dependent mixing of the L-cysteine and GO.RGO-Cys had an excellent conjugated structure and dispersibility in an aqueous solution. In contrast to the GO, the structure of the RGO-Cys was much different.The proportion of the C—O groups of GO fell from 44% to 17.7%.The rich π-π interaction increased the adsorption capacity of RGO-Cys for both anionic and cationic dyes.The maximum adsorption capacity of anionic indigo carmine(IC)and cationic neutral red(NR) was up to 1005.7 mg∙g-1and 1301.8 mg∙g-1, respectively.
Also,the removal of heavy metal ions is applied for GO in addition to those organic dyes. Zhanget al.[65] mixed GO and fuming sulfonic acid in a high-pressure reaction kettle by sulfonation method and reacted at 343.15 K for 24 h. GOS was then obtained after washing and drying. The C—O—S functional groups formed on GO after such modification. The reduction degree of the GOS decreased significantly. The C=O and O—C=O groups somewhat decreased, but the proportion of the C=C and C—O—C/C—O—S groups prominently increased from 40.72% to 59.70% and 13.26%to 27.94%,respectively.The GO part remained hydroxyl and epoxy functional groups and carbonyl and carboxyl groups at the edge of the sheet [66,67], but the GOS was more abundant in oxygencontaining groups. This structural merit benefited the adsorption of the metal ions and organic compounds through coordination and electrostatic interaction. It resulted in excellent adsorption ability for GOs towards U(VI), with an adsorption capacity of 309.09 mg∙g-1.
Besides direct grafting, double grafting of functional groups is also one method. Mahmoodietal.[68] obtained dithiocarbamate-functionalized GO materials (GO-DTC)viasequentially grafting 3-aminopropyltriethoxysilane (APTES) and carbon disulfide (CS2) to the surface of GO, the APTES being a bridge for CS2. FTIR concluded the evolution of GO-DTC from the formation of the S—C—N bond of dithiocarbamate at 1446 cm-1.As the average adsorption energy suggested, electrostatic adsorption or Van der Waals mainly dominated the adsorption. GO-DTC delivered good adsorption performance for basic blue 41 (BB41,128.5 mg∙g-1)and basic red 46(BR46,111 mg∙g-1)viathe following adsorption processes: GO-DTC(S–) + BB41(N+)→GO-DTC-BB41(SN); GO-DTC(S–) + BR46(N+)→GO-DTC-BR46(SN).
Table 5 shows that functional groups grafted GO with individual adsorption performance. Although the whole grafting led to the reduction of oxygen-containing groups in GO, the introduction of new functional groups increased the types and number of functional groups to a certain extent. These new groups provided adsorption sites and enhanced the capacity of GO.It is noteworthy that some grafting processes resulted in the reduction of oxygencontaining groups of GO and,at the same time,repaired the structure of GO itself.
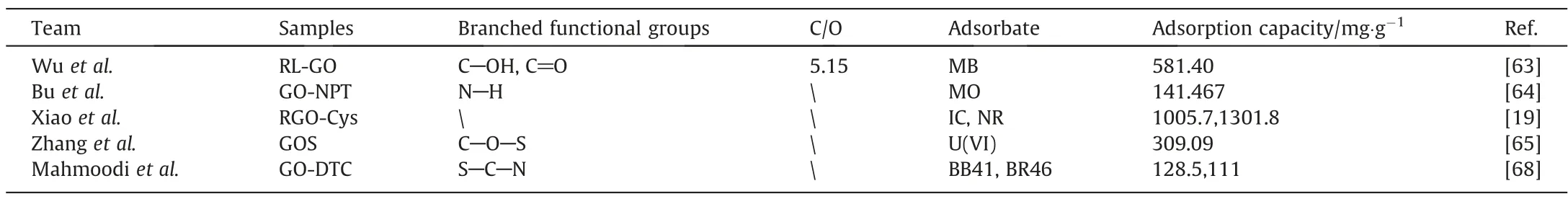
Table 5Surface grafting GOs for adsorption
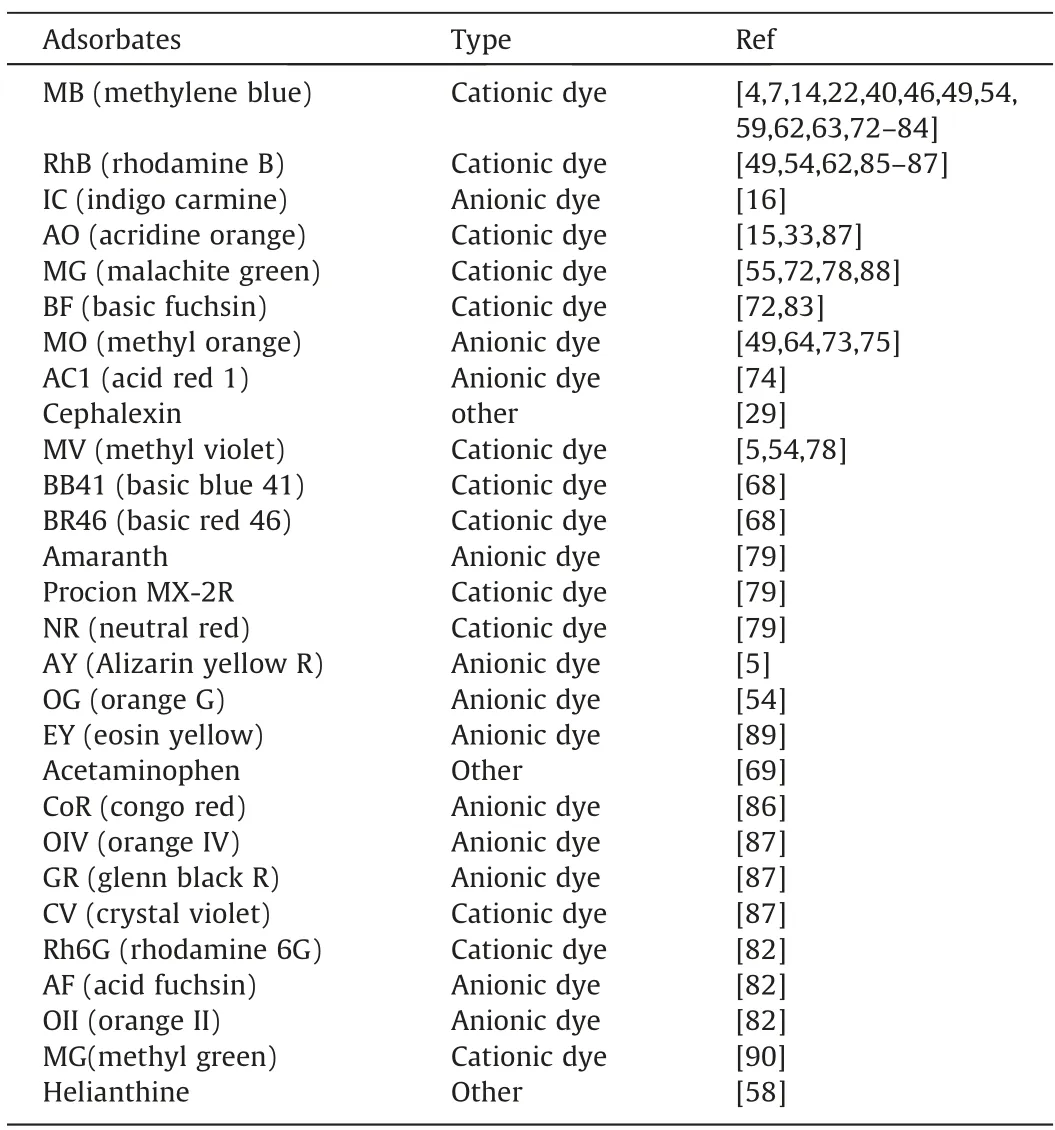
Table 6Organic adsorbates examined in experiments
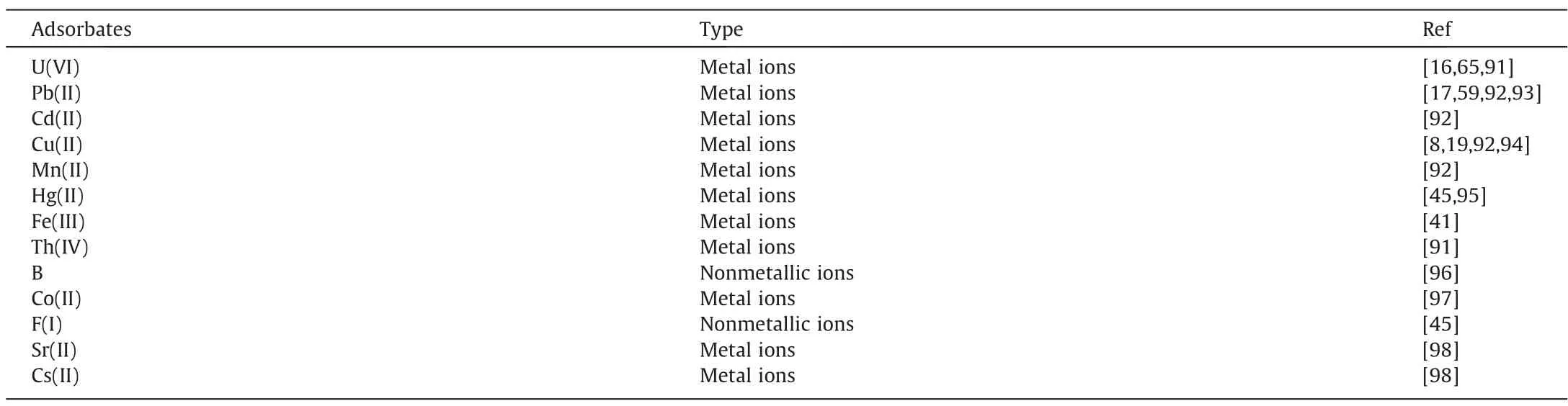
Table 7Elemental ions used in experiments
3.3. On oxidized graphene oxide
In addition to the common reduction modification means, the oxidation of GO is also a method to improve the adsorption active site on the GO surface. This method provides a new angle for the modification of GO.
To our knowledge, only a few reports regarding oxidized graphene oxide (OGO) have been published. Moussaviet al.[69] prepared the OGO by double repeating the Hummers method. After being prepared with the Hummers method, the resulting dried GO suffered secondary oxidation following the Hummers method.FTIR spectra indicated that the OGO contained a high density of oxygen-containing functional groups over GO, including the COO–and peroxide groups, promoting the adsorption rate. Thus,the OGO had an excellent adsorption capacity of acetaminophen,up to 704 mg∙g-1. The modified OGO-based adsorbent was also used to achieve high performance. Alipouret al.[70] used polydopamine-coated double-oxide graphene oxide (PD-GO)sheets to adsorb Nile blue (NB) and paraquat (PQ) in wastewater.These formed catechol groups and phenolic C—O groups verified the successful polymerization of dopamine (DP) in the presence of GO, as correlated new bonds appeared in the infrared spectrum at 1460, 1350, and 1232 cm-1. Notably, GO was found to be partially reduced during DP polymerization due to the absence of C=O stretching vibration observed near 1731 cm-1[71].Compared with GO, the proportion of oxygen-containing groups of the OGO increased further producing more active adsorption sites.But there was the same in the types of groups and the monolayer adsorptive behavior. Through electrostatic interaction and π-π* stacking, the adsorption capacity of PD-GO for NB and PQ reached 128.18 mg∙g-1and 88.517 mg∙g-1, respectively. OGO and its composites have demonstrated promising adsorption properties for various organic pollutants. And currently, this field needs further investigation.
By comparing the adsorption of pristine GO, reduced graphene oxide and oxidized graphene oxide to different pollutants, it can be found that the adsorption capacity of modified GO such as reduced graphene oxide is higher than that of pristine GO and oxidized graphene oxide (Fig. 4). This result also indicates that the adsorption capacity of GO can be effectively improved by reducing agent modification and grafting functional groups. From the perspective of the adsorption mechanism, pristine GO, reduced graphene oxide, and oxidized graphene oxide adsorb organic dyesviahydrogen bonding and a π-π interaction, whereas metal ions adsorbviaion exchange and complexation.This result is consistent with that of WGO and DGO.
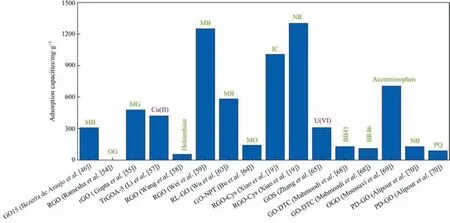
Fig. 4. Adsorption of pristine GO (left one), reduced GO (middle twelve) and oxidized GO (right three) targeting removal of different pollutants.
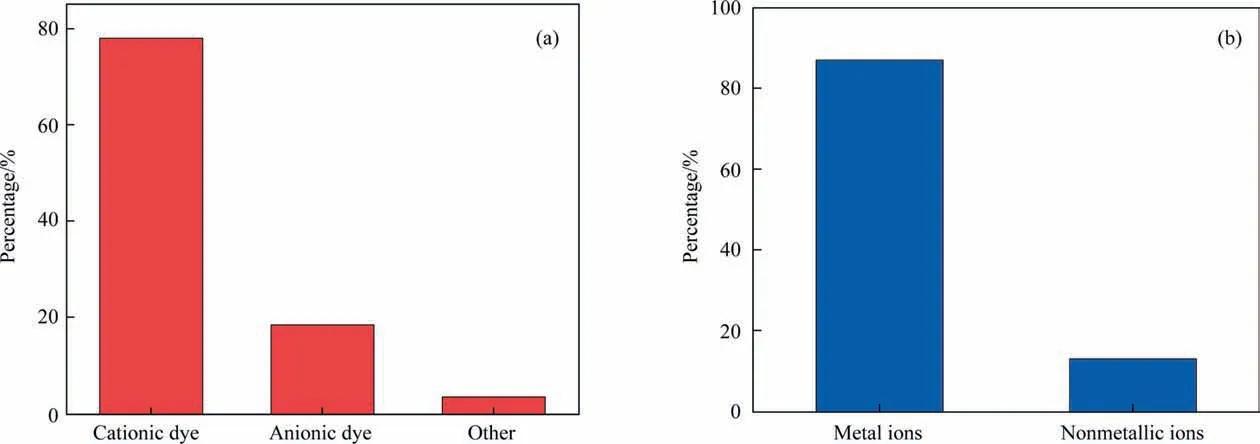
Fig. 5. Statistics of (a) organic and (b) element ionic adsorbates in lab-level publications.
4. Adsorbates in Research
As discussed above, GO has delivered exceptional promise in adsorption at the lab level. But few reviews have ever taken the adsorbates into investigation. For this purpose, the adsorbates reported in the laboratory are summarized.For convenience,these adsorption materials are categorized into three forms:organic dye,ions, and others. Tables 6 and 7 show the adsorbates commonly used. There are many oxygen-containing groups (—OH, —COOH)on the surface of GO. The negatively charged property of GO, first,results in strong attraction with various positively-charged dyes,and second, delivers a performance of tremendous interest to GO itself and the scientific community.Even the modification remains highly probable for GO in favor of adsorb cations and cationic dyes.This is why such abundant work focuses on ‘‘GO- positivelycharged targets” as research objectives other than the ‘‘GOnegatively-charged counterparts”(Fig.5)[99,100].However,there is still the adsorption of various anionic dyes by GO materials,which are grafted with positively charged functional groups.Since the publications centering the ‘‘negatively-charged counterparts”work are limitedly reported, much effort remains to be released for the research of this area.
5. Conclusion and Prospect
In this paper, research progress on the adsorption of GO is reviewed from the perspective of adsorbent and adsorbate. For the adsorbent,the states of existence and surface functional group forms of GO are respectively discussed. Dry or wet state of existence shall influence the structure of GO, affecting the number of active sites of the surface and thus changing the adsorption performance. As to the modification, it changes the original structure of GO itself, such as by reducing the oxygen-containing groups. Furthermore, it can also bring in new active adsorption sites. For adsorption, since there is always a trade-off issue between active adsorption sites and inert sites. Thus, the modification is always on the way to enhancing the utilization of the active adsorption site. As to adsorbate, commonly-researched adsorbates are paid attention to for GO adsorption. Possibly due to the ‘‘good-match”nature of GO itself towards cationic pollutants, three dyes such as methylene blue, malachite green and rhodamine B have been the main targeted adsorbates, while others, especially harmful anionic organic dyes and metal/non-metal ions,gain less attention.Therefore,from the viewpoint of dealing practical environmental/-social risks, there remains a long way to go with GO as a highperformance platform.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The contribution was supported by the National Natural Science Foundation of China (51902007).
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
